Significance
We have identified the transmembrane receptor, LDL receptor-related protein-1 (LRP1), as a regulator of innate immunity and inflammatory responses in macrophages. LRP1 gene deletion in myeloid cells is proinflammatory in mice, as is LRP1 gene deletion in cultured macrophages. In LRP1-expressing macrophages, different LRP1 ligands have opposing effects on LRP1 activity, promoting or attenuating inflammatory mediator expression. NFκB is rapidly regulated by LRP1 ligands and responsible for the initial effects of LRP1 on macrophage gene expression. Regulation of microRNA-155 (miR-155) expression is a key downstream event, which may sustain or inhibit the macrophage inflammatory response. LRP1, NFκB, and miR-155 are thus members of a previously unidentified system, with the potential to control inflammation in a macrophage microenvironment-dependent manner.
Keywords: LDL receptor-related protein-1, tissue-type plasminogen activator, lipopolysaccharide, NFκB, microRNA-155
Abstract
LDL receptor-related protein-1 (LRP1) is an endocytic and cell-signaling receptor. In mice in which LRP1 is deleted in myeloid cells, the response to lipopolysaccharide (LPS) was greatly exacerbated. LRP1 deletion in macrophages in vitro, under the control of tamoxifen-activated Cre-ERT fusion protein, robustly increased expression of proinflammatory cytokines and chemokines. In LRP1-expressing macrophages, proinflammatory mediator expression was regulated by LRP1 ligands in a ligand-specific manner. The LRP1 agonists, α2-macroglobulin and tissue-type plasminogen activator, attenuated expression of inflammatory mediators, even in the presence of LPS. The antagonists, receptor-associated protein (RAP) and lactoferrin (LF), and LRP1-specific antibody had the entirely opposite effect, promoting inflammatory mediator expression and mimicking LRP1 deletion. NFκB was rapidly activated in response to RAP and LF and responsible for the initial increase in expression of proinflammatory mediators. RAP and LF also significantly increased expression of microRNA-155 (miR-155) after a lag phase of about 4 h. miR-155 expression reflected, at least in part, activation of secondary cell-signaling pathways downstream of TNFα. Although miR-155 was not involved in the initial induction of cytokine expression in response to LRP1 antagonists, miR-155 was essential for sustaining the proinflammatory response. We conclude that LRP1, NFκB, and miR-155 function as members of a previously unidentified system that has the potential to inhibit or sustain inflammation, depending on the continuum of LRP1 ligands present in the macrophage microenvironment.
Innate immunity is a phylogenetically conserved system that provides a first line of defense against pathogens (1, 2). Pattern recognition receptors (PRRs), including members of the Toll-like receptor (TLR) family, play an important role in innate immunity, binding microorganism-derived molecules, and initiating proinflammatory cell signaling (1, 3, 4). The effector systems of innate immunity, including proinflammatory cytokines and complement, may be very potent and when regulatory mechanisms fail, shock and death may result (3). Diverse diseases are exacerbated by dysregulated innate immunity, including Crohn’s disease, rheumatoid arthritis, asthma, psoriasis, atherosclerosis, and cancer (3, 5, 6). Understanding mechanisms that control innate immunity is a significant problem in medicine.
LDL receptor-related protein-1 (LRP1) is an endocytic and cell-signaling receptor, which is essential for embryonic development (7, 8). In adults, there is increasing evidence that LRP1 regulates inflammation (9). LRP1-deficient macrophages, isolated from mice in which LRP1 is conditionally deleted in myeloid cells (mLRP1−/− mice), express increased levels of proinflammatory chemokines, including monocyte chemotactic protein/CCL2, MIP-1α/CCL3, and MIP-1β/CCL4 (10–12). These macrophages also migrate more rapidly, due to activation of CCL3-CCR5 signaling (10) and express decreased levels of biomarkers associated with M2 polarization (13).
In syngeneic tumors in mLRP1−/− mice, LRP1-deficient macrophages accumulate in increased number and express increased levels of CCL3 (10). Macrophage infiltration is also increased in atherosclerotic lesions in mLRP1−/− mice (11). However, mechanisms by which LRP1 regulates macrophage physiology remain incompletely understood. LRP1 deficiency is associated with increased NFκB activity in passaged cell lines (12); however, “loss of function” model systems do not address the role of LRP1 as a receptor for diverse ligands (7, 14). In neurons and neuron-like cells, different LRP1 ligands elicit distinct cell-signaling responses by engaging separate LRP1 coreceptors (15–17). If LRP1 regulates macrophage physiology in a ligand-specific manner, this would represent a powerful mechanism by which macrophages may respond to changes in their microenvironment.
