Significance
Compromised CD8+ T-cell immunity is associated with significant morbidity and mortality in the elderly. Whereas the number of naïve CD8+ T cells declines with age, the drivers of loss and consequences for clonal composition are unclear. We show that aging disproportionately impacts small naïve CD8+ T-cell populations. For one CD8+ T-cell population, loss of diversity was minimally attributable to expansion but rather was associated with diminished cell number and selective retention of cells exhibiting markers of heightened self, but not foreign, recognition. Thus, vaccine formulations for the elderly may benefit from targeting naïve antigen-specific populations with relatively high precursor frequency and self-reactivity, and retention of high-quality T cells may be achieved through repeated low-level T-cell receptor stimulation.
Keywords: naive CD8+ T cells, aging, CD44+ virtual memory, influenza A virus, CD5
Abstract
In advanced age, decreased CD8+ cytotoxic T-lymphocyte (CTL) responses to novel pathogens and cancer is paralleled by a decline in the number and function of naïve CTL precursors (CTLp). Although the age-related fall in CD8+ T-cell numbers is well established, neither the underlying mechanisms nor the extent of variation for different epitope specificities have been defined. Furthermore, naïve CD8+ T cells expressing high levels of CD44 accumulate with age, but it is unknown whether this accumulation reflects their preferential survival or an age-dependent driver of CD8+ T-cell proliferation. Here, we track the number and phenotype of four influenza A virus (IAV)-specific CTLp populations in naïve C57BL/6 (B6) mice during aging, and compare T-cell receptor (TCR) clonal diversity for the CD44hi and CD44lo subsets of one such population. We show differential onset of decline for several IAV-specific CD8+ T-cell populations with advanced age that parallel age-associated changes in the B6 immunodominance hierarchy, suggestive of distinct impacts of aging on different epitope-specific populations. Despite finding no evidence of clonal expansions in an aged, epitope-specific TCR repertoire, nonrandom alterations in TCR usage were observed, along with elevated CD5 and CD8 coreceptor expression. Collectively, these data demonstrate that naïve CD8+ T cells expressing markers of heightened self-recognition are selectively retained, but not clonally expanded, during aging.
Given that CD8+ cytotoxic T-lymphocyte (CTL) immunity is critical for the control of viruses and tumors, specific defects are likely to contribute substantially to overall immune dysfunction. Normal aging is associated with increased health risk, as vaccine efficacy wanes and susceptibility to, and severity of, a variety of infections and malignancies is enhanced (1, 2). Whereas aging compromises a number of arms of the immune system (3), studies in mice and humans have demonstrated deficits that are intrinsic to naïve CTL populations (4–6). Thus, a complete understanding of both age-related CTL deficiencies and the underlying mechanisms is critical.
The magnitude of the response, the diversity of T-cell receptor (TCR)-defined clonal recruitment, and the avidity of TCR binding to cognate peptides in complex with MHC class I glycoproteins (pMHCI) are all key determinants of CTL response efficacy (7). Each of these factors is substantially constrained by their respective characteristics in the naive CTL precursor (CTLp) pool (7–10). During aging, both the number and TCR diversity of naïve polyclonal and epitope-specific CD8+ T-cell sets decreases (11–13). In addition, a large proportion (∼60–80%) of the remaining naïve CD8+ T cells, termed virtual memory (TVM) cells, express high levels of CD44, traditionally regarded as a marker of T-cell activation and suggestive of proliferation (14). It is unclear whether the accumulation of TVM cells with age (15) represents preferential retention, de novo generation, or expansion of the TVM subset. TCR repertoire analyses show emerging TCR bias with age (11, 13, 16), limiting the diversity of the TCR repertoire beyond that defined by reduced CTLp numbers. Although it is clear that TCR biases parallel age-related T-cell loss, the relative impact of selective clonal decay versus clonal expansion remains unresolved, as do the key determinants of naïve CTLp survival.
The frequency and TCR usage of naïve and immune CD8+ T cells specific for a range of influenza A virus (IAV) epitopes is well characterized for B6 mice (17–20), providing a convenient experimental system for investigating the characteristics and drivers of age-related CD8+ T-cell decline. Primary responses to the DbNP366–374 and DbPA224–233 epitopes (derived from nucleoprotein and acid polymerase proteins, respectively) are immunodominant in young adult mice, whereas those to a polymerase B subunit 1 epitope (KbPB1703–711) and an epitope derived from a shifted reading frame of PB1 (DbPB1-F262–70) are subdominant (21). This numerical immunodominance hierarchy shifts with aging, with a decrease in DbNP366-specific responses alongside an increase in KbPB1703- and DbPB1-F262–specific responses (13, 22). It has been suggested that this reflects the stochastic decay of CTLp (13), but the rate of decline of individual epitope-specific populations has not been directly assessed during aging.
Here, we directly track the numbers of immune and naive IAV epitope-specific CD8+ T-cell populations through the course of aging, together with the phenotypic and clonotypic characteristics of a naïve CTLp set. We show that the shift in immunodominance across DbNP366, DbPA224, KbPB1703, and DbPB1-F262 for 12-mo-old versus 3-mo mice can be accounted for by differential onset of decline across naïve CTLp populations. Moreover, phenotypic changes in a naïve CTLp set indicates that age-related clonal persistence is associated with the capacity to recognize self-pMHCI, which may, in turn, alter the capacity of CTLps to responding to novel challenges.
Results
Age-Related CD8+ T-Cell Immunodominance Hierarchy Shifts.
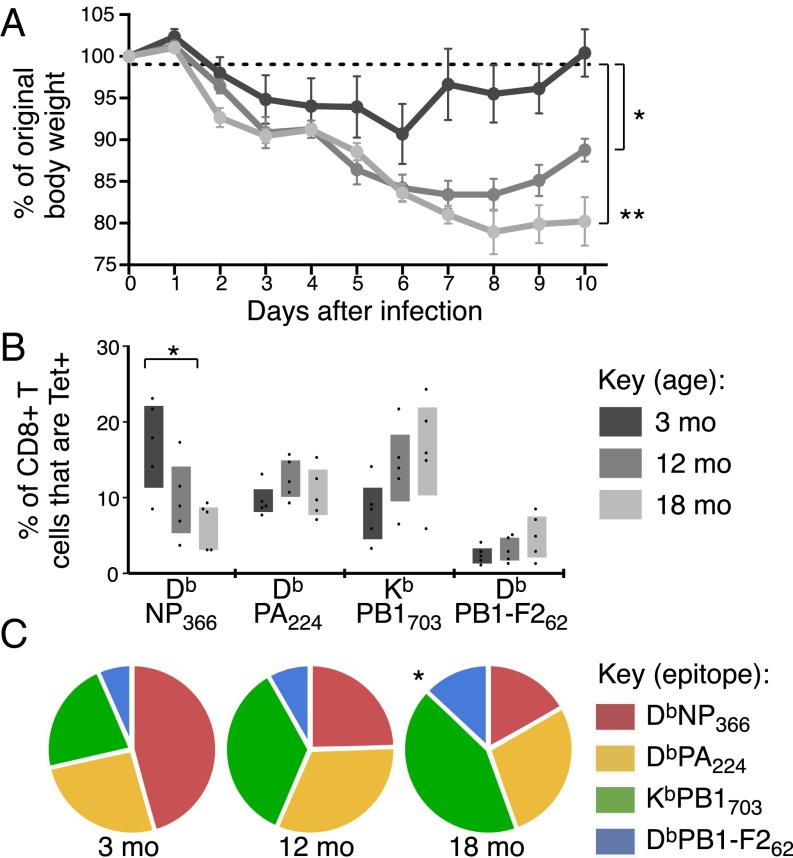
Primary IAV infection of young adult mice results in a well-characterized CD8+ T-cell immunodominance hierarchy, which shifts significantly during aging (13, 22). To establish when this shift occurs, we tracked CD8+ T-cell responses to four IAV-derived epitopes following infection: DbNP366 and DbPA224 (immunodominant), and KbPB1703 and DbPB1-F262 (subdominant), at 3, 12, and 18 mo. Symptoms of infection were more severe in 12- and 18-mo mice, with significantly more weight lost over the course of infection, and an impaired capacity to recover by 10 d after infection (Fig. 1A). This increase in severity may be indicative of a diminished general resilience or a prolonged viral load after infection at older ages (23).
