Glucocorticoids (GCs) belong to a class of endogenous, stress-stimulated steroid hormones found in vertebrates (e.g., cortisol in humans and corticosterone in rodents); they have wide ranging physiologic effects capable of impacting metabolism, immunity, development, stress, cognition, and arousal. GCs exert their cellular effects by binding to the GC receptor (GR), one of a 48-member (in humans) nuclear receptor (NR) superfamily of ligand-activated transcription factors (1). As the first human NR to be cloned (2), GR has provided an invaluable template with which to understand how the structurally related NRs exert their complex cellular effects. Its activity also underscores the importance of small lipophilic ligands in regulating multiple biologic pathways. Like other NR family members, the GR comprises three major functional domains: (i) an N-terminal domain (NTD), which contains a constitutive activation function 1 (AF-1); (ii) a DNA-binding domain (DBD), containing two zinc finger motifs; and (iii) a C-terminal, ligand-binding domain (LBD), with its ligand-dependent AF-2 (Fig. 1A). The human and mouse GRs are encoded by a single NR3C1 gene, which product can be differentially spliced into two major isoforms, GRα and GRβ; the former is responsible for the majority of GR-mediated transcriptional activity (3). Additional variants, generated via translational regulatory mechanisms, together with posttranslational modifications (PTMs), contribute to the complexity and diversification of GR-mediated action (3, 4). These PTMs can dial up, or down, GR-mediated transcriptional activities, to confer distinct biologic functions. Relevant PTMs include phosphorylation, acetylation, methylation, ubiquitination, and SUMOylation (4). The first of these, phosphorylation, has been shown to modulate dimerization and DNA binding, coregulator interaction, and ligand-binding affinity, all of which alter transcriptional activity. A total of nine phosphorylation sites within the human GR NTD has been reported, some of which influence nuclear export and coregulator recruitment (5). Interestingly, NR SUMOylation, which involves the covalent conjugation of SUMO moieties at specific lysines, triggers molecular transrepression pathways that link metabolism and inflammation (6). In two back-to-back publications in PNAS (7, 8), Hua et al. now report the detailed molecular mechanisms by which GR SUMOylation provokes GC-dependent gene repression.
Fig. 1.
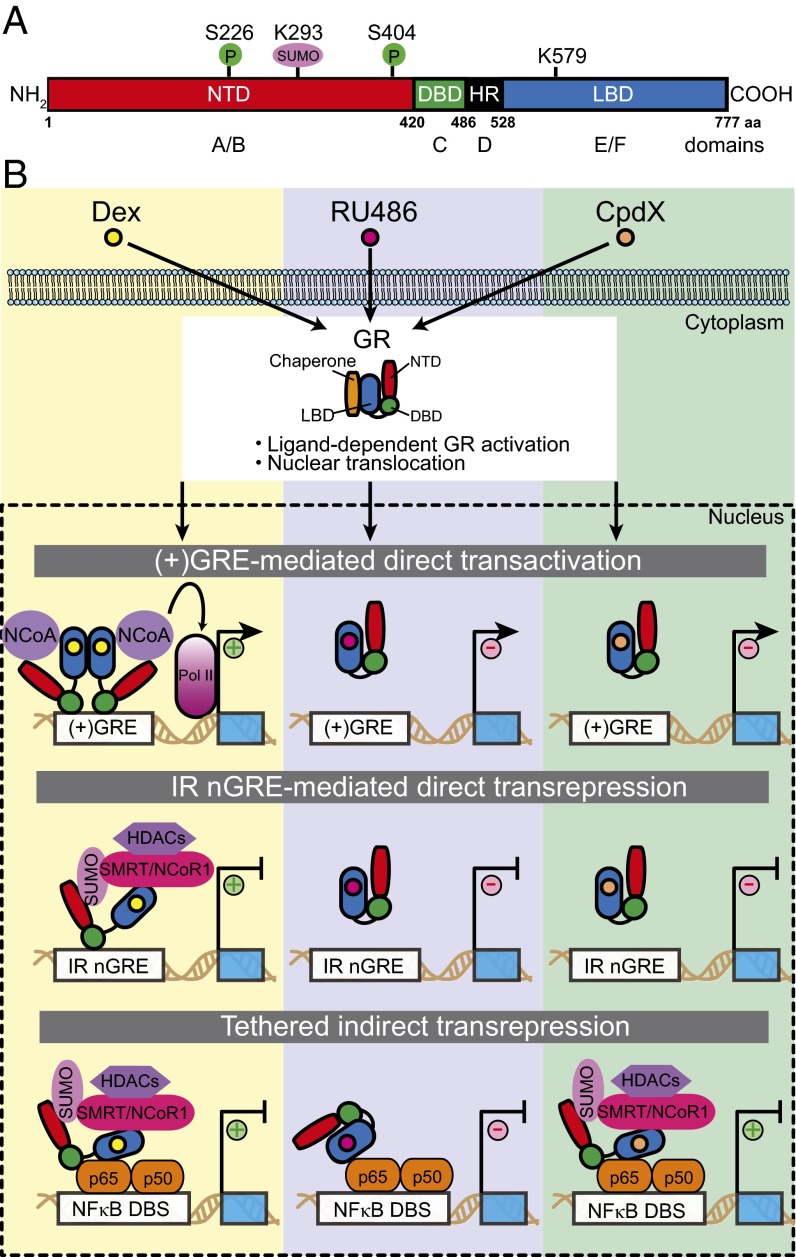
Schematic illustration (A) of the structural domains within the human GR, indicating relevant phosphorylation (P), and SUMOylation sites, and (B) the three molecular mechanisms by which GR regulates expression of its target genes upon binding an agonist (e.g., dexamethasone, Dex), an antagonist (e.g., RU486), or a SEGRA (e.g., CpdX). “+” indicates stimulation of gene activation or repression, whereas “−” indicates no effect on stimulation or repression. DBD, DNA-binding domain; HR, hinge region; LBD, ligand-binding domain; NTD, N-terminal domain.