In this study, we challenged mLRP1−/− mice and control mLRP1+/+ mice with lipopolysaccharide (LPS), which is a major ligand for the PRR, TLR4 (18). The response to LPS was greatly exacerbated in mLRP1−/− mice. Using a second genetic model system, we confirmed that LRP1 gene deletion in macrophages increases expression of proinflammatory mediators. We then showed that expression of proinflammatory mediators is controlled in macrophages by LRP1 ligands in a ligand-specific manner. LRP1 agonists, such as α2-macroglobulin (α2M) and tissue-type plasminogen activator (tPA), suppressed expression of proinflammatory mediators, even in the presence of LPS. Antagonists, such as receptor-associated protein (RAP) and lactoferrin (LF), increased expression of the identical mediators, mimicking the effects of LRP1 gene deletion. The activity of LRP1 ligands was linked to regulation of NFκB and the previously unreported ability of LRP1 to control expression of microRNA-155 (miR-155) (19). LRP1, NFκB, and miR-155 emerge as members of a novel system that may control the macrophage inflammatory response in a microenvironment-sensitive manner.
Results
The Response to LPS Is Exacerbated in mLRP1−/− Mice.
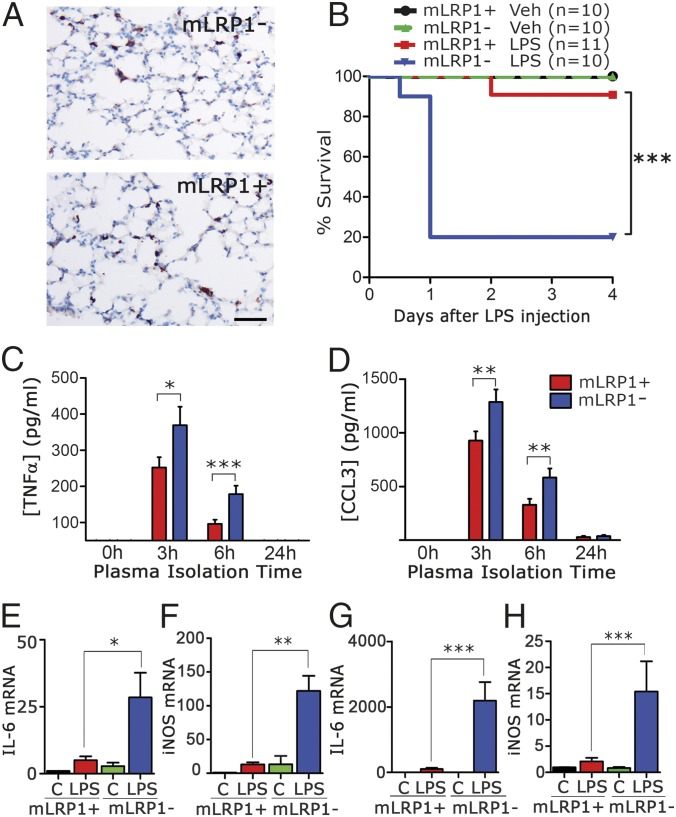
In mLRP1−/− mice, LRP1 is deleted in cells in which the lysozyme-M (LysM) promoter is active, including monocytes, macrophages, neutrophils, and to some extent, dendritic cells (20). mLRP1+/+ mice are homozygous for the floxed LRP1 gene but LysM-Cre-negative (21). Although LRP1 has been identified in neutrophils (22), its function in these cells is not well studied. By contrast, LRP1 is a known regulator of monocytes and macrophages (9). In the absence of experimental immune system challenge, we did not identify abnormal inflammation in 12-wk-old mLRP1−/− mice. TNFα and CCL3 were nearly undetectable by ELISA in plasma from mLRP1−/− mice. In the lungs of mLRP1−/− mice, myeloid cells were restricted mainly to interstitial spaces and unchanged in abundance compared with mLRP1+/+ mice, as determined by CD11b immunohistochemistry (IHC) (Fig. 1A) and image analysis.
Fig. 1.
LPS toxicity is increased in mice in which LRP1 is deleted in myeloid cells. (A) IHC was performed to detect CD11b-positive myeloid cells in sections of lung from untreated mLRP1+/+ and mLRP1−/− mice. (Scale bar, 100 µm.) Image analysis did not reveal a difference in the density of CD11b-positive cells (n = 3). (B) Kaplan–Meier survival curves are shown for mLRP1−/− and mLRP1+/+ mice treated by i.p. injection with 1 mg/kg LPS or vehicle (Veh). Significance was determined by log-rank test (***P < 0.001). (C and D) ELISAs were performed to detect TNFα and CCL3 in plasma samples harvested at the indicated times from LPS-treated mLRP1−/− (blue bar) and mLRP1+/+ (red bar) mice (mean ± SEM; n = 10; *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA with Tukey’s post hoc analysis). (E–H) RNA was harvested from the lungs (E and F) and kidneys (G and H) of mLRP1+/+ and mLRP1−/− mice 24 h after i.p. injection of LPS or vehicle (C). IL-6 and iNOS mRNA were determined by RT-qPCR (n = 4).
mLRP1−/− and mLRP1+/+ mice were injected i.p. with LPS (n = 10–11) or vehicle. The LPS dose was set at 50% of the LD50 to optimize our opportunity to detect increased sensitivity. No toxicity or mortality was observed in vehicle-treated controls, as anticipated (Fig. 1B). One mLRP1+/+ mouse succumbed to LPS. By contrast, 80% of the mLRP1−/− mice succumbed within 24 h of LPS injection. The increase in mortality of LPS-treated mLRP1−/− mice, compared with mLRP1+/+ mice, was statistically significant as determined by log-rank test (P < 0.001).
