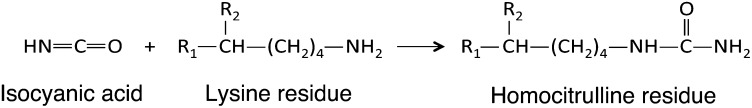Significance
Human longevity is increasing worldwide because of the advances in scientific knowledge and patient care, which leads to the frequent development of age-related pathologies. Aging remains an elusive process associated with genetic and environmental features, and better understanding would promote sustained wellbeing. We show here for the first time, to our knowledge, that carbamylation, a nonenzymatic posttranslational modification of proteins characterized by the spontaneous binding of isocyanic acid mainly derived from urea, is highly associated with aging and life expectancy in three mammalian species. Carbamylation promotes molecular aging through alteration of protein functions, especially long-lived extracellular matrix proteins. Tissue accumulation of carbamylated proteins may be considered a general hallmark of aging, enabling us to establish a link between cumulative metabolic alterations and age-related complications.
Keywords: carbamylation, longevity, nonenzymatic posttranslational modifications, tissue aging, skin
Abstract
Aging is a progressive process determined by genetic and acquired factors. Among the latter are the chemical reactions referred to as nonenzymatic posttranslational modifications (NEPTMs), such as glycoxidation, which are responsible for protein molecular aging. Carbamylation is a more recently described NEPTM that is caused by the nonenzymatic binding of isocyanate derived from urea dissociation or myeloperoxidase-mediated catabolism of thiocyanate to free amino groups of proteins. This modification is considered an adverse reaction, because it induces alterations of protein and cell properties. It has been shown that carbamylated proteins increase in plasma and tissues during chronic kidney disease and are associated with deleterious clinical outcomes, but nothing is known to date about tissue protein carbamylation during aging. To address this issue, we evaluated homocitrulline rate, the most characteristic carbamylation-derived product (CDP), over time in skin of mammalian species with different life expectancies. Our results show that carbamylation occurs throughout the whole lifespan and leads to tissue accumulation of carbamylated proteins. Because of their remarkably long half-life, matrix proteins, like type I collagen and elastin, are preferential targets. Interestingly, the accumulation rate of CDPs is inversely correlated with longevity, suggesting the occurrence of still unidentified protective mechanisms. In addition, homocitrulline accumulates more intensely than carboxymethyl-lysine, one of the major advanced glycation end products, suggesting the prominent role of carbamylation over glycoxidation reactions in age-related tissue alterations. Thus, protein carbamylation may be considered a hallmark of aging in mammalian species that may significantly contribute in the structural and functional tissue damages encountered during aging.
Aging is a complex process resulting from the combination of a large number of genetic and acquired factors leading to a decline of organism functions. Cellular senescence, telomere shortening, decreased proliferative capacity, mitochondrial DNA single mutations, and inflammation influence the aging process (1–3). Protein aging is also actively involved in tissue aging. During their biological life, proteins are exposed to various alterations caused by nonenzymatic posttranslational modifications (NEPTMs), like glycation, oxidation, carbonylation, or carbamylation, that contribute to functional and structural alterations of their properties (4).
Among them, glycation has generally been recognized to significantly contribute to aging processes. Glycation refers to the binding of sugar carbonyl groups to protein amino groups, resulting in the formation of a Schiff base, which rapidly undergoes a molecular rearrangement to form an Amadori product. These products can be further exposed to irreversible oxidative processes, which lead to the generation of a variety of complex compounds called advanced glycation end products (AGEs). Because glycation and oxidative reactions are closely linked, it is more suitable to name the whole pathway “glycoxidation.” It has been recognized that AGEs accumulated in organisms in an age-dependent manner. Nε-carboxymethyl-lysine (CML) and pentosidine, two major AGEs, were found in several tissues, like kidney, bone, eye, skeletal muscle, cartilage, arterial wall, or brain, and shown to be correlated to the risk of adverse aging-related outcomes (5–10). Because AGE formation is cumulative and irreversible, glycoxidation particularly affects extracellular matrix (ECM) proteins because of their long biological life. Indeed, glycation promotes collagen cross-linking involved in stiffness and decreased elasticity of skin (11) but also, modifies matrix proteins of other tissues, contributing, for example, to the development of vessel rigidity. This phenomenon is associated with the higher prevalence of cardiovascular diseases and could predict adverse cardiovascular events in both healthy subjects and high-risk patients (12). Moreover, it has been recently shown that exogenous AGEs brought by diet contributed to endothelial dysfunction, arterial stiffness, and aging (13).
The justified interest expressed for glycoxidation may, however, have distracted attention from other important pathophysiological mechanisms of aging. Indeed, it has been shown that another NEPTM, carbamylation, participated in protein molecular aging. This reaction corresponds to the binding of isocyanic acid to free amino groups and preferentially occurs on the ε-NH2 of lysine residues generating homocitrulline (HCit) (Fig. 1), the most characteristic carbamylation-derived product (CDP) (14, 15) that can be specifically quantified (16). Isocyanic acid is mainly formed by the spontaneous dissociation of urea into cyanate and ammonia (15) but may also derive from thiocyanate through myeloperoxidase action (17, 18). Isocyanic acid generated from biomass burning, biofuel use, or tobacco use has been described as a minor environmental source (19).
Fig. 1.
Carbamylation reaction.
The occurrence of in vivo carbamylation has been known since 1960, when Stark et al. (20) reported that cyanate was able to react with amino acids and proteins. The first deleterious effects of carbamylation in vivo were evidenced in the 1970s. At that time, patients with sickle cell disease were treated with urea or cyanate to promote the carbamylation of HbS, increasing its affinity for oxygen and decreasing its capacity of aggregation. However, these patients developed cataract, which was attributed to the carbamylation of lens proteins (21). Apart from this context, other pathological implications of carbamylation have been discussed only recently (22–24). The intensity of this reaction is particularly amplified during chronic kidney disease (CKD) because of hyperuremia (25, 26). High concentrations of carbamylated plasma proteins constitute a significant risk factor for cardiovascular events and mortality in hemodialysis patients (26, 27). Recently, our laboratory has evidenced in a mouse model that tissue proteins, including skin collagen, were more intensely carbamylated during CKD and accumulated in the organism (28). In addition, carbamylation rate is increased in atherosclerotic plaques because of myeloperoxidase release from inflammatory cells, participating in the development of atherosclerosis in patients with CKD or type 2 diabetes, even in the absence of renal impairment (23, 29, 30).
However, to date, no data are available on the involvement of carbamylation in aging. Such a finding would be of potential relevance, because previous studies have evidenced that carbamylation induced deleterious effects on biological functions of proteins (23, 31–33). In this paper, we have examined the age-related evolution of carbamylation of skin proteins and the two major matrix proteins, type I collagen and elastin, in three mammalian species (human, murine, and bovine). Our results show a progressive increase of carbamylated proteins in skin and suggest that carbamylation may be considered a general feature of tissue aging at least as important as glycation.
Results
CDPs Accumulate in Skin with Age.