In the absence of GC hormone, cytoplasmic GR is rendered inactive by bound chaperones (e.g., Hsp90). A conformational change in the GR LBD that accompanies GC binding causes GR activation and nuclear translocation (9, 10). Once in the nucleus, GR binds to the so-called positive GC response element [i.e., (+)GRE DNA-binding sequence (DBS)]. (+)GRE DBSs lie in the regulatory regions of target genes, and stimulate their expression via GR-dependent recruitment of a transcription initiation complex (Fig. 1B). While the molecular mechanism involved in GC-induced, GR-stimulated gene activity, has been extensively studied (11), agonist-activated GR also has gene-repressive activities conferred by two quite distinct mechanisms (Fig. 1B). The first of these, termed “tethered indirect transrepression,” arises when ligand-activated GR associates with transcription factors [NFκB (p65), AP1 (c-jun), or STAT3] bound to their cognate DBS (12). The second, more recently described repression mechanism, involves a direct binding of ligand-activated GR to an evolutionarily conserved negative GRE [inverted repeat (IR) nGRE DBS]; the result is GC-induced direct transrepression (13–15). The IR nGRE is unrelated to the (+)GRE DBSs described earlier, or a variety of nGREs (13). Until now, the molecular mechanisms governing IR nGRE-mediated direct and tethered indirect transrepression were poorly understood.
In their articles, Hua et al. now decipher these mechanisms (7, 8) (Fig. 1B). The authors demonstrate in vitro and in vivo that SUMOylation of the human and mouse GR [at lysine (K) 293 of human GR] within a conserved region of its NTD, is indispensable for either form of transrepression. For IR nGRE-mediated direct repression, SUMOylation of the GR is mandatory for the assembly of a repressive complex at the IR nGRE DBS; this complex comprises either one, or both, of the corepressors SMRT (silencing mediator for retinoid or thyroid-hormone receptors) and NCoR1 (nulear receptor corepressor 1), as well as histone deacetylase HDAC3. Furthermore, phosphorylation at two serine residues (i.e., S226 and S404 in the human GR NTD) could boost SUMOylation. Depending on the identity of the IR nGRE-containing genes, either SMRT or NCoR1 was required for GR binding to the IR nGREs, and direct transrepression. The authors also found that lysine K579, within the human GR LBD, was necessary for NCoR1 binding to SUMOylated GR and IR nGRE-mediated repression; no such dependency was found for SMRT binding and repression. However, whether SMRT may also directly interact with the GR LBD remains to be determined. Importantly, the repressive complexes of SUMOylated GR and SMRT/NCoR1 corepressors associated with HDAC3 neither bound to a (+)GRE nor inhibited (+)GRE-mediated transactivation. This indicates that formation of the repressive complexes was specific to IR nGREs. Although it is known that the GR DBD can interact in vitro with both the (+)GRE and IR nGRE DBS, it binds much more strongly to the former (16, 17). Seemingly, SUMOylation of the GR, as well as the recruitment and assembly of a SMRT/NCoR1-HDAC3 repressing complex, is required for its efficient binding to IR nGREs. This mode of regulation is in keeping with the observation that only a small fraction of cellular GR is SUMOylated, and would also indicate that there is no absolute need to maintain a dedicated nuclear pool of SUMOylated GR.
Hua et al. (8) also reveal in vitro and in vivo a similar dependence on a SUMOylated GR NTD for corepressor recruitment (SMRT/NCoR1) in tethered indirect transrepression, which is relevant to the GC-induced anti-inflammatory effects. However, SUMOylation was dispensable for the binding of GR to DNA-bound transcription factors (NF-κB/AP-1/STAT3); instead, the GR LBD was needed to interact with NF-κB or AP1, as proven by the loss of DNA-bound p65, or c-Jun, to a LBD-deleted GR. Furthermore, in vivo data provide some clues as to the chronology of events. The recruitment of the SMRT/NCoR1 repressor complexes appeared to precede HDAC3’s binding to the GR-repressive complex bound to NF-κB and AP1. Interestingly, as for IR nGRE-mediated direct transrepression, phosphorylation of the GR NTD dialed-up SUMOylation of NF-κB/AP1-bound GR and increased binding of the SMRT and NCoR1 corepressors.