Plasma was sampled 0, 3, 6, and 24 h after LPS injection. TNFα protein was increased in plasma from mLRP1−/− mice, compared with mLRP1+/+ mice, at 3 and 6 h (Fig. 1C). CCL3 was also increased at 3 and 6 h (Fig. 1D). Quantitative reverse transcription–PCR (RT-qPCR) was performed to assess expression of proinflammatory genes in tissues harvested 24 h after injecting LPS or vehicle. IL-6 and inducible nitric oxide synthase (iNOS) mRNA were significantly increased in the lungs (Fig. 1 E and F) and kidneys (Fig. 1 G and H) of LPS-treated mLRP1−/− mice, compared with mLRP1+/+ mice. In vehicle-treated mLRP1−/− and mLRP1+/+ mice, IL-6 and iNOS mRNA were not significantly different, again indicating that significant inflammation is not present under basal conditions in mLRP1−/− mice.
Analysis of a Second Model of LRP1 Gene Deletion in Macrophages.
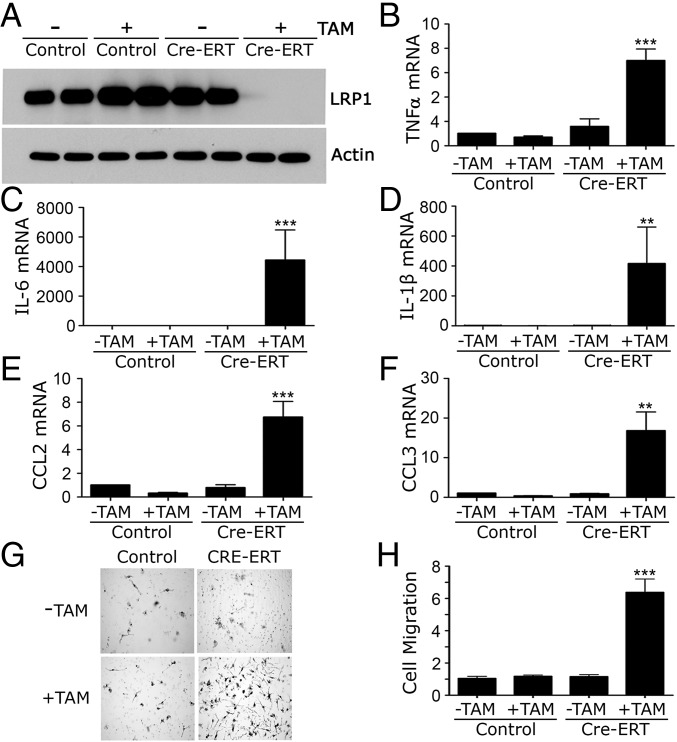
In mLRP1−/− mice, LRP1 is deleted during development (10, 11), allowing for compensatory changes in the physiology of these cells. To address this issue, we generated homozygous LRP1flox/flox mice that express tamoxifen (TAM)-activated Cre-fusion protein (Cre-ERT) (23). Bone-marrow–derived macrophages (BMDMs) were isolated and LRP1 protein expression was studied. Fig. 2A shows that, in the absence of TAM, Cre-ERT-positive and -negative BMDMs expressed similar levels of LRP1. After treatment with 5 µM TAM for 7 d, LRP1 protein expression was neutralized in Cre-ERT-positive cells and unchanged in Cre-ERT-negative cells.
Fig. 2.
LRP1 gene deletion in macrophages in vitro induces a proinflammatory phenotype. BMDMs from Cre-ERT-negative (control) and Cre-ERT-positive (Cre-ERT) LRP1flox/flox mice were treated with 5 µM TAM (+) or vehicle (−) for 7 d. (A) Cell extracts were subjected to immunoblot analysis to detect the LRP1 β-chain and β-actin as a control for load. (B–F) RNA was isolated and RT-qPCR was performed to quantitate mRNA for (B) TNFα, (C) IL-6, (D) IL-1β, (E) CCL2, and (F) CCL3. (G) Cell migration was studied using Transwell systems. Representative images of cells that migrated to the underside surfaces of the membranes are shown. (H) Quantification of cell migration results (mean ± SEM; n = 6; **P < 0.01, ***P < 0.001; one-way ANOVA with a Tukey’s post hoc analysis).
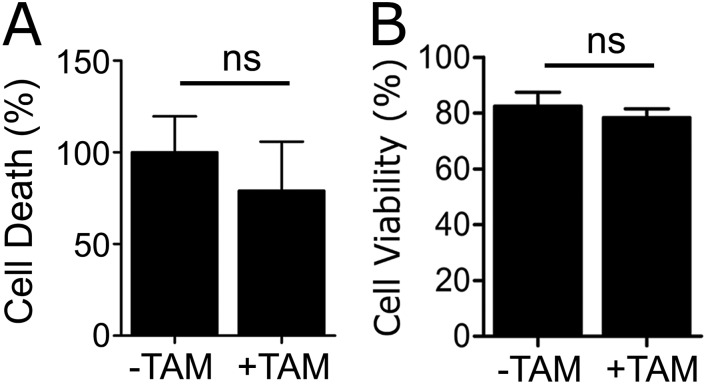
When LRP1 was deleted in Cre-ERT-positive BMDMs, expression of TNFα, IL-6, IL-1β, CCL2, and CCL3 was increased (Fig. 2 B–F). TAM did not affect cytokine expression in Cre-ERT-negative cells. LRP1 gene deletion also stimulated cell migration (P < 0.001) (Fig. 2 G and H), mimicking the results obtained with BMDMs from mLRP1−/− mice (10). TAM-induced LRP1 gene deletion did not increase apoptosis, determined 8 h after transferring cells to serum-free medium (SFM), or alter the number of viable cells, determined by trypan blue exclusion at 24 h (Fig. S1).