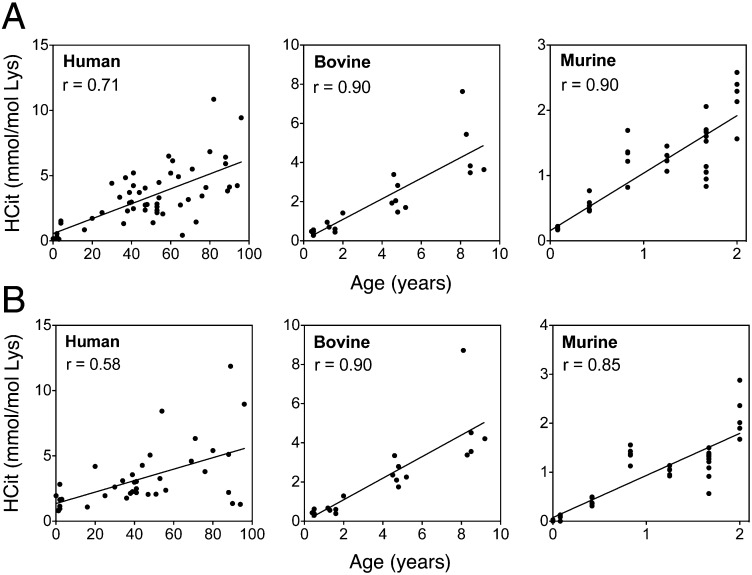
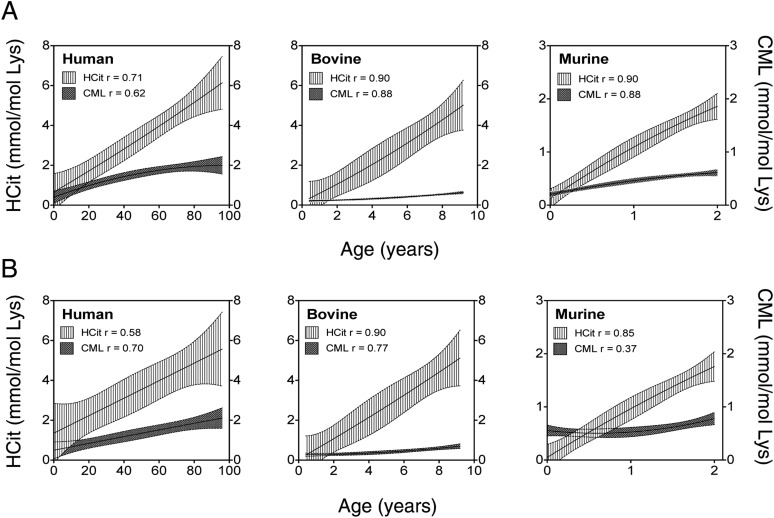
Protein carbamylation rate was evaluated in skin extracts by HCit quantification. HCit concentrations significantly increased with age in all species (Fig. 2A and Table S1). HCit increased from 0.08 mmol/mol Lys in 1-d-old mice up to 2.2 mmol/mol Lys in 2-y-old mice, representing a 29-fold increase. The magnitudes of increase between young and old individuals in bovines and humans were 11.5- and 8.1-fold, respectively. It is noteworthy that HCit concentrations were higher in these two species compared with mice (4.8 mmol HCit/mol Lys in 8-y-old bovines and 5.5 mmol HCit/mol Lys in >70-y-old humans). A comparable evolution of HCit contents was evidenced in skin matrix proteins. Type I collagen carbamylation increased in all species (r ≥ 0.58, P < 0.001) (Fig. 2B and Table S1). For the sake of clarity, HCit values in the youngest groups are given in Table S2. Also, human elastin showed an age-dependent increase of HCit content (Fig. S1). It did not exceed 2.5 mmol/mol Glu in younger subjects (<4 y old), whereas it reached 11.7 mmol/mol Lys in older subjects (>70 y old), representing a 4.7-fold increase.
Fig. 2.
Age-related increase of skin HCit content. HCit was assayed by liquid chromatography–MS/MS in (A) total skin extract and (B) skin type I collagen in human (n = 60), bovine (n = 20), and murine (n = 35) individuals. HCit content progressively increased with age in all studied species. All Spearman coefficients of correlation (r) showed significant HCit increase with P < 0.001.
Table S1.
Linear regression equations of carbamylated protein accumulation in skin as a function of age
| Tissue and species | Mean life expectancy (y) | Regression equation | Spearman coefficient | Significance |
| Total extract | ||||
| Human | 80 | y = 0.0565x + 0.6008 | 0.71 | P < 0.001 |
| Bovine | 20 | y = 0.5267x + 0.0367 | 0.90 | P < 0.001 |
| Murine | 2 | y = 0.8814x + 0.1555 | 0.90 | P < 0.001 |
| Type I collagen | ||||
| Human | 80 | y = 0.0439x + 1.3500 | 0.58 | P < 0.001 |
| Bovine | 20 | y = 0.5506x − 0.0078 | 0.90 | P < 0.001 |
| Murine | 2 | y = 0.8599x + 0.0736 | 0.85 | P < 0.001 |
Linear regression equations and Spearman coefficients of correlation were calculated from graphics of HCit concentrations as a function of age (shown in Fig. 2).
Table S2.
Skin HCit concentrations in the youngest groups of three species
| Tissue and species | Age of the youngest group | HCit (mmol/mol Lys) |
| Total extract | ||
| Human | 1–4 y | 0.66 ± 0.56 |
| Bovine | 6 mo | 0.42 ± 0.13 |
| Murine | 0–1 mo | 0.12 ± 0.06 |
| Type I collagen | ||
| Human | 1–4 y | 1.48 ± 0.69 |
| Bovine | 6 mo | 0.42 ± 0.12 |
| Murine | 0–1 mo | 0.05 ± 0.02 |
Fig. S1.
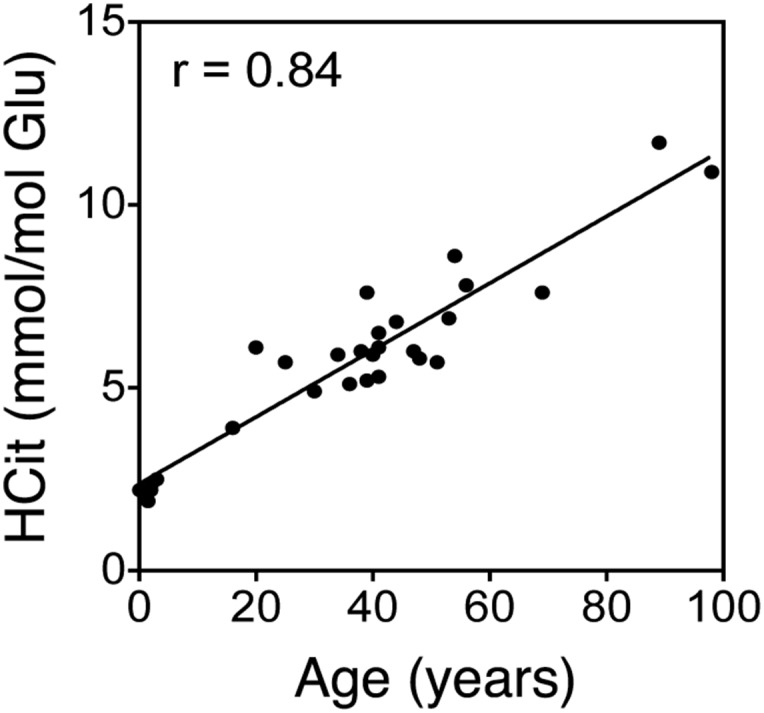
Evaluation of carbamylation of human skin elastin with age. HCit concentrations were evaluated by LC-MS/MS in elastin extracted from human skin (n = 28). Spearman coefficient of correlation (r) showed a significant HCit increase with P < 0.001.
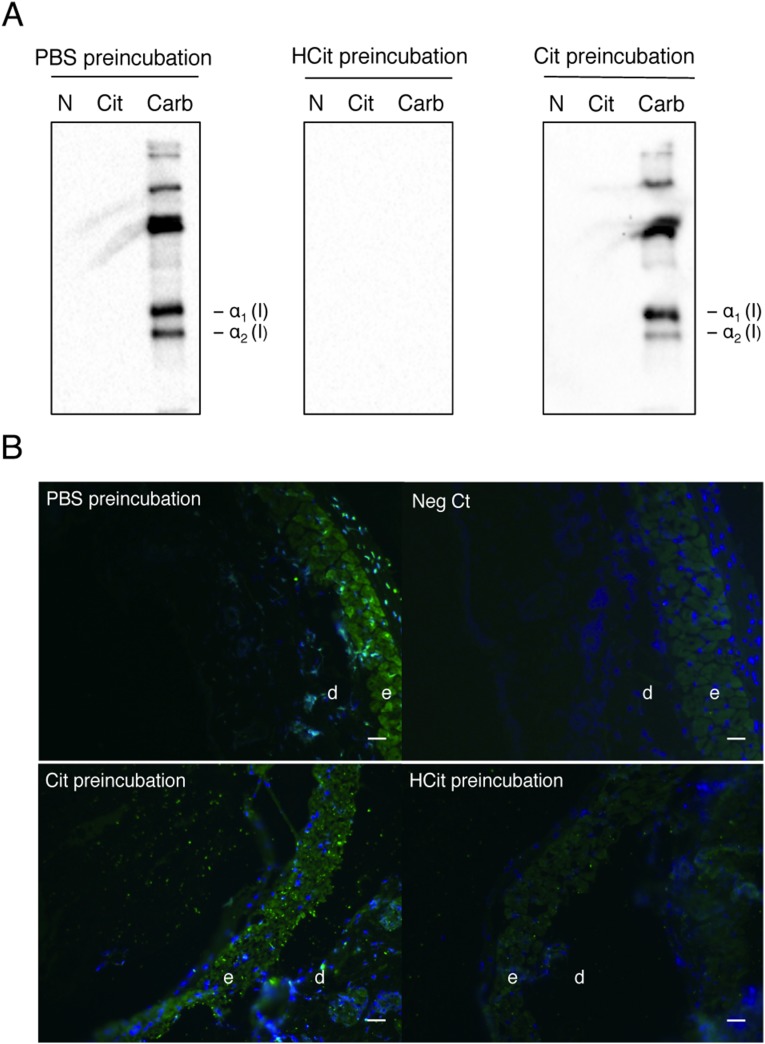
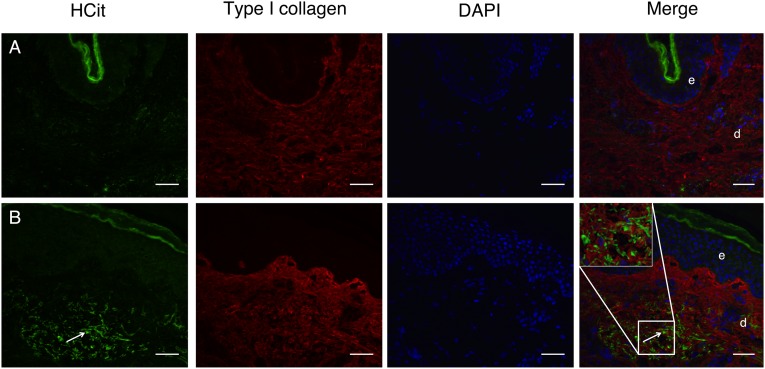
The increase of skin carbamylation intensity with age was confirmed by immunohistochemistry studies on skin samples from young and old humans. For that purpose, an affinity-purified rabbit polyclonal antibody against HCit was produced, and its specificity was verified (Fig. S2). With this anti-HCit–specific antibody, we localized carbamylated proteins into old or young human skin sections. A significant example is displayed in Fig. 3. A weak fluorescence was observed in the dermis or the epidermis of a sample obtained from a 20-y-old human (Fig. 3A). By contrast, an intense staining of HCit was observed in a skin sample obtained from an 80-y-old human, particularly in the reticular dermis (Fig. 3B).