Despite their side effects, the potent anti-inflammatory effects of GCs have led to their widespread use in treating inflammatory and allergic disorders. Clearly any property of GCs that could discriminate between harmful and beneficial effects would be a substantial step forward in understanding the GR functions. That step came when it was found that many of the clinically debilitating effects caused by prolonged GC therapy were related to its transcriptional activities and specifically (+)GRE-mediated direct transactivation (15) and IR nGRE-mediated direct repression (13). On the other hand, the process of GR-mediated tethered indirect transrepression segregated with therapeutically beneficial, anti-inflammatory effects (15). Not surprisingly, this distinction led to the search for “dissociated” ligands (SEGRAs, SElective GR Agonists): agonists that would fit the indirect transrepressive profile but avoid any direct effects. Thus far, the search for these elusive compounds has been unsuccessful; compounds lacking (+)GRE-mediated transactivation have been identified, but their IR nGRE-mediated direct transrepression activity remains largely intact (13). Until now, the search for the beneficial SEGRA has suffered from our lack of understanding about the relevant molecular mechanisms. In this respect, the findings now reported by Hua et al. (7, 8) should pave the way to educated designs and screens for such compounds; in short, they tell us what to look for. Better still, encouraging data to support the notion that these compounds exist come from the preliminary characterization of a putative SEGRA, CpdX, which selectively induces GR-mediated NF-κB/AP1 indirect transrepression activity yet maintains the anti-inflammatory properties (8).
There are still intriguing questions to be solved regarding the molecular mechanisms involving GR-induced transrepression. For example, can SMRT directly interact with the GR LBD like NCoR1? Is the retention of an active repressive complex that contains SMRT (but not NCoR1) by a GR construct lacking a LBD, indicative of SMRT recruitment to other regions of the GR, such as the NTD or DBD? Future in vivo studies are required to establish the most beneficial pharmacokinetic profile of CpdX (e.g., duration and dosage of treatment), and determine whether its administration could interfere with the regulatory activities mediated by endogenous GC. Last but not least, it is also important to find out whether administration of CpdX at the time point of lowest diurnal levels of endogenous GC (i.e., during the resting phase of the circadian cycle) could be beneficial.
The isolation of the GR gene and that of other NR family members, followed by their functional characterization, revolutionized our understanding of the signaling pathways triggered by small lipophilic molecules. These data have led to some largely unanticipated discoveries as to how these molecules, and their associated regulatory apparatuses, have evolved to become such master regulators of our physiology. The demonstration of distinct direct and indirect transcriptional repression mechanisms for GR function illustrates this point. Given the latest discoveries, and those made since the initial cloning of the NRs, we anticipate a further unraveling of the GR’s functional complexity that could help us to discover agents with clinically useful anti-inflammatory functions, which are free of the drawbacks that we currently associate with GCs.
Footnotes
References
- 1.Mangelsdorf DJ, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenberg SM, et al. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:e001. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galliher-Beckley AJ, Cidlowski JA. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life. 2009;61(10):979–986. doi: 10.1002/iub.245. [DOI] [PubMed] [Google Scholar]
- 6.Treuter E, Venteclef N. Transcriptional control of metabolic and inflammatory pathways by nuclear receptor SUMOylation. Biochim Biophys Acta. 2011;1812(8):909–918. doi: 10.1016/j.bbadis.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Hua G, Paulen L, Chambon P. GR SUMOylation and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex is mandatory for GC-induced IR nGRE-mediated transrepression. Proc Natl Acad Sci USA. 2016;113:E626–E634. doi: 10.1073/pnas.1522821113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua G, Ganti KP, Chambon P. Glucocorticoid-induced tethered transrepression requires SUMOylation of GR and formation of a SUMO-SMRT/NCoR1-HDAC3 repressing complex. Proc Natl Acad Sci USA. 2016;113:E635–E643. doi: 10.1073/pnas.1522826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricketson D, Hostick U, Fang L, Yamamoto KR, Darimont BD. A conformational switch in the ligand-binding domain regulates the dependence of the glucocorticoid receptor on Hsp90. J Mol Biol. 2007;368(3):729–741. doi: 10.1016/j.jmb.2007.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandevyver S, Dejager L, Libert C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic. 2012;13(3):364–374. doi: 10.1111/j.1600-0854.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 11.Meijsing SH, et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324(5925):407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlenhaut NH, et al. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol Cell. 2013;49(1):158–171. doi: 10.1016/j.molcel.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surjit M, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145(2):224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Topete D, Cidlowski JA. One hormone, two actions: Anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1-2):20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134(1):54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Luisi BF, et al. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- 17.Hudson WH, Youn C, Ortlund EA. The structural basis of direct glucocorticoid-mediated transrepression. Nat Struct Mol Biol. 2013;20(1):53–58. doi: 10.1038/nsmb.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]