Fig. S1.
LRP1 gene deletion does not compromise cell viability. BMDMs were isolated from Cre-ERT-positive-LRP1flox/flox mice and treated with 5 µM TAM (+TAM) or vehicle (−TAM). (A) Apoptosis was measured using the Cell Death ELISA. (B) Cell viability was determined by trypan blue exclusion (mean ± SEM; n = 4; ns, not statistically significant; Mann–Whitney test).
LRP1 Ligands Regulate Macrophage Physiology in a Ligand-Specific Manner.
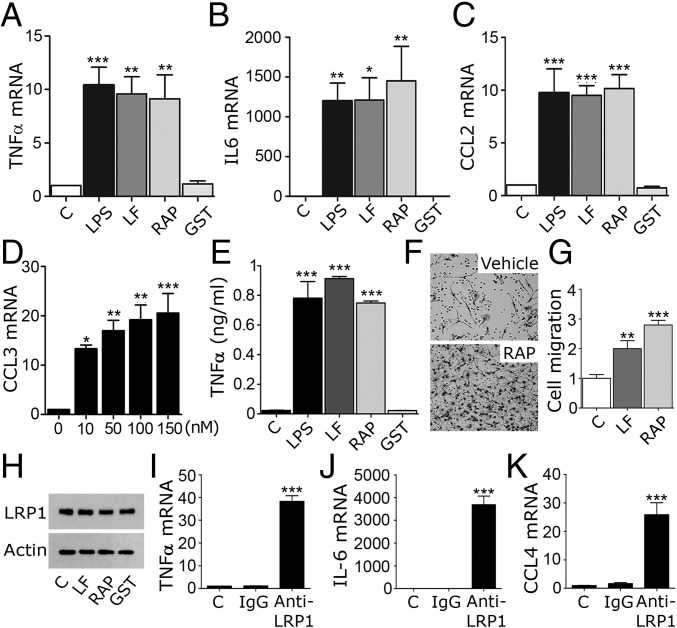
In neurons and neuron-like cells, α2M* and tPA function as LRP1 agonists, activating cell-signaling factors such as c-Src, ERK1/2, and Akt (15–17). LF and RAP function as antagonists, blocking cell signaling in response to agonists (16). We treated BMDMs from wild-type C57BL/6J mice with RAP (150 nM), LF (150 nM), or LPS (0.1 µg/mL) for 8 h. Fig. 3 A–C shows that RAP and LF increased expression of TNFα, IL-6, and CCL2 mRNA, similarly to LPS. RAP and LF also increased expression of IL-1β and CCL4 (Fig. S2 A and B). Fig. 3D shows that RAP increased CCL3 mRNA in a dose-dependent manner; 10 nM RAP was sufficient to induce a significant response. RAP and LF also increased TNFα protein levels in BMDM-conditioned medium, similarly to LPS (Fig. 3E).
Fig. 3.
LF and RAP induce expression of proinflammatory cytokines in BMDMs. (A–C) BMDMs from C57BL/6J mice were treated with LPS (0.1 µg/mL), LF (150 nM), RAP expressed as a GST fusion protein (150 nM), purified GST (150 nM), or vehicle (C) for 8 h. RT-qPCR was performed to determine mRNA levels for TNFα, IL-6, and CCL2. (D) CCL3 mRNA was determined in BMDMs treated for 8 h with increasing concentrations of RAP. (E) BMDMs were stimulated for 8 h with LPS, LF, RAP, GST, or vehicle as in A. TNFα in conditioned medium was determined by ELISA. (F) BMDMs were treated with LF, RAP, or vehicle (C). Cell migration was studied using Transwell systems. Representative images of migrated cells are shown. (G) Quantification of cell migration results (n = 6). (H) BMDMs were treated with LF, RAP, GST (each at 150 nM), or vehicle (C) for 8 h. Cell extracts were subjected to immunoblot analysis to detect the LRP1 β-chain and β-actin. (I–K) BMDMs were treated with LRP1-neutralizing antibody (anti-LRP1) or isotype-matched IgG for 8 h. mRNA levels were determined for TNFα, IL-6, and CCL4 (mean ± SEM; n ≥ 6; *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA followed by Dunnett’s multiple comparison test).
Fig. S2.