Fig. S2.
Specificity of anti-HCit antibodies. Anti-HCit antibodies were submitted to a preliminary incubation with PBS (PBS preincubation), 5 mM free HCit (HCit preincubation), or 5 mM free citrulline (Cit preincubation). Specificity was characterized by (A) Western blotting using native (N), citrullinated (Cit), or carbamylated (Carb) type I collagen and (B) immunohistofluorescence on mice skin sections with a staining of HCit (green) and nuclei (blue). A negative control (Neg Ct) was performed using secondary antibodies only. For both techniques, antibodies did not show cross-reactivity with citrulline, showing their specificity against HCit. d, Dermis; e, epidermis. (Scale bar: 40 µm.)
Fig. 3.
Localization of carbamylated proteins in human skin. HCit (green) and type I collagen (red) were stained using specific antibodies. Nuclei were stained with DAPI (blue). A poor labeling of carbamylated proteins was found in (A) young skin (20-y-old human) compared with (B) old skin (80-y-old human) showing intense staining (arrows). d, Dermis; e, epidermis. (Scale bar: 40 µm.)
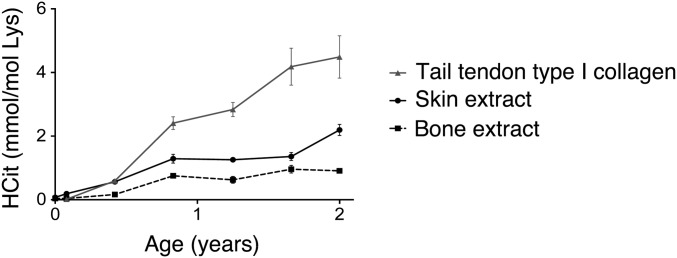
Carbamylation was also shown to increase with age in two other ECM-rich tissues: bone and tail tendon type I collagen. For example, a progressive accumulation of HCit content with age was observed in mice (22-fold increase in both cases from 1 to 24 mo) (Fig. S3).
Fig. S3.
Comparison of HCit accumulation of different tissues during aging in mice. Tissue carbamylation was evaluated by HCit assay in type I collagen extracted from tail tendons, skin, and bones. Each point represents mean ± SD (n = 5).
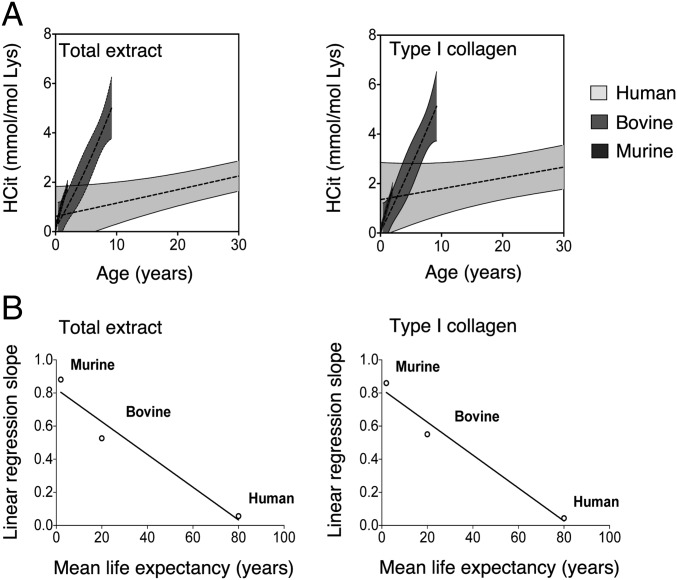
Carbamylation Accumulation Rate Is Inversely Correlated with Life Expectancy.
Slopes of linear regression analysis representing rates of HCit accumulation (Table S1) were plotted against the mean life expectancy of three species. A significant relationship (P < 0.001) was found between accumulation speed of HCit and life expectancy in both skin extracts and skin type I collagen (Fig. 4). Rates of HCit accumulation were notably accelerated in mice, which have the shortest lifespan estimated at 2 y, compared with humans, who have the longest lifespan estimated at 80 y. HCit accumulation in total skin extracts was about 16-fold faster in mice than in humans and 1.6-fold faster than in bovines (Table S1). Similarly, HCit accumulation in type I collagen was accelerated in mice (slope equal to 0.8599) and slower in humans (slope equal to 0.0439).
Fig. 4.
Age-related HCit accumulation rate in skin of human, bovine, and murine species. Accumulation rate was evaluated in skin total extracts and type I collagen. (A) Nonlinear regressions (95% confidence interval; shaded zones) and linear regressions (dotted lines) of HCit concentrations over time were calculated for each species. For better clarity, the graphs focus on a 30-y period. (B) Slopes obtained from equations of linear regression (Table S1) were plotted as a function of mean life expectancy.
HCit Is More Abundant than CML in Skin.
Because skin glycoxidation has previously been shown to increase with age (34, 35), we quantified in skin and collagen samples, in additional to HCit, CML, a characteristic AGE formed by glycoxidation. As expected, a significant increase of CML concentrations was observed over time in total skin and collagen of three species, with Spearman coefficients ranging between 0.62 and 0.88 and between 0.37 and 0.70, respectively (P < 0.01) (Fig. S4). However, HCit concentrations were constantly higher than CML concentrations. HCit was about 3.0-, 3.2-, and 2.6-fold more important than CML in younger murine, bovine, and human individuals, respectively. In older mice and humans, the difference in the elevation remained similar that in younger ones, whereas it reached a factor of 8.6 in older bovines. For example, HCit and CML concentrations were equal to 5.15 and 1.96 mmol/mol Lys, respectively, in >70-y-old humans.
Fig. S4.
Comparison of carbamylation and glycation rates in skin during aging in human, bovine, and murine species. HCit and CML were measured by LC-MS/MS to evaluate carbamylation and glycation, respectively, of proteins in (A) total skin extracts and (B) skin type I collagen. Nonlinear regressions (95% confidence intervals) and Spearman coefficients of correlations (r values) were calculated. HCit contents were higher than CML contents during aging in all studied species.
Carbamylation- and Glycoxidation-Modified Sites of Skin Type I Collagen Were Analyzed by Orbitrap Liquid Chromatography–MS.