Fig. 1.
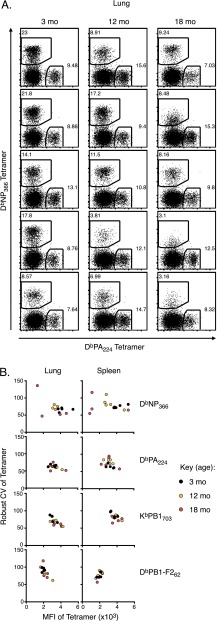
Shift in IAV CTL immunodominance hierarchy with aging. B6 mice were infected i.n. with IAV at 3, 12, and 18 mo. (A) Percent of original body weight at d 0 (mean ± SEM). (B) The frequency of epitope-specific CD8+ T cells as assessed by tetramer staining in the lung 10 d after infection. Bars represent interquartile range (IQR). (C) Prevalence of each epitope-specific CD8+ T-cell population as a proportion of total tetramer+ CD8+ T cells in the lung 10 d after infection. *P ≤ 0.05 and **P ≤ 0.01 compared with 3-mo mice. Data are representative of two independent experiments (n = 4–5).
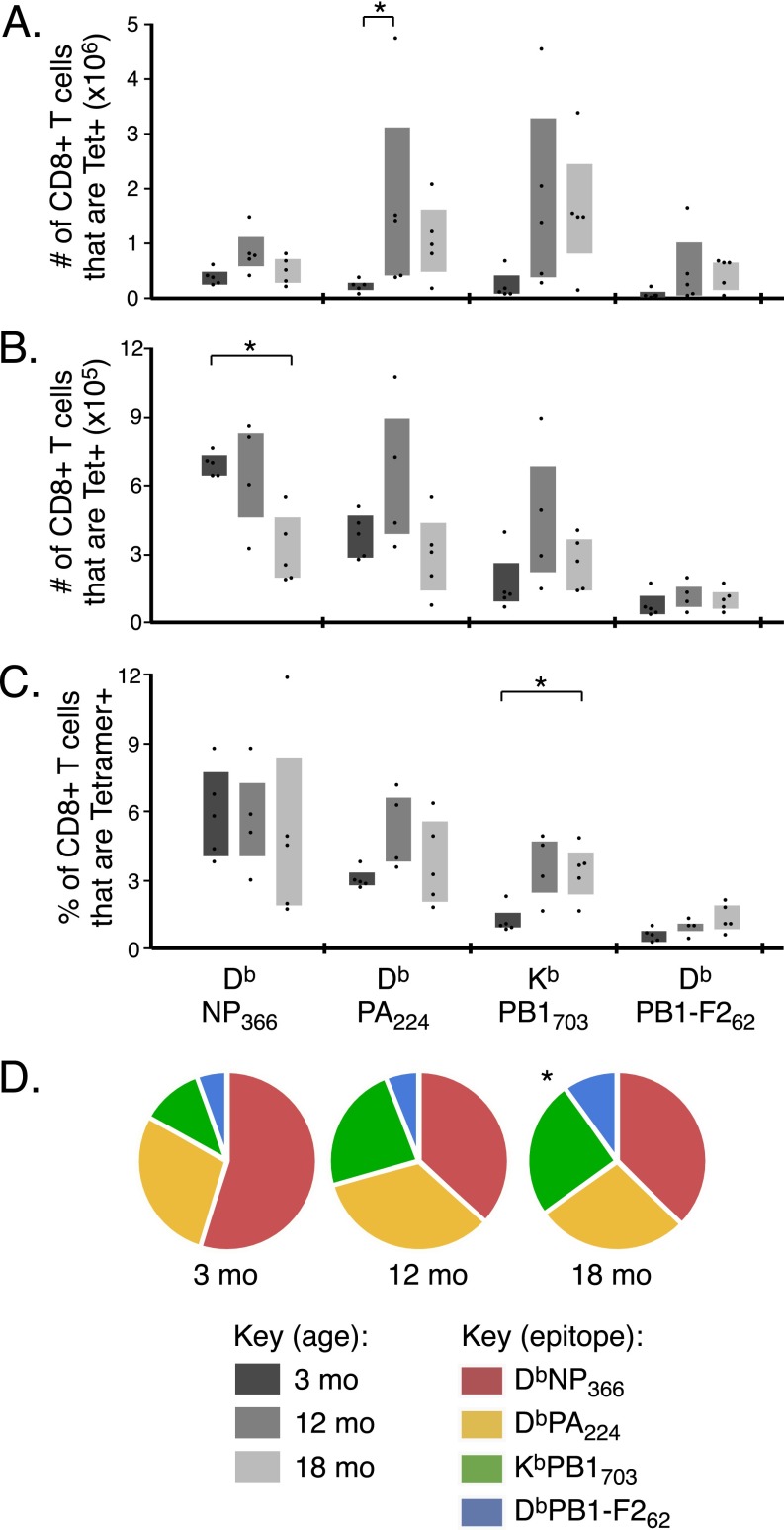
We used tetramer staining to enumerate epitope-specific CD8+ T cells in the lung (Fig. S1A). We also assessed individual epitope-specific CD8+ T-cell responses as a proportion of total CD8+ T cells (Fig. 1B) and of total tetramer+ CD8+ T cells (Fig. 1C) to control for the effects of differential viral load and/or lung inflammation. The relative frequency of DbNP366-specific CD8+ T cells was substantially diminished by 12 mo and decreased further by 18 mo (Fig. 1 B and C). In contrast, the relative magnitude of the DbPA224-specific response was largely stable (Fig. 1 B and C) and the KbPB1703- and DbPB1-F262–specific sets increased in magnitude and proportion with age, resulting in a significantly different distribution of epitope-specific responses (P ≤ 0.05 at 18 mo; Fig. 1C). This effect was consistent for lung (Fig. 1 B and C) and spleen (Fig. S1 B–D). Collectively, these data demonstrate that, as early as 12 mo, a significant quantitative change occurs in the CD8+ T-cell response to IAV, with this change differentially affecting distinct epitope specificities.
Fig. S1.
CD8 T-cell responses in lung and spleen. C57BL/6 mice were infected i.n. with IAV at 3, 12, and 18 mo. The absolute number of CD8+ T cells in the lung (A) and the absolute number (B) or the frequency (C) of CD8+ T cells in the spleen that are DbNP366-, DbPA224-, KbPB1703- or DbPB1-F262–specific, as assessed by tetramer staining at 10 d after infection. (D) The proportion of total tetramer+ CD8+ T cells specific for each of the 4 IAV epitopes in the spleen at 10 d after infection. Bar graphs represent interquartile range, *P ≤ 0.05 compared with 3-mo mice. Data represents two independent experiments (n = 4–5).
Kinetics of Naïve CD8+ T-Cell Loss and the Acquisition of CD44.
We hypothesized that quantitative changes in the relative response magnitude reflects variation in the relative prevalence of the naïve CTLps retained with age. Age-related “holes” have been inferred previously for naïve IAV-specific CTLp repertoires, with the DbNP366-specific response being particularly compromised (13). To directly enumerate CTLps during aging, naive epitope-specific CTLps were isolated at 3, 9, 12, 15, and 18 mo by using a tetramer-based magnetic enrichment protocol (24, 25).