LF and RAP increase expression of proinflammatory mediators. BMDMs from C57BL/6J mice were treated with LPS (0.1 µg/mL), LF (150 nM), RAP, which was expressed as a GST-fusion protein (150 nM), purified GST (150 nM), or vehicle (C) for 8 h. RT-qPCR was performed to quantify mRNA levels for (A) IL-1β and (B) CCL4 (mean ± SEM; n = 6; **P < 0.01, ***P < 0.001; one-way ANOVA followed by Dunnett’s post hoc analysis).
RAP and LF stimulated BMDM cell migration (Fig. 3 F and G), mimicking the effects of LRP1 gene deletion (10). Although inflammatory mediators are known to decrease LRP1 protein levels in macrophages (24, 25), LRP1 protein was unchanged in BMDMs treated with LF or RAP for 8 h (Fig. 3H). Modest changes in LRP1 mRNA were observed in cells treated for 8 h with LF (44 ± 6%) or RAP (52 ± 5%), suggesting that longer incubations may have decreased the LRP1 protein level.
We hypothesized that RAP and LF mimic LRP1 gene deletion by blocking LRP1 signaling initiated by agonists produced endogenously by BMDMs. LRP1 ligands are numerous and diverse (7, 9, 14). To test our hypothesis, we treated BMDMs with an antibody that binds to the LRP1 heavy chain (anti-LRP1) and blocks ligand binding. Fig. 3 I–K shows that anti-LRP1 induced expression of TNFα, IL-6, and CCL4, similarly to RAP and LF. In control experiments, isotype-matched IgG was without effect.
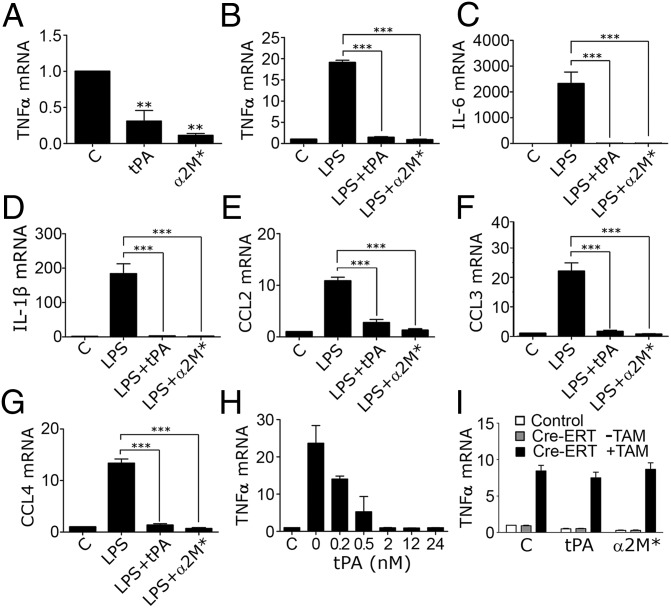
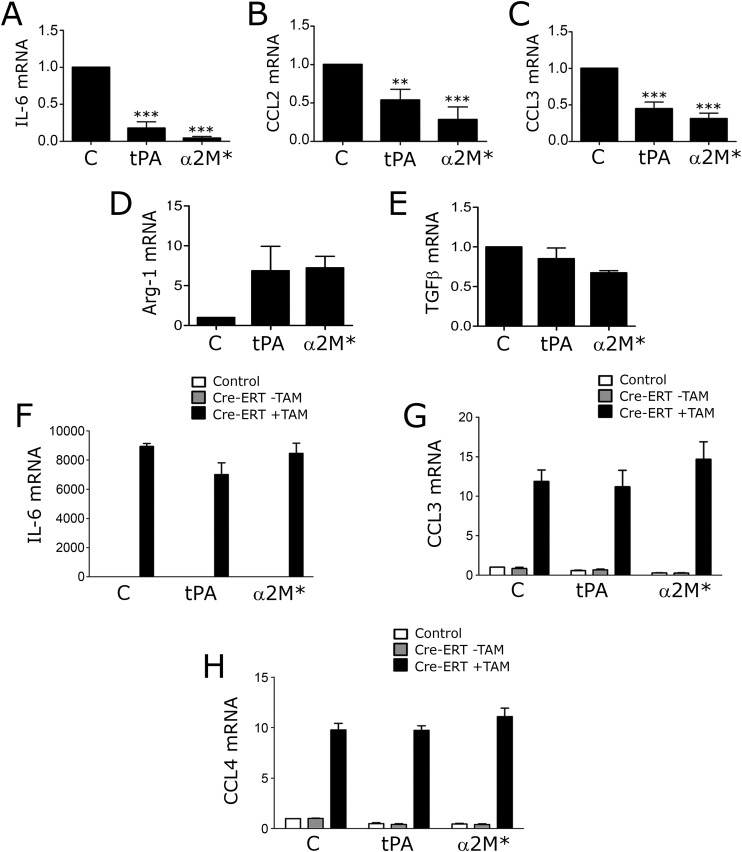
Next, we studied the LRP1 agonists: α2M* (10 nM) and tPA (12 nM). We used an enzymatically inactive tPA variant (EI-tPA) to avoid effects unrelated to receptor-binding (26). Fig. 4A shows that, in wild-type BMDMs, EI-tPA and α2M* significantly decreased TNFα expression. EI-tPA and α2M* also decreased expression of IL-6, CCL2, and CCL3 (Fig. S3 A–C). Arginase-1 mRNA was increased; TGFβ mRNA was not significantly changed (Fig. S3 D and E). Because the basal level of expression of proinflammatory cytokines in BMDMs was low, we tested whether α2M* and EI-tPA attenuate the response to LPS (0.1 µg/mL). Fig. 4 B–G shows that α2M* and EI-tPA blocked the increase in expression of TNFα, IL-1β, IL-6, CCL2, CCL3, and CCL4 induced by LPS. The activity of EI-tPA was dose dependent; the minimum EI-tPA concentration that had a significant effect (P < 0.05) was 0.5 nM (Fig. 4H).