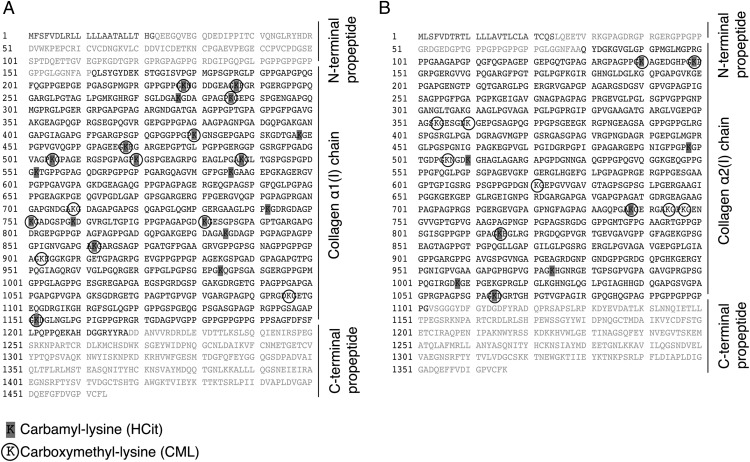
Type I collagen preparations obtained from skin samples of four human subjects ages from 77 to 90 y old were analyzed. In the α1(I)- and α2(I)-chains, 20 and 9 carbamylation sites (generating HCit) and 15 and 11 glycation sites (generating CML) have been identified, respectively. Among them, K277, K538, K586, and K862 in α1(I)-chain and K498 in α2(I)-chain have been identified in several peptides and may be considered preferential targets for carbamylation. Also, 12 lysine residues in α1(I)-chain and 5 lysine residues in α2(I)-chain could be modified by either carbamylation or glycation (Fig. 5 and Tables S3 and S4).
Fig. 5.
Carbamylation and glycation sites in human type I collagen. Carbamylated and glycated lysine residues were identified by Orbitrap liquid chromatography–MS in type I collagen extracted from four human skin samples; (A) 20 carbamyl-lysine residues and 15 CML residues were identified in α1(I)-chain, and (B) 9 carbamyl-lysine and 11 CML residues were identified in α2(I)-chain. Details are given in Tables S3 and S4.
Table S3.
Carbamylation sites in type I collagen from four human skin samples
| Age (y) | |||||
| Lysine sites | 77 | 88 | 89 | 90 | Sum |
| α1-Chain | |||||
| 228 | 2 | 3 | 1 | 6 | |
| 237 | 2 | 1 | 2 | 3 | 8 |
| 277* | 3 | 1 | 4 | 3 | 11 |
| 286 | 2 | 2 | |||
| 430 | 2 | 1 | 2 | 1 | 6 |
| 448 | 1 | 1 | 2 | ||
| 468 | 1 | 1 | 2 | 2 | 6 |
| 505 | 1 | 3 | 1 | 1 | 6 |
| 520 | 1 | 1 | 1 | 3 | |
| 538* | 3 | 3 | 3 | 4 | 13 |
| 552 | 1 | 1 | 2 | ||
| 586* | 3 | 2 | 8 | 4 | 17 |
| 742 | 2 | 2 | |||
| 751 | 1 | 1 | 4 | 2 | 8 |
| 759 | 1 | 1 | 2 | ||
| 781 | 1 | 1 | 1 | 3 | |
| 835 | 1 | 1 | 2 | ||
| 862* | 3 | 1 | 4 | 2 | 10 |
| 984 | 1 | 1 | 1 | 3 | |
| 1,152 | 2 | 2 | |||
| α2-Chain | |||||
| 140 | 2 | 3 | 2 | 2 | 9 |
| 149 | 3 | 2 | 3 | 8 | |
| 498* | 3 | 2 | 5 | 4 | 14 |
| 510 | 1 | 2 | 1 | 4 | |
| 738 | 1 | 3 | 2 | 6 | |
| 815 | 1 | 1 | 2 | 2 | 6 |
| 974 | 1 | 1 | 1 | 3 | |
| 1,008 | 2 | 1 | 3 | ||
| 1,064 | 1 | 1 | 2 | 2 | 6 |
Collagen samples were analyzed by Orbitrap LC-MS. Values depict the numbers of spectra corresponding to the peptides that contain a carbamyl-lysine (HCit) residue.
Lysine residues frequently carbamylated.
Table S4.
Glycation sites in type I collagen from four human skin samples
| Age (y) | |||||
| Lysine sites | 77 | 88 | 89 | 90 | Sum |
| α1-Chain | |||||
| 228 | 3 | 2 | 3 | 8 | |
| 237 | 5 | 4 | 4 | 13 | |
| 286 | 1 | 2 | 3 | ||
| 430 | 2 | 2 | 4 | 8 | 16 |
| 468 | 2 | 1 | 1 | 4 | |
| 505 | 2 | 1 | 1 | 1 | 5 |
| 520 | 1 | 2 | 2 | 5 | |
| 538 | 4 | 5 | 3 | 12 | |
| 709 | 2 | 3 | 2 | 7 | |
| 751 | 1 | 1 | 2 | ||
| 781 | 2 | 2 | 2 | 6 | |
| 862 | 4 | 1 | 5 | ||
| 903 | 2 | 1 | 3 | ||
| 1,096 | 1 | 2 | 3 | ||
| 1,152 | 4 | 1 | 4 | 5 | 14 |
| α2-Chain | |||||
| 140 | 3 | 3 | 2 | 8 | |
| 149 | 2 | 2 | 3 | 7 | |
| 354 | 1 | 5 | 2 | 8 | |
| 360 | 12 | 1 | 8 | 7 | 28 |
| 506 | 4 | 2 | 1 | 7 | |
| 621 | 1 | 2 | 1 | 4 | |
| 738 | 2 | 4 | 1 | 7 | |
| 744 | 2 | 2 | |||
| 747 | 5 | 5 | 4 | 14 | |
| 815 | 1 | 1 | 1 | 3 | |
| 1,064 | 2 | 2 | |||
Collagen samples were analyzed by Orbitrap LC-MS. Values depict the numbers of spectra corresponding to the peptides that contain a CML residue.
Overall, considering that the triple helix is composed of two α1-chains and one α2-chain, about 45% of total lysine residues could be carbamylated to form HCit, whereas 35% could be glycated to form CML. Also, 25% of lysine residues could be either carbamylated or glycated.
Carbamylated Proteins Are Partly Eliminated from the Organism.
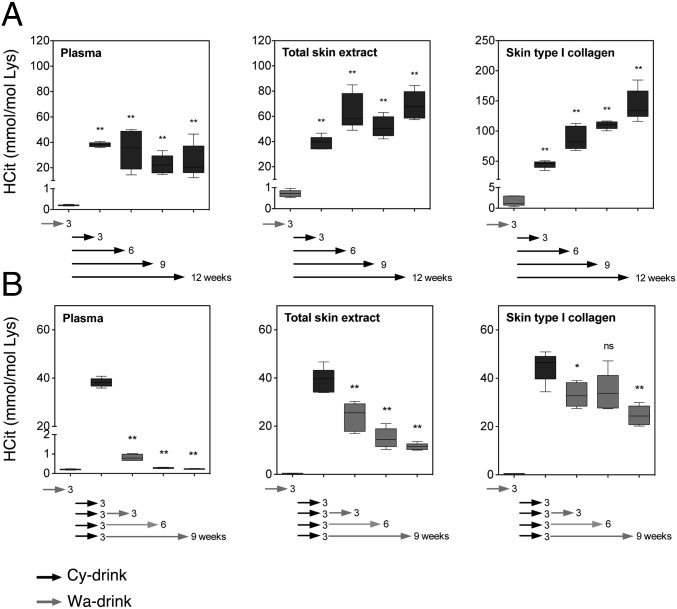
To study the potential turnover of carbamylated proteins in vivo, we used a mouse model of dietary cyanate-induced carbamylation corresponding to a feeding with cyanate-supplemented water [drinking water containing 15 mM sodium cyanate (Cy-drink)]. After 3 wk, HCit significantly increased in plasma and skin in the Cy-drink group compared with the group given nonsupplemented water (Wa-drink), with values being higher than 38 mmol HCit/mol Lys vs. less than 1 mmol HCit/mol Lys, respectively. When cyanate consumption was continued until 12 wk, HCit contents further accumulated in skin, whereas they did not vary anymore in plasma (Fig. 6A). By contrast, when Cy-drink over 3 wk was replaced by Wa-drink, HCit progressively decreased over time. The decrease was almost complete in plasma (−99%), whereas it was only partial in total skin extract (−70%) and especially, collagen (−45%) after 9 wk (P < 0.01) (Fig. 6B).
Fig. 6.