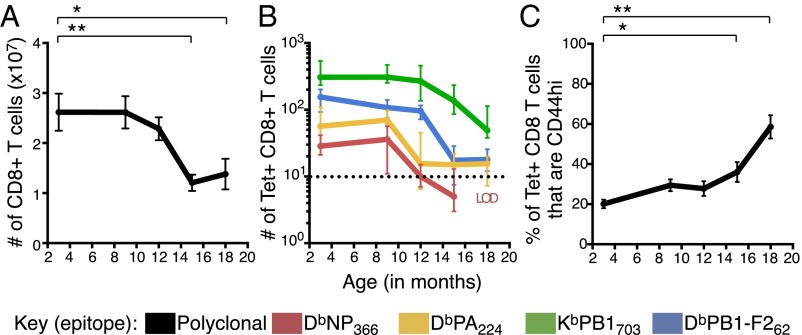
The total CD8+ T-cell number remained relatively stable until 12 mo, but, within a further 3 months, had dropped to less than 50% of the original “young adult” frequency (Fig. 2A), with this profile being maintained out to 18 mo. This broadly parallels the marked loss of total naïve CD8+ T cells in humans by 60–80 y of age (12).
Fig. 2.
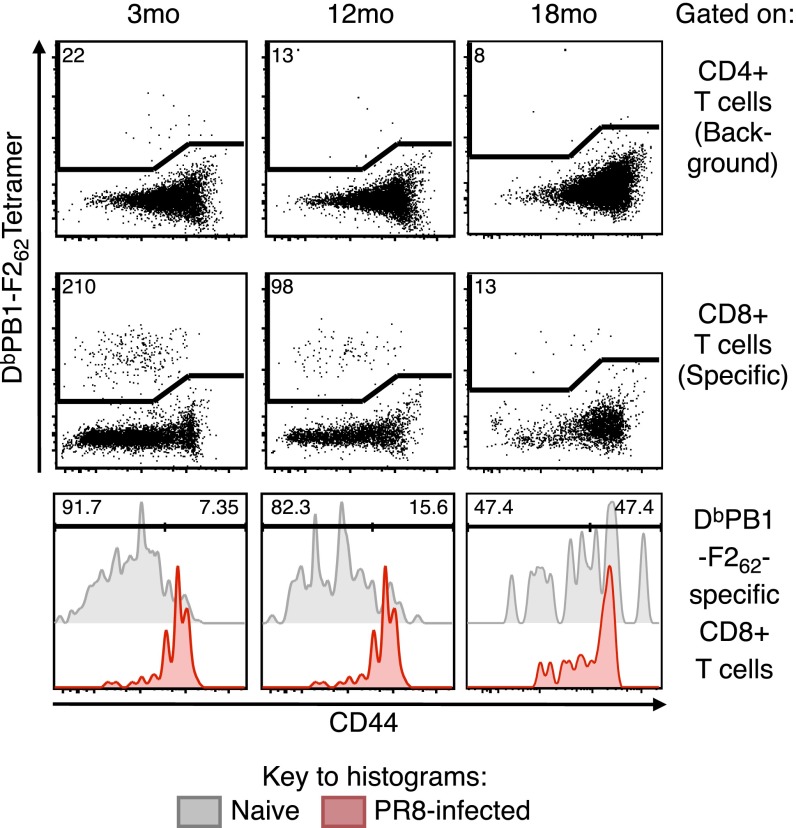
Decay kinetics of total and naïve epitope-specific CD8+ T cells, and acquisition of CD44 with aging. (A) Total number (median ± IQR) of CD8+ T cells in the spleen and pooled lymph nodes/mouse with age. (B) Number (median ± IQR) of DbNP366-, DbPA224-, KbPB1703- or DbPB1-F262–specific CTLps from spleen and pooled lymph nodes/mouse with age. Dashed line = LOD of 10 events, based on tetramer staining in CD3+CD4+ T-cell population (Fig. S4, Top). (C) The frequency (mean ± SEM) of naïve epitope-specific CTLps that are CD44hi during aging. *P ≤ 0.05, **P ≤ 0.01 compared with 3-mo mice. Data in C were pooled across specificities, but results with fewer than 10 events (LOD) were excluded. Data were pooled across 26 independent experiments (n = 6–12 per timepoint).
In young adult (3–6 mo) mice, earlier work has shown an inverse relationship between the frequency of naïve epitope-specific CTLps and CTL response magnitude following IAV infection (10), with low CTLp counts for the immunodominant epitopes (DbNP366 and DbPA224), and high numbers for the subdominant epitopes [DbPB1-F262 and nonstructural protein 2 (KbNS2)114–121]. In keeping with this trend, CTLps specific for the subdominant KbPB1703 epitope were also found in relatively large numbers (median of ∼308 per mouse) in 3-mo mice (Fig. 2B).
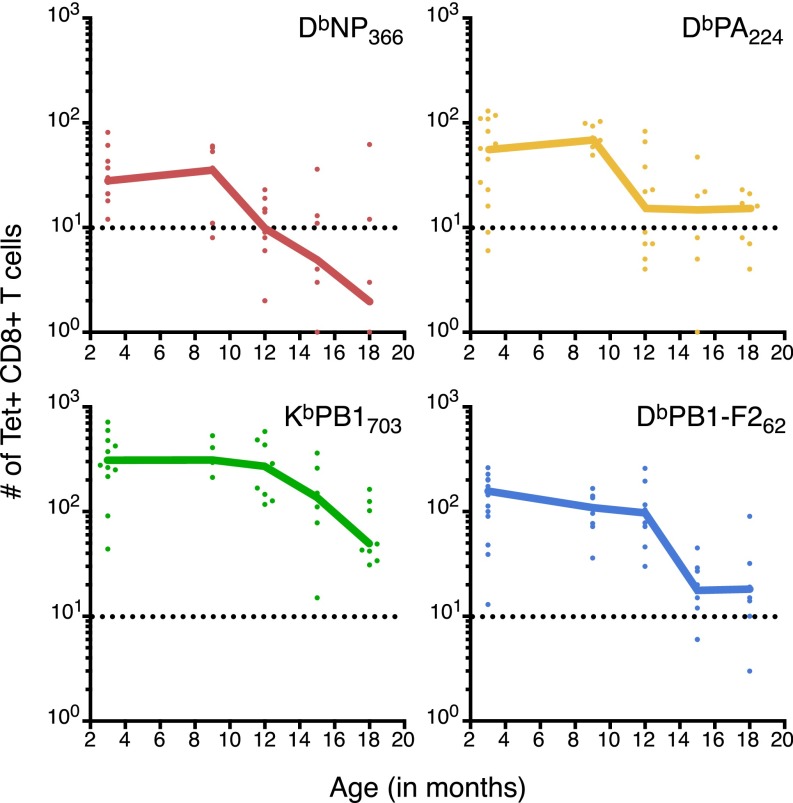
When naïve CTLp populations for individual epitope specificities were tracked during aging, the KbPB1703- and DbPB1-F262–specific sets declined in parallel with total CD8+ T-cell numbers, showing minimal loss at 12 mo (Fig. 2B), followed by a marked decrease between 12 and 15 mo to less than 50% of the frequency observed at 3 mo. In contrast, the DbNP366- and DbPA224-specific CTLp frequencies began to drop between 9 and 12 mo, with the DbNP366-specific population reduced almost to the limit of detection (LOD) by 12 mo (Fig. 2B). There was, however, significant between-mouse variability in specific CTLp number during the decline phase (Fig. S2), suggesting that while the decline occurs rapidly, the timing can vary across individuals.
Fig. S2.
Decline of naïve epitope-specific CTLp with individual datapoints. The number of DbNP366-, DbPA224-, KbPB1703- or DbPB1-F262–specific CD8+ T cells recovered per mouse during aging. Line graphs represent median with individual datapoints displayed, derived from the dataset used in Fig. 2B. Dashed line represents the assay LOD of 10 events. Data were pooled across 26 independent experiments with a minimum of n = 6 and a maximum of n = 12.
Intriguingly, epitope specificities with lower initial CTLp numbers (i.e., DbNP366 and DbPA224) tended to exhibit a relatively early onset of loss (Fig. 2B). The earlier disappearance of DbNP366-specific CTLps by 12 mo is commensurate with inversion of the IAV-specific CTL immunodominance hierarchy at 12 mo, which is even more apparent by 18 mo (Fig. 1). It also corresponds with variability in tetramer MFI and robust coefficient of variation (CV) (a measure of variability of fluorescence intensity for tetramer+ events) for DbNP366-specific CTLs recovered from lung and spleen of IAV-infected 18-mo mice (Fig. S3), suggestive of an extremely limited pool of responders. In contrast, the apparently delayed onset of loss for the naïve KbPB1703- and DbPB1-F262–specific CTLps is consistent with their increased contribution to IAV-specific immune responses with aging.