Fig. 4.
α2M* and EI-tPA inhibit expression of inflammatory mediators by BMDMs. (A) BMDMs from C57BL/6J mice were treated for 8 h with El-tPA (12 nM), α2M* (10 nM), or vehicle (C). TNFα mRNA was determined by RT-qPCR. (B–G) BMDMs were pretreated with LPS (0.1 µg/mL) for 30 min and then with El-tPA (12 nM), α2M* (10 nM), or vehicle (C) for 8 h. mRNA levels were determined for TNFα, IL-6, IL-1β, CCL2, CCL3, and CCL4. (H) BMDMs were pretreated with LPS and then with 0.2–24 nM EI-tPA for 8 h. TNFα mRNA was determined. EI-tPA concentrations of ≥0.5 nM yielded significant results (P < 0.05). (I) BMDMs from Cre-ERT-negative (control) and Cre-ERT-positive (Cre-ERT) LRP1flox/flox mice were treated with TAM (+TAM) or vehicle (−TAM) for 7 d. The Cre-ERT-negative cells and Cre-ERT-positive cells that were not treated with TAM both expressed LRP1. The cells were then treated with EI-tPA, α2M*, or vehicle (C) for 8 h. TNFα mRNA was determined (mean ± SEM; n = 4; **P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett's or Tukey’s post hoc analysis).
Fig. S3.
α2M* and EI-tPA express antiinflammatory activity by an LRP1-dependent mechanism. (A–E) BMDMs were isolated from wild-type C57BL/6J mice and treated for 8 h with El-tPA (12 nM), α2M* (10 nM), or vehicle (C). RTqPCR was performed to determine mRNA levels for IL-6, CCL2, CCL3, Arg-1, and TGFβ (mean ± SEM; n = 4; **P < 0.01, ***P < 0.001; one-way ANOVA with Dunnett’s post hoc test). (F–H) LRP1 was deleted in BMDMs by treating cells isolated from Cre-ERT-positive-LRP1flox/flox mice with 5 µM TAM (+TAM) for 7 d (solid bars). LRP1-expressing BMDMs included cells isolated from Cre-ERT-negative-LRP1flox/flox mice (open bars) and cells isolated from Cre-ERT-positive-LRP1flox/flox mice and treated with vehicle (−TAM, shaded bars). The cells were treated with EI-tPA, α2M*, or vehicle (C) for 8 h. mRNA levels for IL-6, CCL3, and CCL4 were determined by RT-qPCR (mean ± SEM; n = 4).
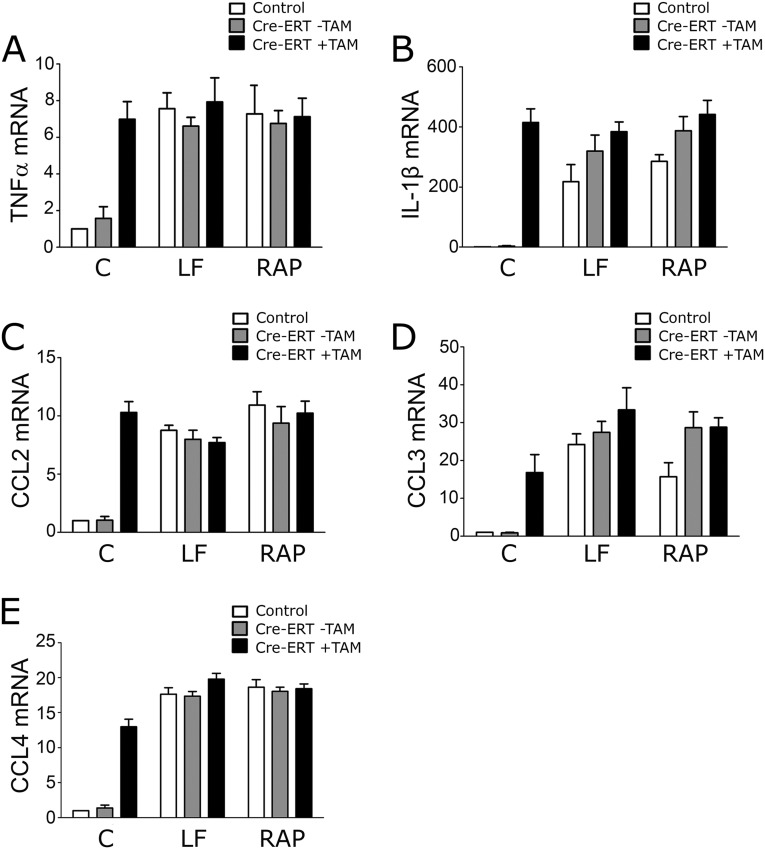
To prove that the activity of EI-tPA and α2M* requires LRP1, we used the Cre-ERT-LRP1flox/flox model system. Confirming the mechanism of EI-tPA activity was considered particularly important because tPA is an FDA-approved drug (27), known to bind to receptors in addition to LRP1 (28). TAM-treated Cre-ERT-positive BMDMs expressed increased levels of mRNA for TNFα (Fig. 4I), IL-6, CCL3, and CCL4 (Fig. S3 F–H) as anticipated; however, when LRP1 was deleted, EI-tPA and α2M* failed to attenuate cytokine expression, indicating an essential role for LRP1. Similarly, in TAM-treated Cre-ERT-LRP1flox/flox BMDMs, RAP and LF failed to further stimulate cytokine expression (Fig. S4), supporting the conclusion that LRP1 is the target for LF and RAP.