Turnover of carbamylated proteins in mice. Mice received Cy-drink for 3, 6, 9, or 12 wk. (A) Each group was compared with the control group receiving Wa-drink for 3 wk. Other groups of mice received Cy-drink for 3 wk before receiving Wa-drink for 3, 6, or 9 wk. (B) Each group was compared with the control group given Wa-drink for 3 wk. HCit concentrations were evaluated in plasma, total skin extract, and skin type I collagen by liquid chromatography–MS/MS. Whiskers indicate the minimum and maximum values. ns, Nonsignificant (n = 5). *P < 0.05; **P < 0.01.
Discussion
Aging processes arouse a major interest in biomedical research, which tries to understand how the loss of physiological integrity coupled with cumulative dysfunctions occurring in cells and tissues are responsible for the increased risk of disease and evolution to death. Its global understanding is still obscure and complex because of the multifactorial causes. Genetic and environmental factors are involved in this phenomenon, some of them being related to the occurrence of chemical reactions associated to intrinsic aging. Many NEPTMs occur during the biological life of proteins, leading to protein molecular aging. These chemical reactions interfere with normal functions by disrupting molecular conformations, altering enzymatic activities, reducing degradation capacities, or interfering with receptor recognitions (4, 36). Among them, glycoxidation has been well-described, and its major contribution to the development of pathophysiological complications of various diseases, such as diabetes mellitus, has been established (37–40). This reaction had also been shown to participate in the physiological aging process with an accumulation of AGEs in tissues with age. It has been shown that glycoxidation rate increased as a function of age in skin collagen of various mammalian species or cartilage collagen in humans, potentially contributing to age-related impairments (11, 34, 41). However, other biochemical reactions are involved in molecular aging, such as carbamylation, which is caused by the reaction of proteins with isocyanic acid (4, 42–45). Isocyanic acid is mainly derived from urea dissociation but may also be generated from thiocyanate metabolism or brought by environment (15, 19, 23). Because isocyanic acid sources are found in physiological conditions, a basal carbamylation level of tissues is expected. However, nothing has been reported to date about tissue carbamylation during aging. In this study, we have evaluated carbamylation of skin proteins in three mammalian species with distinct longevities and shown for the first time, to our knowledge, that carbamylation is a general age-related process with significant intensity.
The study has been carried out in murine, bovine, and human species from younger to older ages. We show that skin content in carbamylated proteins singularly increases over time regardless of lifespan, showing the physiological occurrence of the carbamylation process. Previous works have shown that the increase of carbamylated proteins could be considered a risk factor in patients with kidney disease, atherosclerosis, or coronary artery disease (22, 23, 25). Our results show that this reaction is not restricted to disease conditions but occurs physiologically during aging, inducing tissue accumulation of HCit at the highest ages of life.
To identify the accumulation sites of CDPs within skin, we performed an immunohistochemical study on human skin sections. HCit labeling was particularly intense in reticular dermis compared with papillary dermis. Its high content in matrix proteins, especially thick and mature elastic and type I collagen fibers, and the long half-life of these proteins may explain why the reticular layer is more prone to carbamylation. Indeed, type I collagen and elastin are especially highly carbamylated in older subjects compared with younger ones after a continuous process starting from birth. Proteomic analysis showed that an average of 45% of total lysine residues could be modified by carbamylation in both α1- and α2-collagen chains. However, four of them were preferentially carbamylated, despite theoretical random character of this modification.
Previous in vitro studies in our laboratory have shown the deleterious effects of carbamylation on type I collagen, including local modifications in the triple helical structure. It has been shown that the modification of only four HCit residues per α-chain is sufficient to induce local destabilization, leading to a decrease of thermal stability, or fibrillogenesis impairment or alter sensitivity to proteolysis by matrix metalloproteinases (42, 43). In addition, collagen carbamylation leads to functional damages, because carbamylated collagen is able to alter oxidative functions of inflammatory cells (42). Elastin carbamylation was never suspected before this study. It is known that in vitro glycation is responsible for the alteration of its assembly, proteolytic degradation, or physical properties (46), suggesting that the chemical modifications brought by carbamylation may also impact functional and structural properties of elastin. Herein, it is likely that accumulation of matrix CDPs contributes to skin aging by reducing elasticity and increasing rigidity of skin matrix proteins.
Another major finding of our study is the relationship between skin protein carbamylation and life expectancy. We show that HCit increase in skin and especially, collagen is more pronounced in species with shorter lifespans. Indeed, HCit increases by 29-fold in mice from birth to elderly, whereas it increases only by 11.5- and 8.1-fold in bovines and humans, respectively. However, although the rate of HCit accumulation in mice is the highest, absolute levels reached at the end of life are lower than in humans. Carbamylation is a cumulative and time-dependent reaction explaining that species with longer lifespans exhibit higher skin HCit contents. We expected that HCit accumulation would be more intense in humans than in mice and consequently, that HCit content would be even higher. This hypothesis has not been confirmed by our experimental results, which could be partly explained by the higher blood urea concentrations found in murine species compared with human species (8–11 vs. 2–7 mmol/L, respectively). Consequently, mice tissues are more exposed to isocyanic acid over time, leading to an increased carbamylation rate and a faster accumulation of CDPs. In addition, it is likely that animal species with longer lifespans develop efficient turnover, repair, or degradation mechanisms restricting tissue accumulation of carbamylated proteins. Similar processes have already been described. For example, it has been shown that fibroblasts from longer-lived primate species exhibit a significant elevation in immunoproteasome activities compared with those from shorter-lived species, which could explain the increased turnover of oxidized or otherwise damaged proteins (47).
However, skin carbamylation might reflect a systemic carbamylation affecting other tissues, such as vessels, brain, or heart. Because of their adverse effects on proteins properties and cell interactions, accumulating CDPs might participate in metabolic disorders, explaining the inverse correlation between CDP accumulation speed and longevity.
To approach the potential turnover of CDPs, we used a mouse model of dietary cyanate-induced carbamylation. HCit strongly increases in plasma and skin when mice are given Cy-drink (with factors equal to 180 and 86, respectively). When mice are then given Wa-drink for 9 wk, plasma proteins are totally renewed because of their short half-life, whereas carbamylated skin proteins are only partially eliminated from organism, surmising that CDPs could be under homeostatic control. We can hypothesize that this decrease is associated with shorter half-life of some carbamylated proteins or elimination of intracellular CDPs by proteolytic systems, such as proteasome pathway or autophagy, as previously reported for glycated or oxidized proteins (48–50). However, skin collagen exhibits the weakest HCit decrease because of its slower turnover or the contribution of some mechanisms taking part in the renewal and remodeling of ECM proteins.
Finally, we compared glycation and carbamylation rates during aging, because an accumulation of AGEs had been previously shown, whereas nothing was known about carbamylation. Pentosidine and CML, two majors AGEs, accumulate in skin collagen of various mammalian species, indicating a correlation between glycoxidation of protein content and longevity (34). Interestingly, our study shows that HCit content in skin is significantly higher than CML content. Especially, proteomic analysis shows that type I collagen of aged human skin contains more carbamylated than glycated sites. This difference might be explained by the successive reactions required for the formation of CML, contrary to HCit. CML is one of the most prevalent AGEs in vivo (51), but it seems that HCit is a better marker of skin aging and that carbamylation is a major modification of tissue proteins. Nevertheless, intercurrent diseases, such as chronic renal failure or diabetes mellitus, may cause an imbalance in favor of either HCit or CML formation (15, 25, 37, 52). Because these diseases are commonly intertwined, carbamylation and glycation could induce synergistic deleterious effects on tissue proteins, which could accelerate age-related complications.
In summary, this study shows for the first time, to our knowledge, that carbamylation is a physiological process responsible for the accumulation of CDPs in skin during aging. Being an adverse event for protein structure and function, we hypothesize that this NEPTM participates in long-term dysfunctions associated with skin aging. With carbamylation being described as profibrotic and proinflammatory, skin CDP accumulation is potentially engaged in elasticity loss or infectious complications. Pathophysiological consequences of this accumulation and longevity-associated mechanisms preventing carbamylation are puzzling questions that have to be further addressed to better understand the molecular mechanisms underlying tissue aging.
Materials and Methods
Tissue Samples.
Mice.
Experiments were performed in C57BL/6J mice purchased from Charles River. Animals were fed ad libitum and housed in a room with a constant ambient temperature and a 12-h light–dark cycle. All animal procedures were conducted in accordance with French government policies (Services Vétérinaires de la Santé et de la Production Animale, Ministère de l’Agriculture), and the study protocol was approved by the institutional animal care committee (Comité d’éthique en expérimentation animale de Reims Champagne Ardenne registration 56).