Fig. S3.
Variability in DbNP366-specific tetramer staining with aging. (A) DbNP366- vs. DbPA224–specific tetramer staining on CD8 T cells from the lungs of 3-, 12-, or 18-mo mice at 10 d after IAV infection. (B) Scatter plots of tetramer MFI vs. robust coefficient of variation (a measure of variation across the central 68% of tetramer+ events) as a demonstration of consistent staining patterns with DbPA224-, KbPB1703- or DbPB1-F262–specific tetramer but increased variation with age for NP366-specific tetramer in lung and spleen from 3-, 12-, or 18-mo mice. Data are representative of two independent experiments (n = 5).
Given the well-established accumulation (up to 80%) of CD44hi CD8+ T cells in aged, uninfected mice (14, 16), we asked when the naïve CTLp sets (defined by IAV specificity in uninfected mice) transitioned to being predominantly CD44hi. The total naïve epitope-specific CD8+ T cells that were CD44hi increased from 26% at 12 mo to 60% by 18 mo, with the majority of this shift occurring between 15 and 18 mo (Fig. 2C). Thus, within a relatively narrow time frame, naïve viral epitope-specific CD8+ T-cell populations undergo synchronized quantitative and qualitative changes that correlate with a diminished ability to respond to virus infection.
Aged Naïve CD8+ T Cells Show No Evidence of Marked Clonal Expansion.
Differential decline of epitope-specific populations suggests that this process is not stochastic, and increased CD44 expression suggests that surviving cells may have undergone proliferation. To determine whether these aged naïve CTLp populations were maintained, at least in part, by proliferation, we characterized the TCRα- and β-chain repertoires during the course of aging, using single-cell nested multiplex RT-PCR and sequencing (17) (Fig. S4) of the DbPB1-F262–specific CTLp set, given its high prevalence at 18 mo. The naïve and immune DbPB1-F262–specific TCR repertoires of young adult mice have been thoroughly analyzed (26), providing a well characterized reference set.
Fig. S4.
Representative dot plots of cells sorted for TCR clonotyping. For enumeration and sorting of DbPB1-F262–specific CD8 T cells in 3-, 12-, and 18-mo mice, cells were gated on singlets, live, lymphocytes, dump, and CD3+ events. From this gate, CD4+ events (Top) were used to define background at a median of 10 events, then CD8+ events (Middle) were assessed for DbPB1-F262–specific events. Numbers in the dot plots indicate the number of events sorted. Sorted cells were defined as CD44lo vs. CD44hi at the time of sorting by using DbPB1-F262–specific tetramer+ cells from IAV-infected mice (Bottom). Numbers in the histograms indicate proportion of DbPB1-F262–specific cells that are CD44lo or CD44hi.
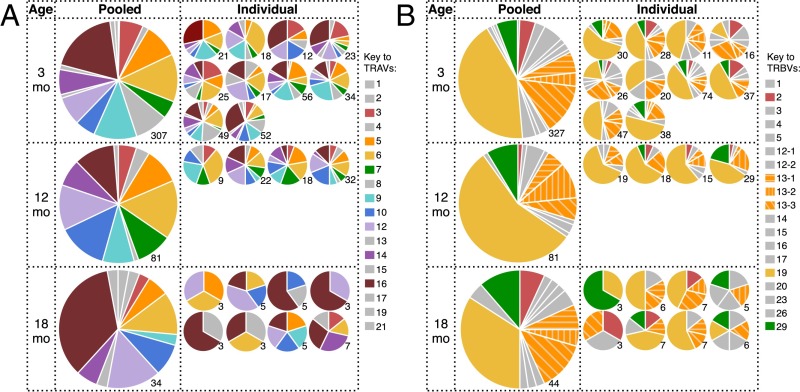
First, we looked for evidence of expansion of naïve CD8+ T-cell clones. The DbPB1-F262–specific TCRαβ-chain repertoires at 3 and 12 mo contained rare duplicate complementarity determining region 3 (CDR3) nucleic acid sequences, with none being found at 18 mo. Even analysis of TCRα or TCRβ CDR3 amino acid sequences demonstrated that the naïve repertoire was almost completely diverse at all timepoints, with only a small number of clonotypes being found more than once, but never more than twice, in individuals (Table 1). This degree of diversity is typical of a naïve epitope-specific repertoire from young adult mice (26) but is distinct from expanded populations, where clonal expansions (due either to antigen or homeostatic proliferation) are readily detected by the repeated identification of a specific CDR3 nucleic acid sequence (11, 16, 18). Thus, extensive proliferation does not occur during aging in DbPB1-F262–specific CTLps.