Fig. S4.
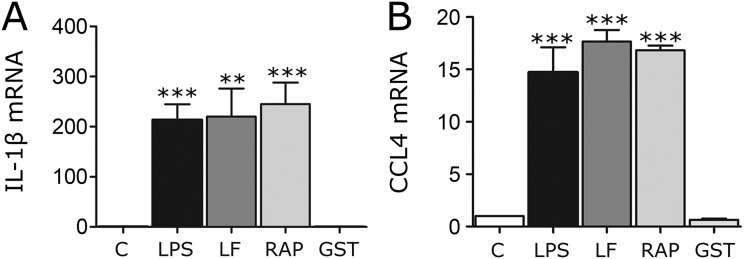
RAP and LF do not further stimulate expression of proinflammatory mediators when LRP1 is deleted. LRP1 was deleted in BMDMs by treating cells isolated from Cre-ERT-positive-LRP1flox/flox mice with 5 µM TAM (+TAM) for 7 d (solid bars). LRP1-expressing BMDMs included cells isolated from Cre-ERT-negative-LRP1flox/flox mice (open bars) and cells isolated from Cre-ERT-positive-LRP1flox/flox mice and treated with vehicle (−TAM, shaded bars). The cells were treated with LF (150 nM), RAP (150 nM), or vehicle (C). RT-qPCR was performed to determine expression of: (A) TNFα; (B) IL-1β; (C) CCL2; (D) CCL3; and (E) CCL4 (mean ± SEM; n = 4).
NFκB Functions in the Pathway by Which LRP1 Ligands Regulate Inflammation.
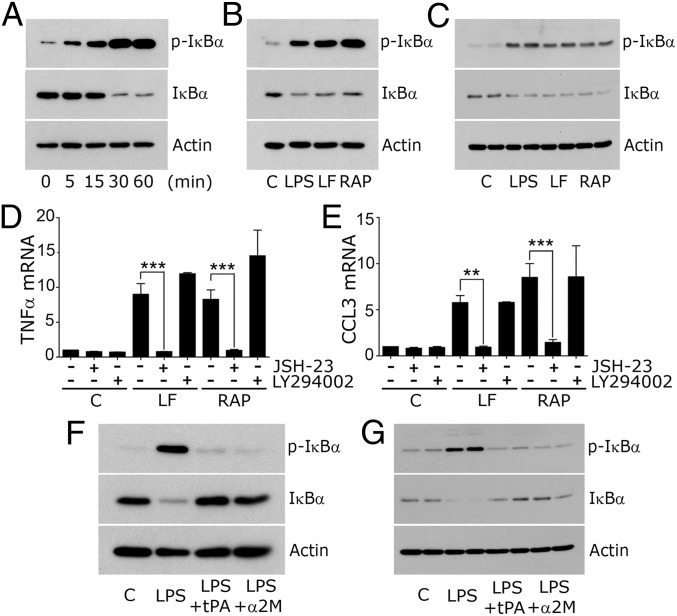
When wild-type BMDMs were treated with RAP, IκBα was phosphorylated within 5 min (Fig. 5A). Total IκBα decreased substantially by 30 min, confirming NFκB activation. LF also induced IκBα phosphorylation and decreased the total abundance of IκBα (Fig. 5B). The effects of RAP and LF on phospho-IκBα and total IκBα were sustained for 8 h (Fig. 5C).
Fig. 5.
LRP1 regulates NFκB activation. (A) BMDMs from C57BL/6J mice were treated with 150 nM RAP for the indicated times. (B) BMDMs were treated with vehicle (C), LPS (0.1 µg/mL), LF (150 nM), or RAP (150 nM) for 30 min. (C) The same incubations were conducted for 8 h. Immunoblot analysis was performed to detect phospho-IκB and total IκB. (D and E) BMDMs were pretreated with JSH-23 (10 μM), LY294002 (20 μM), or vehicle for 16 h and then with LF, RAP, or vehicle (C) for 8 h. mRNA levels were determined for TNFα and CCL3 (mean ± SEM; n = 6; **P < 0.01, ***P < 0.001; one-way ANOVA with Tukey’s post hoc test). (F) BMDMs were treated with El-tPA (12 nM) or α2M* (10 nM) together with LPS (0.1 µg/mL) or with LPS or vehicle (C) alone for 1 h. Immunoblot analysis was performed. (G) The same experiment was performed for 8 h.