In aging experiments, mice were randomly assigned to seven groups according to their age (newborn and 1, 5, 10, 15, 20, and 24 mo old; n = 5 each). Mice were killed under xylazine [2% (wt/vol) Rompun; 6 μg/g body weight; Bayer] and ketamine (Clorketam 1000; 120 μg/g body weight; Vetoquinol SA) anesthesia, and blood was collected in heparinized tubes after cardiac puncture. Skin, posterior limb bones, and tail were extracted. Plasma samples obtained after blood centrifugation and tissues were frozen at −80 °C until analysis.
In the model of dietary cyanate-induced carbamylation, 8-wk-old mice were randomly assigned to nine groups (n = 5 each). Four groups received Cy-drink for 3, 6, 9, or 12 wk. Three groups received Cy-drink for 3 wk and then, Wa-drink for 3, 6, or 9 wk. Two control groups received Wa-drink for 3 or 12 wk. Water was renewed two times per week in all series. Mice were killed under anesthesia (ketamine and xylazine), and blood and tissues were collected as described above.
Bovines.
Skin samples were provided by the Soredex Slaughterhouse in accordance with French government policies (Services Vétérinaires d’Inspection, Ministère de l’Agriculture). Animals were ranked in four groups according to their age (6 mo old and 1, 4, and 8 y old; n = 5 each).
Humans.
Skin samples were obtained from the Forensic Institute of the University Hospital of Reims. Macroscopically normal skin samples were excised in the abdomen within 2 d after death and frozen at −80 °C. Patients with CKD and diabetes mellitus were excluded. The protocol, conducted according to French regulation policies, included 60 individuals ages between 1 mo old and 98 y old.
Extraction Protocols.
Tissue extraction.
Tissues (∼100 mg) were homogenized with 1 mL 0.5 M acetic acid in Lysing Matrix D Tubes with the FastPrep-24 System (MP Biomedicals). After homogenization, samples underwent pepsin digestion (10% wt/wt) for 24 h at 37 °C.
Collagen extraction.
Type I collagen was extracted from mice tail tendons and human, bovine, and mice skin as previously described (28). Briefly, tendons were incubated in 0.5 M acetic acid for 24 h at 4 °C, and the resulting solution was submitted to precipitation with 0.7 M NaCl. However, skin samples were homogenized using the FastPrep-24 System in 0.5 M acetic acid containing 0.1% (wt/wt) pepsin and incubated in this solution for 24 h at 4 °C. After centrifugation at 10,000 × g for 30 min, all precipitates were solubilized with 0.5 M acetic acid and dialyzed against distilled water for 3 d at 4 °C. Samples were then freeze-dried and stored at −80 °C until analysis.
Elastin extraction.
Human aorta samples (200 mg) were ground in a ball mill under liquid nitrogen cooling (Retsch Technology), and elastin was extracted according to a previously described protocol, which is summarized in Table S5 (53). The protocol was carried out at room temperature under shaking. Between each step, a volume of 1.5 mL corresponding buffer was added, samples were centrifuged at 14,000 × g for 2 min, and the supernatant was carefully removed.
Table S5.
Protocol of skin elastin extraction
| Day 1 |
| Washing two times with 1 M NaCl solution containing 0.02% (wt/vol) NaN3 for 2 h |
| Addition of a solution of ethanol:water (containing 0.02% NaN3; 1:1 vol/vol) overnight |
| Day 2 |
| Consecutive washing with pure ethanol, chloroform:methanol (2:1 vol/vol), ether, acetone, and pure ethanol for 1 h each |
| Addition of 10% (wt/vol) cyanogen bromide in 97% (vol/vol) formic acid overnight |
| Day 3 |
| New cleavage with 10% cyanogen bromide in 97% formic acid for 6 h |
| Washing three times with ethanol:water (containing 0.02% NaN3; 1:1 vol/vol) for 10 min each |
| Incubation with 0.3 M Tris⋅HCl (containing 0.02% NaN3), 1.5 M 2-mercaptoethanol, and 4 M guanidinium buffer overnight |
| Day 4 |
| Two repetitions of the precedent step for 3 h each |
| Washing three times with ethanol:water (containing 0.02% NaN3; 1:1 vol/vol) for 10 min each |
| Incubation with 100 mM NH4HCO3 (containing 0.02% NaN3) and 8,000 U N-benzoyl-l-arginine ethyl ester trypsin overnight at 37 °C |
| Day 5 |
| Addition of 0.3 M Tris⋅HCl (containing 0.02% NaN3), 1.5 M 2-mercaptoethanol, and 4 M guanidinium buffer for 1 h |
| Extraction two times with 0.1% (vol/vol) acetonitrile:formic acid (1:1 vol/vol) for 10 min each |
| Consecutive washing with ethanol:water (containing 0.02% NaN3; 1:1 vol/vol), ethanol:water (7:3 vol/vol), and pure ethanol for 10 min each |
| Drying under laminar airflow |
HCit and CML Quantification.
HCit and CML were evaluated by liquid chromatography–MS/MS as described in SI Materials and Methods and previous studies (16, 28). Results were expressed as ratios to lysine (HCit to Lys or CML to Lys), except in the case of elastin, where HCit was expressed as a ratio to glutamate content (HCit to Glu), because most of Lys residues are involved in elastin cross-linking.
Analysis of Carbamylation and Glycation Sites of Collagen.
Purified collagen samples were solubilized in 18 mM acetic acid at 4 °C overnight. Samples were then denatured for 40 min at 60 °C and subsequently treated with 5 mM DTT for 40 min at 70 °C under mild shaking. The samples were then neutralized using 1 M NaOH, incubated for 40 min at room temperature in the dark after adding 12.5 mM iodoacetamide, and digested with trypsin (Thermo Scientific/Pierce) at an enzyme to substrate ratio of 1:40 (wt/wt) at 37 °C. After 12 h of incubation, the same amount of enzyme was added. All digestions were stopped after 24 h by adding TFA to a final concentration of 0.5% (vol/vol). Liquid chromatography–MS/MS analysis was carried out by separating the collagen on an Ultimate 3000 RSLCnano System (trap column: Acclaim PepMap RP-C18; 300 µM × 5 mm, 5 µm, and 100 Å; separation column: Acclaim PepMap RP-C18; 75 μm × 250 mm, 2 µm, and 100 Å; Thermo Fisher Scientific) coupled to an Orbitrap Fusion Tribrid Mass Spectrometer equipped with a Nanospray Flex Ion Source (Thermo Fisher Scientific). Parameters of HPLC and MS are described in SI Materials and Methods.
Immunohistochemistry.
Samples of human and bovine skins were cleaned free of adipose tissues, embedded in a cryomatrix (Shandon; Thermo Scientific), and frozen in liquid nitrogen before storage at −80 °C. From cryomatrix block, 6-µm-thick serial sections were performed with a microtome (MICROM Cryo-Star HM 560). After hydration and washes with PBS, sections were incubated with anti-HCit antibody (polyclonal antibody from rabbit; 1:40 vol/vol; produced and characterized as discussed in SI Materials and Methods; Covalab) overnight at 4 °C and then, antitype I collagen antibody (polyclonal antibody from goat; 1:50 vol/vol; Abcam) under the same conditions. After serial washes with PBS, sections were incubated with anti-rabbit IgG Alexa Fluor 488 antibody (1:100 vol/vol; Invitrogen) for 30 min at room temperature and then, anti-goat IgG Alexa Fluor 633 antibody (1:100 vol/vol; Life Technologies) under the same conditions. The sections were counterstained with DAPI (Dapi-Fluoromount G; Clinisciences).
Statistical Analysis.
Nonlinear regression (second-order polynomial function with 95% confidence interval) and linear regression were computed by using GraphPad Prism software. A nonparametric Spearman test was used for the calculation of correlation coefficients. Comparisons of carbamylation rates were studied by the Mann–Whitney u test. Differences were considered statistically significant when P was ≤0.05.
SI Materials and Methods
Production and Characterization of Anti-HCit Antibodies.
Affinity-purified rabbit polyclonal antibodies against HCit were produced by Covalab Society. For this purpose, rabbits were immunized with carbamylated carrier proteins according to an optimized immunization protocol including four injections. The production of antibodies was monitored by immunopurification and ELISA tests. In addition, we verified the specificity of the antibodies, because anti-HCit antibodies could react with citrullinated proteins (Fig. S2). Citrullination is a physiological enzymatic posttranslational modification catalyzed by peptidyl-arginine deiminase that converts arginine to citrulline. Citrulline and HCit both contain a ureido group and only differ by one carbon in their atom number, meaning that citrulline could be recognized by HCit antibodies. Consequently, specificity of antibodies was evaluated by Western blotting against carbamylated or citrullinated type I collagen extracted from Sprague–Dawley tail tendons.