Table 1.
Number of clonotypes found at a frequency of >1 within individual mice
| Age | TCRα | TCRβ | TCRαβ |
| 3 mo | 5 (307) | 10 (327) | 0 (175) |
| 12 mo | 3 (81) | 3 (81) | 2 (50) |
| 18 mo | 0 (34) | 0 (44) | 0 (27) |
No clones found at a frequency of >2. Number in parentheses is total number of sequences.
Preferential Retention of TCRs in pMHCI-Specific Repertoire with Age.
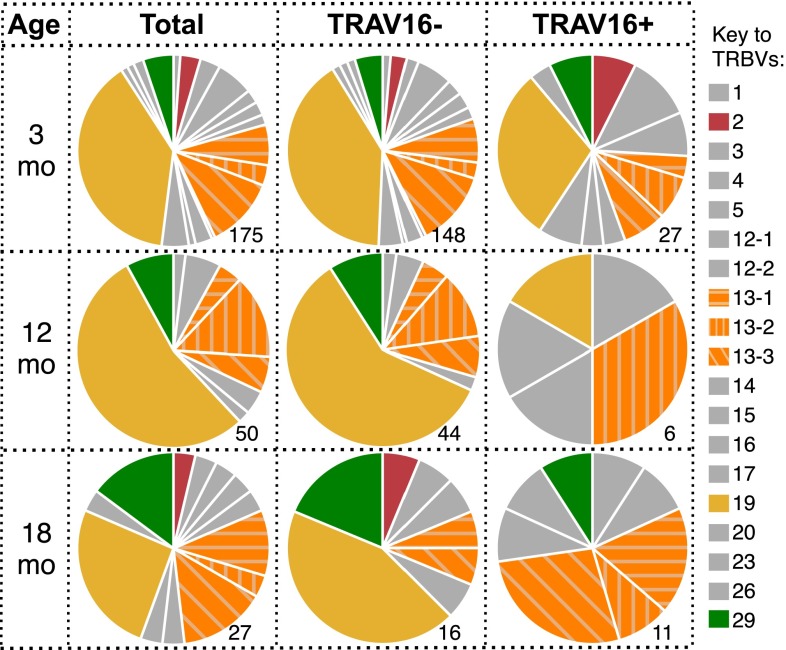
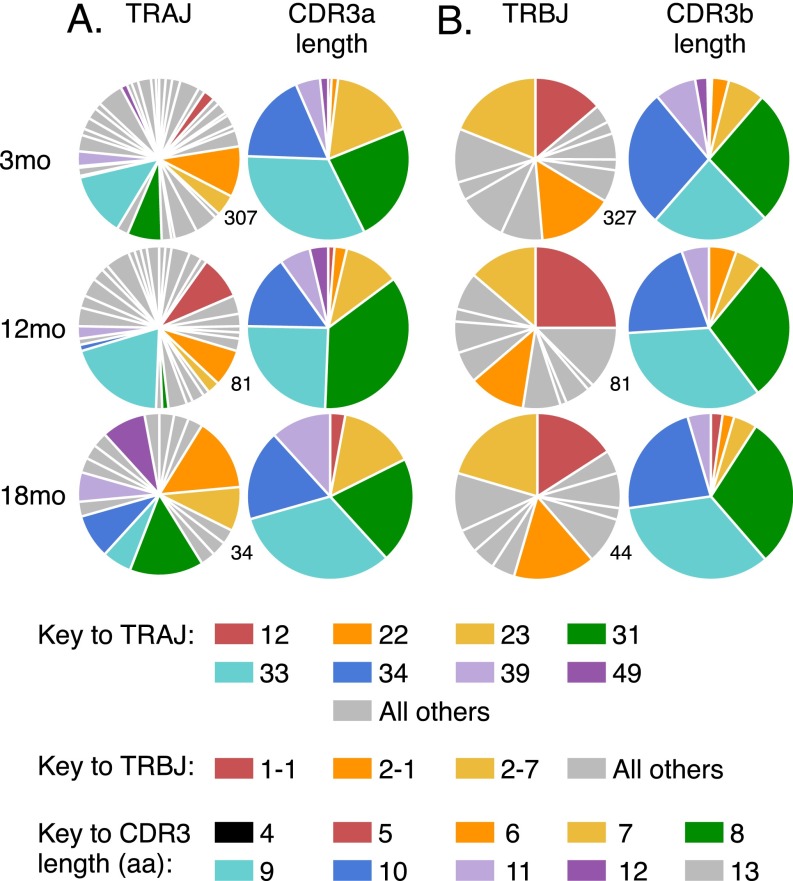
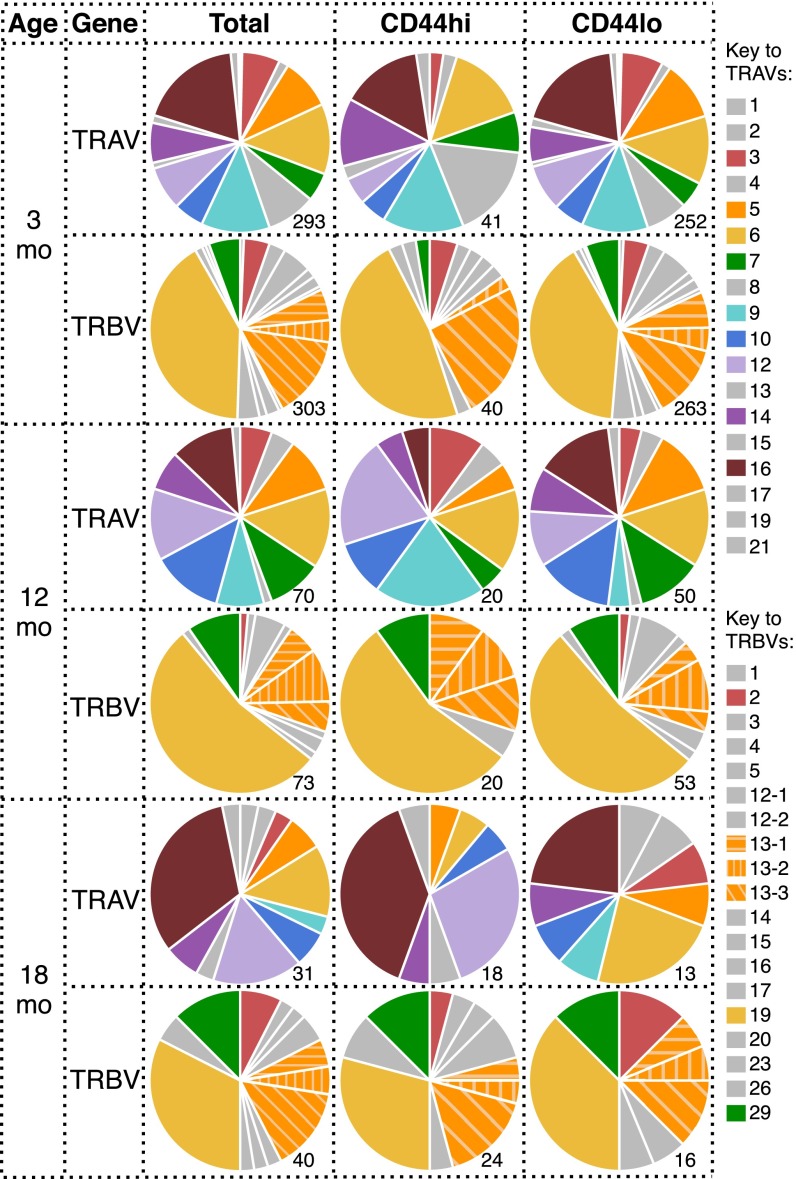
We next asked whether the loss of pMHCI-specific CD8+ T-cell numbers with age is purely stochastic, or whether particular T-cell clones are selectively retained with age. Analysis of TCRα and TCRβ chain V region gene (TRAV and TRBV, respectively) usage in the naïve DbPB1-F262–specific CD8+ T-cell population at 3 mo showed profiles that were consistent with earlier results (26), being characterized by diversity in TRAV usage and a prominent (38.8%) TRBV19 bias (Fig. 3 A and B, Top). Interestingly, the distribution of both TRAV and TRBV usage was subtly but significantly altered from 3 to 18 mo, and 12–18 mo, but not 3–12 mo (Fig. 3 and Table S1). Most notably, the frequency of TRAV16+ TCRs increased from 19.1% at 3 mo to 40.7% at 18 mo (Fig. 3A). Analysis of paired TRAV/TRBV usage with regard to the TRAV16+ population demonstrated that 55% of the paired TRAV16+ TCRs at 18 mo used TRBV13, compared with only 12.5% of the TRAV16− TCRs at 18 mo and 18.5% of TRAV16+ TCRs at 3 mo (Fig. S5). In addition, we did not detect TRBV19 in the 18 mo TRAV16+ repertoire (0/11 paired sequences), whereas it was present in 44% of TRAV16− TCRs at 18 mo and in 30% of TRAV16+ TCRs at 3 mo (Fig. S5). Examination of TRAJ and TRBJ usage and CDR3 lengths demonstrated that the overall diversity and gene usage was broadly maintained with aging (Fig. S6). Collectively, these data demonstrate nonrandom alterations to the naïve DbPB1-F262–specific TCR repertoire with aging, which, due to an absence of any substantial clonal expansion, is likely to reflect selective survival of a subset of CTLps rather than increased division.
Fig. 3.
TRAV usage shifts during aging in naïve DbPB1-F262–specific CTLps. Proportion of naïve DbPB1-F262–specific CTLps in pooled or individual 3-, 12-, and 18-mo mice using distinct TRAV (A) or TRBV (B) chains. Numbers indicate number of sequences sampled.
Table S1.
Results of mvabund analyses examining the association between TCR (TRAV and TRBV) counts and age group
| TRAV | TRBV | |||
| Statistical test | Sum-of-LR statistic | P value | Sum-of-LR statistic | P value |
| Overall effect | ||||
| age | 161.6938 | 0.001 | 122.5833 | 0.002 |
| Post hoc pairwise comparisons | ||||
| 3 mo vs. 12 mo | 25.5653 | 0.18 | 32.39681 | 0.114 |
| 3 mo vs. 18 mo | 124.5643 | 0.001 | 98.80578 | 0.001 |
| 12 mo vs. 18 mo | 80.15084 | 0.003 | 41.13005 | 0.013 |
Fig. S5.
TRBV usage in TRAV16+ and TRAV16− aged naïve DbPB1-F262–specific CD8+ T cells. TRBV usage in total naïve PB1-F262–specific CD8+ T cells compared with TRAV16− or TRAV16+ cells in 3-, 12-, and 18-mo mice. Numbers beside pie graphs indicate the number of paired TRAV/TRBV sequences (as a subset of data from Fig. 4) sampled.
Fig. S6.