Carbamylation was performed by incubation of collagen with 0.1 M NaCNO in 0.15 M phosphate buffer (pH 7.4) for 48 h at 37 °C (42). Citrullination was achieved by dialysis of collagen against a 0.1 M Tris (pH 7.6) buffer containing 10 mM CaCl2 and 5 mM DTT overnight at 4 °C followed by an incubation of collagen with 2 U/mL peptidyl-arginine deiminase for 48 h at 37 °C. After addition of 2 mM EDTA, the sample was dialyzed against 2 mM EDTA (pH 2.6) solution overnight at 4 °C and then, 10 mM Tris (pH 7.6) buffer overnight at 4 °C; 5 µg native, carbamylated, or citrullinated collagens were separated by SDS/PAGE with 5% (wt/vol) polyacrylamide and transferred to a PVDF membrane. The membrane was then incubated with anti-HCit antibodies (1:300 vol/vol) overnight at 4 °C. In separate series, anti-HCit antibodies were preliminary incubated with either 5 mM free HCit or free citrulline for 2 h at 37 °C to control their specificity. Anti-rabbit antibodies (HRP-conjugated antibodies from donkey; 1:10,000 vol/vol; Amersham) were used as secondary antibodies. No signal was detected after preincubation of antibodies with free HCit, whereas preincubation with free citrulline did not alter the signal, showing the specificity of the antibodies (Fig. S2A). In addition, we tested anti-HCit antibodies on mice skin sections. Samples were embedded, and sections were realized as described above. After hydration and washes with PBS, sections were incubated with anti-HCit antibodies (1:40 vol/vol) overnight at 4 °C. As described above, anti-HCit antibodies were preliminary incubated with either 5 mM free HCit or free citrulline for 2 h at 37 °C to verify their specificity. After serial washes with PBS, sections were incubated with anti-rabbit antibodies (Alexa Fluor 488-conjugated antibodies from donkey; 1:100 vol/vol; Invitrogen) for 30 min at room temperature. The sections were counterstained with DAPI (Dapi-Fluoromount G; Clinisciences). Like previously seen, no signal was noticed when anti-HCit antibodies were preincubated with free HCit. By contrast, preliminary incubation with citrulline did not disturb the reaction of antibodies with tissue HCit (Fig. S2B), showing the absence of cross-reaction with citrullinated proteins and the specificity against HCit.
HCit, CML, Lys, and Glu Quantification by Liquid Chromatography–MS/MS.
All samples were subjected to acid hydrolysis by 6 M hydrochloric acid for 18 h at 110 °C. Hydrolysates were twice evaporated to dryness under a nitrogen stream. Dried samples were resuspended in 100 μL 125 mM ammonium formate containing 1 μM d7-citrulline, 1 µM d2-CML, and 65 µM d8-lysine [used as internal standards (ISs)] and filtered using Uptidisc PTFE Filters (4 mm, 0.45 μm; Interchim). Samples were then 10-fold diluted in 125 mM ammonium formate containing three ISs. For Lys and Glu quantitation, a second dilution at 1:20 (vol/vol) was performed in 5 mM ammonium formate buffer (pH 2.9) containing ISs. Diluted hydrolysates were subjected to liquid chromatography (LC) –MS/MS analysis (API4000; ABSciex) to quantify HCit, CML, Lys, and Glu. LC was performed using a Kinetex HILIC Column (100 × 4.6 mm, 2.6 µm; Phenomenex) with 5 mM ammonium formate (pH 2.9) as mobile phase A and 100% acetonitrile as mobile phase B. The flow rate was constant at 0.9 mL/min during all separation steps. Parameters of HPLC and MS for HCit and Lys assays have been described elsewhere (16, 28). Parameters for CML and Glu are described below.
CML quantification.
Before analysis, hydrolyzed samples were diluted 10-fold in 125 mM ammonium formate (containing 1 µM d2-CML used as IS). The gradient program was as follows: 0–0.3 min, 90% B; 0.3–1.5 min, gradient to 5% B; 1.5–2 min, 5% B; 2–3.1 min, gradient to 40% B; 3.1–3.5 min, 40% B; 3.5–4 min, gradient to 90% B; and 4–5.5 min, 90% B. The injection volume was 10 µL, and oven temperature was set at 25 °C. Detection was performed in positive ion mode, and electrospray ionization source parameters were as follows: curtain gas, 50 psi; collision gas, 6 psi; nebulization gas 1, 40 psi; nebulization gas 2, 60 psi; ion spray voltage, 5,500 V; and source temperature, 600 °C. Parameters used for mass detection by multiple reaction monitoring were as follows: 205.1 > 130.1 Da, collision energy (CE): 19 eV and collision cell exit potential (CXP): 6 eV for CML and 207.1 > 84.1 Da, CE: 29 eV and CXP: 15 eV for d2-CML.
Glu quantification.
Before injection, hydrolyzed samples were 10-fold diluted in 125 mM ammonium formate buffer and then, 5-fold diluted in 5 mM ammonium formate buffer (pH 2.9; both containing 65 µM d8-lysine used as IS). The gradient program was as follows: 0–0.5 min, 90% B; 0.5–1 min, gradient to 50% B; 1–2 min, 50% B; 2–2.5 min, gradient to 10% B; 2.5–3.6 min, 10% B; 3.6–3.8 min, gradient to 90% B; and 3.8–5.5 min, 90% B. Injection volume was 1 μL, and oven temperature was set at 30 °C. Detection was performed in positive ion mode, and electrospray ionization source parameters were as follows: curtain gas, 40 psi; collision gas, 6 psi; nebulization gas 1, 40 psi; nebulization gas 2, 60 psi; ion spray voltage, 4,500 V; and source temperature, 650 °C. Parameters used for mass detection by multiple reaction monitoring were as follows: 148.1 > 102.0 Da, CE: 27 eV and CXP: 8 eV for Glu and 155.0 > 92.0 Da, CE: 19 eV and CXP: 6 eV for d8-lysine.
Nitrogen and argon were used as nebulization and collision gases, respectively. Mass spectra and chromatograms were acquired and processed with Analyst software, version 1.5.1 (ABSciex).
LC-MS/MS Parameters for Identification of Carbamylation and Glycation Sites.
Collagen samples were digested as described in the text. Peptide mixture was then loaded on the trap column and washed with water containing 0.1% (vol/vol) TFA for 15 min (30 µL min−1). Then, they were separated on the separation column using solvent A [0.1% (vol/vol) formic acid in water] or B [0.08% (vol/vol) formic acid in acetonitrile] according to this program: gradient from 1% to 35% B (90 min) and from 35% to 85% B (5 min) followed by 85% B (5 min). Data were acquired using data-dependent MS/MS mode. Each high-resolution full scan in the Orbitrap (m/z 300–1500; R = 120,000) was followed by high-resolution product ion scans in the Orbitrap (collision-induced dissociation; 35% normalized collision energy; R = 15,000) within 5 s, starting with the most intense signal in the full-scan mass spectrum (quadrupole isolation; window: 2 Th). Dynamic exclusion (exclusion duration: 60 s; exclusion window: ±2 ppm) was enabled to allow detection of less abundant ions. Data acquisition was controlled with Xcalibur 3.0.63. Peptides were identified by automated de novo sequencing followed by matching against the human section of the Swiss-Prot database using the software PEAKS Studio 7.5 (Bioinformatics Solutions). The mass error tolerances for precursor and fragment ions were set to 5 ppm and 0.015 Da, respectively. Carbamidomethyl cysteine was set as fixed modification, whereas oxidations of Met, Pro, Lys, carbamyl-lysine, and CML were set as variable modifications. The peptide score threshold was decreased until a false discovery rate no higher than 0.1% was achieved. Only modifications that were confidently localized in at least two peptides were accepted and are indicated in this study.
Acknowledgments
The authors thank Catherine Desroches, Nathalie Leroy, and Eymeric Lagonotte for skillful technical assistance as well as the Tissue and Cell Imaging Platform of the Faculty of Medicine. We also thank the American Memorial Hospital Committee, the University of Reims Champagne-Ardenne, and the CNRS for financial support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 1121.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1517096113/-/DCSupplemental.
References
- 1.Grimes A, Chandra SBC. Significance of cellular senescence in aging and cancer. Cancer Res Treat. 2009;41(4):187–195. doi: 10.4143/crt.2009.41.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brack C, Lithgow G, Osiewacz H, Toussaint O. EMBO WORKSHOP REPORT: Molecular and cellular gerontology Serpiano, Switzerland, September 18-22, 1999. EMBO J. 2000;19(9):1929–1934. doi: 10.1093/emboj/19.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40(2):333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillery P, Jaisson S. Usefulness of non-enzymatic post-translational modification derived products (PTMDPs) as biomarkers of chronic diseases. J Proteomics. 2013;92:228–238. doi: 10.1016/j.jprot.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Li JJ, Surini M, Catsicas S, Kawashima E, Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiol Aging. 1995;16(1):69–76. doi: 10.1016/0197-4580(95)80009-g. [DOI] [PubMed] [Google Scholar]
- 6.Albon J, Karwatowski WS, Avery N, Easty DL, Duance VC. Changes in the collagenous matrix of the aging human lamina cribrosa. Br J Ophthalmol. 1995;79(4):368–375. doi: 10.1136/bjo.79.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63(4):582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Schinzel R, Münch G, Heidland A, Sebekova K. Advanced glycation end products in end-stage renal disease and their removal. Nephron. 2001;87(4):295–303. doi: 10.1159/000045934. [DOI] [PubMed] [Google Scholar]
- 9.Odetti P, et al. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043:710–717. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 10.Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol (1985) 2007;103(6):2068–2076. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- 11.Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 12.Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: A novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol. 2015;185:263–268. doi: 10.1016/j.ijcard.2015.03.167. [DOI] [PubMed] [Google Scholar]
- 13.Grossin N, et al. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol Nutr Food Res. 2015;59(5):927–938. doi: 10.1002/mnfr.201400643. [DOI] [PubMed] [Google Scholar]
- 14.Gillery P, Jaisson S. Post-translational modification derived products (PTMDPs): Toxins in chronic diseases? Clin Chem Lab Med. 2014;52(1):33–38. doi: 10.1515/cclm-2012-0880. [DOI] [PubMed] [Google Scholar]
- 15.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–S107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaisson S, Gorisse L, Pietrement C, Gillery P. Quantification of plasma homocitrulline using hydrophilic interaction liquid chromatography (HILIC) coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402(4):1635–1641. doi: 10.1007/s00216-011-5619-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13(10):1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 18.Sirpal S. Myeloperoxidase-mediated lipoprotein carbamylation as a mechanistic pathway for atherosclerotic vascular disease. Clin Sci (Lond) 2009;116(9):681–695. doi: 10.1042/CS20080322. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JM, et al. Isocyanic acid in the atmosphere and its possible link to smoke-related health effects. Proc Natl Acad Sci USA. 2011;108(22):8966–8971. doi: 10.1073/pnas.1103352108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark GR, Stein WH, Moore S. Reactions of the cyanate present in aqueous urea with amino acids and proteins. J Biol Chem. 1960;235(11):3177–3181. [Google Scholar]
- 21.Nicholson DH, Harkness DR, Benson WE, Peterson CM. Cyanate-induced cataracts in patients with sickle-cell hemoglobinopathies. Arch Ophthalmol. 1976;94(6):927–930. doi: 10.1001/archopht.1976.03910030465005. [DOI] [PubMed] [Google Scholar]
- 22.Jaisson S, et al. Increased serum homocitrulline concentrations are associated with the severity of coronary artery disease. Clin Chem Lab Med. 2015;53(1):103–110. doi: 10.1515/cclm-2014-0642. [DOI] [PubMed] [Google Scholar]
- 23.Apostolov EO, Basnakian AG, Ok E, Shah SV. Carbamylated low-density lipoprotein: Nontraditional risk factor for cardiovascular events in patients with chronic kidney disease. J Ren Nutr. 2012;22(1):134–138. doi: 10.1053/j.jrn.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, et al. Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies. Autoimmun Rev. 2014;13(3):225–230. doi: 10.1016/j.autrev.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Berg AH, et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med. 2013;5(175):175ra29. doi: 10.1126/scitranslmed.3005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koeth RA, et al. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24(5):853–861. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalim S, et al. Carbamylation of serum albumin and erythropoietin resistance in end stage kidney disease. Clin J Am Soc Nephrol. 2013;8(11):1927–1934. doi: 10.2215/CJN.04310413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrement C, Gorisse L, Jaisson S, Gillery P. Chronic increase of urea leads to carbamylated proteins accumulation in tissues in a mouse model of CKD. PLoS One. 2013;8(12):e82506. doi: 10.1371/journal.pone.0082506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asci G, et al. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 2008;13(6):480–486. doi: 10.1111/j.1440-1797.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 30.Shiu SW, et al. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin Sci (Lond) 2014;126(2):175–181. doi: 10.1042/CS20130369. [DOI] [PubMed] [Google Scholar]
- 31.Oimomi M, et al. Carbamylation of insulin and its biological activity. Nephron. 1987;46(1):63–66. doi: 10.1159/000184303. [DOI] [PubMed] [Google Scholar]
- 32.Park KD, Mun KC, Chang EJ, Park SB, Kim HC. Inhibition of erythropoietin activity by cyanate. Scand J Urol Nephrol. 2004;38(1):69–72. doi: 10.1080/00365590310006291. [DOI] [PubMed] [Google Scholar]
- 33.Koro C, et al. Carbamylation of immunoglobulin abrogates activation of the classical complement pathway. Eur J Immunol. 2014;44(11):3403–3412. doi: 10.1002/eji.201444869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sell DR, et al. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci USA. 1996;93(1):485–490. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sell DR, Kleinman NR, Monnier VM. Longitudinal determination of skin collagen glycation and glycoxidation rates predicts early death in C57BL/6NNIA mice. FASEB J. 2000;14(1):145–156. doi: 10.1096/fasebj.14.1.145. [DOI] [PubMed] [Google Scholar]
- 36.Chondrogianni N, et al. Protein damage, repair and proteolysis. Mol Aspects Med. 2014;35:1–71. doi: 10.1016/j.mam.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflamm. 2013;2013:403460. doi: 10.1155/2013/403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simm A. Protein glycation during aging and in cardiovascular disease. J Proteomics. 2013;92:248–259. doi: 10.1016/j.jprot.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Castellani RJ, et al. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic Biol Med. 2001;31(2):175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 41.Verzijl N, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 42.Jaisson S, et al. Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem Biol. 2006;13(2):149–159. doi: 10.1016/j.chembiol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Jaisson S, et al. Carbamylation differentially alters type I collagen sensitivity to various collagenases. Matrix Biol. 2007;26(3):190–196. doi: 10.1016/j.matbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Mun KC, Golper TA. Impaired biological activity of erythropoietin by cyanate carbamylation. Blood Purif. 2000;18(1):13–17. doi: 10.1159/000014403. [DOI] [PubMed] [Google Scholar]
- 45.Kraus LM, Traxinger R, Kraus AP. Uremia and insulin resistance: N-carbamoyl-asparagine decreases insulin-sensitive glucose uptake in rat adipocytes. Kidney Int. 2004;65(3):881–887. doi: 10.1111/j.1523-1755.2004.00456.x. [DOI] [PubMed] [Google Scholar]
- 46.Yoshinaga E, et al. N(ε)-(carboxymethyl)lysine modification of elastin alters its biological properties: Implications for the accumulation of abnormal elastic fibers in actinic elastosis. J Invest Dermatol. 2012;132(2):315–323. doi: 10.1038/jid.2011.298. [DOI] [PubMed] [Google Scholar]
- 47.Pickering AM, Lehr M, Miller RA. Lifespan of mice and primates correlates with immunoproteasome expression. J Clin Invest. 2015;125(5):2059–2068. doi: 10.1172/JCI80514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickering AM, et al. The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432(3):585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaisson S, Gillery P. Impaired proteostasis: Role in the pathogenesis of diabetes mellitus. Diabetologia. 2014;57(8):1517–1527. doi: 10.1007/s00125-014-3257-1. [DOI] [PubMed] [Google Scholar]
- 50.Shang F, Taylor A. Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: Implications in the pathogenesis of age-related macular degeneration. Mol Aspects Med. 2012;33(4):446–466. doi: 10.1016/j.mam.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gkogkolou P, Böhm M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol. 2012;4(3):259–270. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251(2):87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 53.Schmelzer CEH, Jung MC, Wohlrab J, Neubert RHH, Heinz A. Does human leukocyte elastase degrade intact skin elastin? FEBS J. 2012;279(22):4191–4200. doi: 10.1111/febs.12012. [DOI] [PubMed] [Google Scholar]