TRAJ and TRBJ usage and CDR3 length in aged naïve DbPB1-F262–specific CD8+ T cells. TRAJ usage and CDR3a length (A) or TRBJ usage and CDR3β length (B) in total naïve DbPB1-F262–specific CD8+ T cells in 3-, 12-, and 18-mo mice. Numbers beside pie graphs indicate the number of sequences (derived from the same dataset used for Fig. 4) sampled.
TCR Usage in Aged CD8+ T Cells Modestly Segregates with CD44 Phenotype.
Although we found no evidence of proliferation for CD44hi CTLps from young or aged mice, experiments by others have indicated that the expression of particular TCRs may drive the acquisition of a CD44hi phenotype. In particular, Renkema et al. found that age-related accumulation of CD44hi cells only occurred in TCR transgenic animals if the endogenous TCRα chain was free to rearrange (6).
To specifically determine whether changes in TCR characteristics observed with aging were associated with the CD44hi TVM phenotype, individual naïve DbPB1-F262–specific CD8+ T cells were recorded as CD44hi or CD44lo at the time of sorting, before TCRα and TCRβ analysis (Fig. S4, Bottom). The distribution of TRAV usage for the CD44hi and CD44lo CTLps was similar at 3 and 12 mo (Fig. S7, Top and Middle). Likewise, TRBV usage for these two sets was remarkably similar at all timepoints analyzed (Fig. S7). Although there was some indication of differential TRAV distribution for CD44hi and CD44lo cells at 18 mo (e.g., TRAV16+ and TRAV12+ TCRs) (Fig. S7, Bottom), we had previously noted that duplicate, naive clonotypes (Table 1) were derived from both CD44hi and CD44lo cells. These results indicate that the expression of such TCRs does not dictate the level of CD44 expression and is, in turn, not a reflection of expansion. Thus, at least for this epitope, there is no evidence of extensive expansion of naïve CD8 T cells with aging and CD44 expression does not definitively mark naïve cells that have divided in either young or older mice. The accumulation of the CD44hi population with age therefore reflects either de novo up-regulation of CD44 or selective survival of CD44hi TVM cells.
Fig. S7.
TRAV usage shifts with CD44 expression in aged naïve DbPB1-F262–specific CD8+ T cells. TRAV and TRBV usage in total CD44-scored naïve DbPB1-F262–specific CD8+ T cells compared with CD44lo or CD44hi cells, in 3-, 12-, and 18-mo mice. Numbers beside pie graphs indicate the number of CD44-scored sequences (as a subset of data from Fig. 4) sampled.
Characteristics of Aged Naïve IAV-Specific CD8+ T Cells.
Naive CTLp may be differentially retained but mechanisms driving this selective retention are still unclear. It has been suggested that TCRs with high affinity for their self-selecting ligand are preferentially retained during aging, due to repeated, low-level stimulatory encounters with self that drive CD44 expression and survival (16). CD5 is a negative regulator of TCR signaling whose expression on naïve cells reflects the strength of TCR signal received during thymic development and, thus, TCR-self-pMHC avidity (27, 28). Additionally, CD5 levels correlate with nonself (foreign) pMHCI reactivity in polyclonal CD8+ T-cell populations (28, 29).
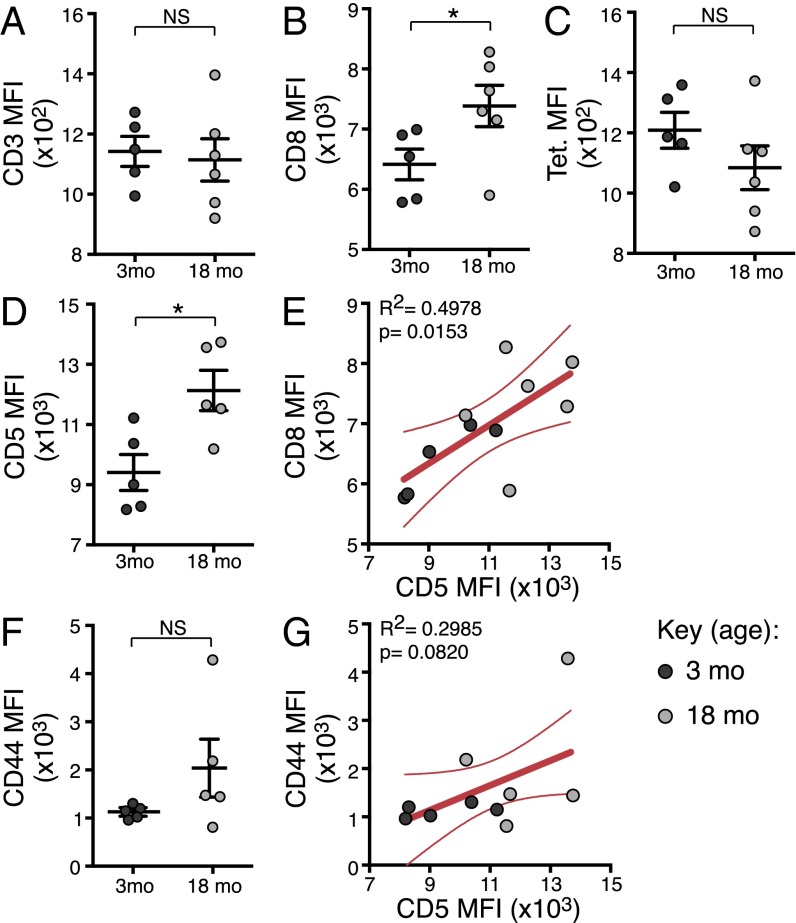
We therefore addressed whether naive epitope-specific CTLp that persist in aged mice exhibit superior avidity for self or foreign cognate pMHCI. Given that avidity is a function of the amount of TCR, the contribution of coreceptors and the TCR-pMHC affinity, we measured the median fluorescence intensity (MFI) of CD3 (TCR levels), CD8 (a key coreceptor), DbPB1-F262 tetramer (foreign pMHCI avidity), and CD5 (surrogate marker for self-pMHCI avidity). There was no difference in the MFI for CD3, but CD8 was significantly higher in 18- compared with 3-mo mice (Fig. 4 A and B), illustrating there is no deficiency in TCR expression, although the coreceptor may be slightly more abundant. Intriguingly, whereas the tetramer MFI was unchanged, the MFI for CD5 was significantly higher in 18-mo mice (Fig. 4 C and D), suggesting that foreign pMHC avidity is unaffected but self-pMHC avidity increases with aging. We also noted a significant positive correlation between CD8 and CD5 expression (P = 0.0153; Fig. 4E). Elevated levels of CD5 have been seen to accompany a shift to a CD44 intermediate phenotype in naïve cells from young adult mice (29), and we saw a trend for both higher CD44 MFI (Fig. 4F) and correlation of CD44 and CD5 MFI (Fig. 4G) in 18-mo naïve DbPB1-F262–specific cells. Altogether, these data suggest that naïve CTLps with increased self-pMHCI reactivity are selectively retained during aging, but the increase in self-recognition does not impact on overall TCR avidity for foreign pMHCI epitopes.
Fig. 4.
CD5 expression increases during aging in naïve DbPB1-F262–specific CTLps. MFI of CD3 (A), CD8 (B), tetramer (C), CD5 (D), and CD44 (F) expression on naive DbPB1-F262–specific CTLps from individual 3-mo or 18-mo mice (showing mean ± SEM). Linear regression analysis of CD5 vs. CD8 MFI (E) and CD44 vs. CD5 MFI (G), showing line of best fit and 95% confidence interval. Data represents two independent experiments (n = 5). Cells from two 3-mo and three 18-mo mice were pooled for each sample to ensure enough events for consistent phenotyping. *P ≤ 0.05.
Discussion
The present analysis in B6 mice demonstrates that age-related changes in the well-characterized immune IAV-specific CD8+ T-cell immunodominance hierarchy are a consequence of analogous changes in CTLp frequency. Moreover, this study is the first to our knowledge to demonstrate that the maintenance of a naïve epitope-specific CTLp population seems related to self-pMHC recognition but not selective clonal expansion. The maintenance of CTLps with enhanced self-recognition, in turn, results in subtle, but significant, shifts in the epitope-specific TCR repertoire, but has no apparent impact on foreign pMHC avidity, providing insight into mechanisms underlying naïve CD8+ T-cell attrition with age.
The age-related decline in naïve epitope-specific CTLps may occur stochastically, leading to an equivalent rate of loss across all epitope specificities or could reflect the differential fitness of particular CTLp sets in an aged environment. Earlier experiments demonstrated that the IAV-specific immunodominance hierarchy shifts in B6 mice, with infection of aged naïve mice resulting in substantial loss of the immunodominant DbNP366-specific CD8 T-cell response, a maintenance of the prominent DbPA224-specific response, and a relative increase in the subdominant KbPB1703- and DbPB1-F262–specific responses (13, 22). A previous study also indirectly suggested that age-related “holes” emerge in the naïve CTLp TCR repertoire, particularly for those epitope-specific sets with lower precursor frequencies (13). Our direct enumeration of naïve CTLp frequency suggests, somewhat surprisingly, that there is a pattern of differential loss for epitope-specific CTLp populations. The DbNP366- and DbPA224–specific CTLps were substantially diminished by 12 mo, although the KbPB1703- and DbPB1-F262–specific sets were largely maintained. Additionally, the initial size of naive CTLp populations in young adult mice varies for the IAV epitopes and correlates with the selective loss of particular specificities from the IAV CD8+ T-cell response, Thus, the initial, postthymic size of any given naïve CTLp pool may be a primary determinant of the onset of age-related decline and the shift in immunodominance. Taken together with our observation that cells retained with age have higher CD5 expression, shown by others to reflect superior self-pMHCI recognition (27, 28), it is tempting to speculate that an enhanced capacity to recognize self-pMHCI may both promote CTLp survival over time and represent a key determinant of initial naïve CTLp frequency. Certainly, for CD4+ T cells, Chu et al. showed that, in the absence of negative selection, the size of a naive CD4+ T-cell population positively correlated with its ability to interact with pMHCII complexes (30). Given our data, self-pMHCI affinity represents an intriguing potential determinant of naïve CTLp population size.
Other studies have suggested that proliferation of CTLp may occur with age, driving CD44 expression (16), but the contribution of loss of number vs. expansion to narrowed TCR diversity with aging had not been defined. In young adult mice, naïve CD44hi TVM cells represent a distinct subset, characterized by markers of homeostatic proliferation, exquisite sensitivity to cytokine stimulation, and arising independently of antigenic stimulation (31, 32). Naïve CTLps with a TVM phenotype accumulate with age (14, 16), an effect that is only seen for TCR transgenic animals if the endogenous TCRα is free to rearrange, indicating a requirement for TCR-mediated signals (6). However, when we used TCR sequencing analysis to assess the age-related repertoire (for DbPB1-F262) at the individual cell level, there was no evidence that clonal expansion is associated with either age or TVM phenotype, because the TCR repertoires at all ages remained completely diverse and were not dominated by selective clonal expansions. Our results would suggest that any selective, TCR-pMHCI–dependent increase in homeostatic proliferation within the CD44hi TVM or aged naïve T-cell compartments is modest and not sufficient to narrow TCR diversity in any substantial way. Consequently, we propose that the restricted TCR diversity of aged CD8+ T-cell immune responses is due to the physical loss of CTLp number rather than a reflection of homeostatic expansion restricting clonal diversity. It remains to be seen whether the enhanced proliferative capacity attributed to TVM from young adult mice is also true of TVM from aged animals. Given their prevalence with aging (15), an improved understanding of their functional potential is imperative if we are to effectively tailor vaccines for the elderly.
Despite finding no evidence of clonal expansion for naïve DbPB1-F262–specific CTLps, we observed significant age-related changes in TCR use together with a general increase in CD5 expression. This observation is consistent with the idea that self-pMHCI recognition provides a survival signal for naïve CTLps (33). Studies have suggested that self-pMHC reactivity is predictive of reactivity to foreign cognate pMHC (28, 29). A study by Rudd et al. demonstrated that naïve HSV-1 gB-8p–specific CTLps were significantly enriched with age for CD44hi cells and also for a Vβ10+ subset (16). Of note, Vβ10+ T cells in young adult mice had higher expression of CD5 and were selectively expanded following HSV-1 infection, suggesting that cells selectively retained with age were more reactive to both self and foreign pMHCI. However, whereas we observed selective retention of TRAV16 and increased CD5 expression, the MFI of tetramer binding on DbPB1-F262–specific CTLp did not change with aging in our experiments. It should be noted that TRAV16 is not preferentially recruited into the DbPB1-F262–specific immune response to IAV (26), nor has it been found to pair with TRBV19, which is preferentially recruited, in naïve CTLps from 18-mo mice (Fig. S5). Collectively, these data illustrate that there is selective retention of TRAV16 in DbPB1-F262–specific CTLps in aged mice, which is characterized by superior self, but not foreign, pMHCI recognition. This set of results highlights that self and foreign pMHCI recognition are not necessarily coupled.
Another key point of difference between our study and the Rudd et al. study is that there was clear evidence of proliferation in naive Vβ10+ HSV-1 gB-8p–specific cells, whereas we saw no evidence of proliferation for naïve DbPB1-F262–specific CTLps. We speculate that, over time, naïve Vβ10+ gB-8p–specific CTLps may be driven to proliferate because of a more ubiquitous self-antigen, cross-reactivity with a commensal or environmental antigen, and/or the average avidity of CTLps for their restricting self-ligand may be higher than TRAV16+ DbPB1-F262–specific CTLps. Given that these three parameters would differ substantially across epitope specificities, we propose that distinct epitope specificities will respond differently to aging, resulting in substantial homeostatic proliferation for some, but not all, CTLp populations.
Collectively, our results suggest that modifications in CTL immunodominance hierarchies that occur with age may be driven by analogous, asynchronous changes in CTLp numbers. Our data also indicate that the decrease in CD8+ T-cell responses with age is minimally attributable to clonal narrowing of the available repertoire due to selective homeostatic proliferation. However, changes in the TCR repertoire are observed with advancing age and are associated with increased self, but not foreign, pMHCI recognition. Collectively, these data provide insights into the mode and mechanism of naïve epitope-specific CTLp attrition and the relative contribution of such changes to diminished immune response after infection.
Materials and Methods
Mice and Infections.
Female B6 mice were bred and housed in specific pathogen-free conditions in the Department of Microbiology and Immunology at the University of Melbourne. Mice were anesthetized by isofluorane inhalation and infected intranasally (i.n.) with 1 × 104 pfu of the HKx31 (H3N2) IAV strain in 30 µL of PBS. All animal experimentation was approved and conducted under guidelines set by the University of Melbourne Animal Ethics Committee.
Tetramer and Antibody Staining.
Spleen and lung tissue from IAV-infected mice (d10) were processed (34) and enriched lymphocyte populations were stained with PE-labeled DbNP366 or KbPB1703 and APC-labeled DbPA224 or DbPB1-F262 MHCI tetramers (University of Melbourne Tetramer Facility), stained with Fixable Live/Dead AquaBlue viability dye (Life Technologies), blocked with anti-CD16/32 mAb (2.4G2), and stained with anti-CD8α-PacBlue (BD Pharmingen; 53-6.7) and anti-CD3ε-PerCPCy5.5 (BD Pharmingen; 145-2C11). Cells were acquired on a FACS Canto II flow cytometer (BD Biosciences), and data were analyzed by using FlowJo software (Treestar), followed by Pestle and SPICE software (National Institute of Allergy and Infectious Diseases).
Enumeration and Isolation of Naïve Epitope-Specific CD8+ T Cells.
Tetramer-based magnetic enrichment was used for the identification and isolation of naïve epitope-specific CTLps, as has been described (10, 24, 25). Briefly, single-cell suspensions of pooled spleen and major LNs (auxiliary, brachial, cervical, inguinal, and mesenteric) from individual mice were stained with PE- or APC-labeled DbNP366, DbPA224, KbPB1703, or DbPB1-F262 MHCI tetramers, washed and labeled with anti-PE-mAb and anti-APC-mAb conjugated magnetic microbeads, and tetramer-bound cells enriched over a magnetic LS column (Miltenyi Biotec). Enriched cells were then stained with Fixable Live/Dead AquaBlue viability dye (Life Technologies) and a panel of conjugated mAbs to identify epitope-specific cells (CD8α+, CD3ε+, CD11c−, CD11b−, F4/80−, B220−, NK1.1−, CD4−). For phenotypic characterization conjugated mAbs to CD44 and CD5 were also used. The entire enriched sample was acquired on a LSRII flow cytometer with FACSDiva software (BD Biosciences), whereas data were analyzed with FlowJo software (TreeStar).
Single-Cell RT-PCR and Sequencing.
Individual DbPB1-F262–specific CD8+ T cells were individually sorted into wells of a 96-well Twin.tec PCR plate (Eppendorf) by using a BD FACSAria III (BD Biosciences). Representative plots of sorted cells are displayed in Fig. S4. mRNA transcripts were reverse-transcribed to cDNA by using Sensiscript (Qiagen). The CDR3α and CDR3β regions were amplified by nested multiplexed PCRs (17, 26).
Statistics.
Data for immune responses in Figs. 1–3 were analyzed in Prism or SPICE by using standard unpaired, nonparametric tests; either Wilcoxon signed-rank sum or Mann–Whitney. Differences in TRAV or TRBV use over time (Fig. 4) were analyzed by using the sum-of-likelihood ratio (sum-of-LR), as described in SI Materials and Methods and Dataset S1.
SI Materials and Methods
To detect evidence of an age effect on TCR diversity (Fig. 4), we used the manyglm() function, within R’s mvabund package (version 3.11.4) (35, 36). manyglm() fits separate, univariate, generalized linear models (GLM), which, in this case, relates the count of each TCR from each mouse to the explanatory variable age group (3, 12, or 18 mo). Negative binomial regression was found to be the best model of the mean-variance relationship (identified by using residuals vs. fitted values plots). A multivariate test statistic, referred to as the sum-of-LR statistic, is derived from the separate GLM fits, which tests the null hypothesis that the mean vector of counts from clusters is the same for each group (e.g., the mean vector of TCR counts in mice is the same for each age group). R’s anova() function is applied to a manyglm object to perform resampling-based hypothesis testing (the default settings were implemented), which produces a P value assessing the strength of evidence against the null hypothesis of no association between a factor and multivariate count data. To identify which age groups show statistically significant differences in TCR diversity, pairwise comparisons between each age group were also performed by using this approach. The nominal level of significance of the pairwise comparison was adjusted for multiple testing by using a Bonferroni correction [Bonferroni corrected level of significance is 0.017 (i.e., 0.05/3, where 3 is the number of tests)]. Results of mvabund analyses examining the association between TCR (TRAV and TRBV) counts and age group are shown in Table S1. Presented are tests for the overall effect of age as well as pairwise comparisons between each age group. P values for the pairwise comparisons are compared with the Bonferroni corrected level of significance (0.017) (Table S1).
Supplementary Material
Acknowledgments
We thank Stephen J. Turner for critical reading of the manuscript. This work was supported by National Health and Medical Research Council (NHMRC) Program Grant AI1071916 (to P.C.D. and N.L.L.G.) and Project Grant AI1046333 (to N.L.L.G.), National Institutes of Health Grants U19AI117891 (to P.G.T. and A.H.) and AI107625 (to P.G.T.), a Sylvia and Charles Viertel Senior Medical Research Fellowship (to N.L.L.G.), an Australian Research Council Future Fellowship (to J.M.M.), and NHMRC Biomedical Postgraduate Scholarship AI520643 (to T.C.). S.G.Z. was supported under NHMRC Centre of Research Excellence Grant AI1035261, awarded to the Victorian Centre for Biostatistics (ViCBiostat).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1525167113/-/DCSupplemental.
References
- 1.CDC 2013. Influenza Activity - United States, 2012-13 Season and Composition of the 2013-14 Influenza Vaccine, Report Mamw. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm6223a5.htm. Accessed January 6, 2016.
- 2.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24(10):1609–1614. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 3.Nikolich-Žugich J. Aging of the T cell compartment in mice and humans: From no naive expectations to foggy memories. J Immunol. 2014;193(6):2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decman V, et al. Cell-intrinsic defects in the proliferative response of antiviral memory CD8 T cells in aged mice upon secondary infection. J Immunol. 2010;184(9):5151–5159. doi: 10.4049/jimmunol.0902063. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Fisher EM, Murasko DM. Intrinsic defects in CD8 T cells with aging contribute to impaired primary antiviral responses. Exp Gerontol. 2013;48(6):579–586. doi: 10.1016/j.exger.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Žugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192(1):151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tscharke DC, Croft NP, Doherty PC, La Gruta NL. Sizing up the key determinants of the CD8(+) T cell response. Nat Rev Immunol. 2015;15(11):705–716. doi: 10.1038/nri3905. [DOI] [PubMed] [Google Scholar]
- 8.Cukalac T, et al. The influenza virus-specific CTL immunodominance hierarchy in mice is determined by the relative frequency of high-avidity T cells. J Immunol. 2014;192(9):4061–4068. doi: 10.4049/jimmunol.1301403. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol. 2012;188(9):4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Gruta NL, et al. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J Clin Invest. 2010;120(6):1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M, et al. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182(2):784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42(5):400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yager EJ, et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205(3):711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decman V, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188(4):1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu B-C, Martin BE, Stolberg VR, Chensue SW. Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191(12):5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudd BD, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci USA. 2011;108(33):13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dash P, et al. Paired analysis of TCRα and TCRβ chains at the single-cell level in mice. J Clin Invest. 2011;121(1):288–295. doi: 10.1172/JCI44752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc Natl Acad Sci USA. 2004;101(14):4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Gruta NL, et al. Epitope-specific TCRbeta repertoire diversity imparts no functional advantage on the CD8+ T cell response to cognate viral peptides. Proc Natl Acad Sci USA. 2008;105(6):2034–2039. doi: 10.1073/pnas.0711682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner SJ, Diaz G, Cross R, Doherty PC. Analysis of clonotype distribution and persistence for an influenza virus-specific CD8+ T cell response. Immunity. 2003;18(4):549–559. doi: 10.1016/s1074-7613(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 21.Thomas PG, et al. Hidden epitopes emerge in secondary influenza virus-specific CD8+ T cell responses. J Immunol. 2007;178(5):3091–3098. doi: 10.4049/jimmunol.178.5.3091. [DOI] [PubMed] [Google Scholar]
- 22.Valkenburg SA, et al. Early priming minimizes the age-related immune compromise of CD8⁺ T cell diversity and function. PLoS Pathog. 2012;8(2):e1002544. doi: 10.1371/journal.ppat.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123(8):1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 24.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28(6):859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cukalac T, et al. Paired TCRαβ analysis of virus-specific CD8(+) T cells exposes diversity in a previously defined ‘narrow’ repertoire. Immunol Cell Biol. 2015;93(9):804–814. doi: 10.1038/icb.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38(2):263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulton RB, et al. The TCR’s sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015;16(1):107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu HH, Moon JJ, Kruse AC, Pepper M, Jenkins MK. Negative selection and peptide chemistry determine the size of naive foreign peptide-MHC class II-specific CD4+ T cell populations. J Immunol. 2010;185(8):4705–4713. doi: 10.4049/jimmunol.1002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188(6):2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206(2):435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206(10):2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Gruta NL, Turner SJ, Doherty PC. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: Correlation of cytokine profile and TCR avidity. J Immunol. 2004;172(9):5553–5560. doi: 10.4049/jimmunol.172.9.5553. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Naumann U, Wright ST, Warton DI. mvabund - an R package for model-based analysis of multivariate abundance data. Methods Ecol Evol. 2012;3:471–474. [Google Scholar]
- 36.Warton DI, Wright ST, Wang Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol Evol. 2012;3:89–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.