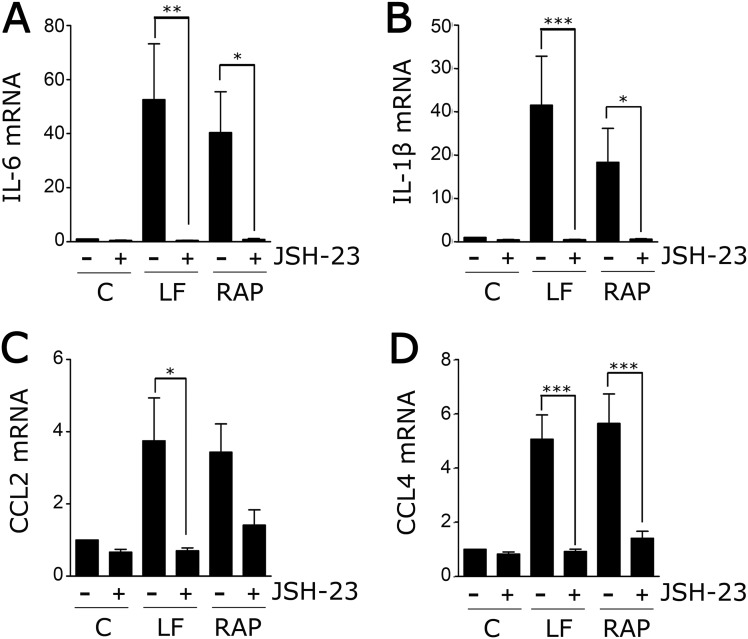
To test whether NFκB is necessary in the pathway by which LF and RAP increase cytokine expression in BMDMs, cells were treated with the NFκB nuclear translocation inhibitor, JSH-23. As a control, cells were treated with the PI3K inhibitor, LY294002. JSH-23 blocked the increase in expression of TNFα (Fig. 5D) and CCL3 (Fig. 5E) caused by LF and RAP. LY294002 was without effect. Fig. S5 shows that JSH-23 also blocked expression of IL-6, IL-1β, CCL2, and CCL4.
Fig. S5.
The effect of LRP1 antagonists on cytokine expression require NFкB. BMDMs were isolated from wild-type mice and pretreated for 16 h with JSH-23 (10 μM) (+) or vehicle (−) and then with LF (150 nM), RAP (150 nM), or vehicle (C) for 8 h. RNA was isolated and RT-qPCR was performed to determine mRNA levels for (A) IL-6; (B) IL-1β; (C) CCL2; and (D) CCL4 (mean ± SEM; n = 3; *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA with Tukey’s post hoc analysis).
Next, we examined the ability of LRP1 agonists to block NFκB activation in response to LPS. Fig. 5F shows that α2M* and EI-tPA blocked IκBα phosphorylation and the decrease in total IκB induced by LPS treatment for 1 h. The effects of α2M* and EI-tPA were sustained for 8 h (Fig. 5G).
LRP1 Regulates miR-155 Expression.
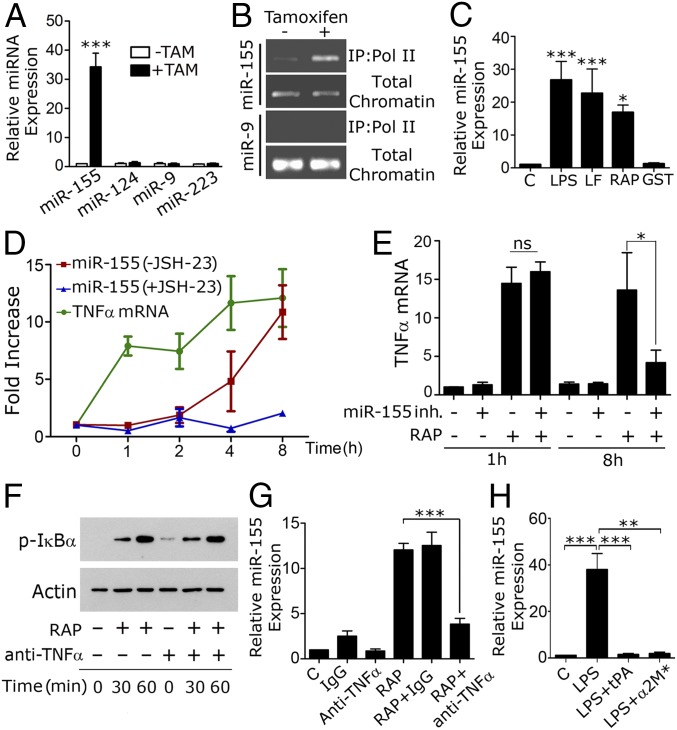
MicroRNAs are regulators of inflammation in various cells, including macrophages (19, 29–31). Fig. 6A shows that LRP1 gene deletion in Cre-ERT-LRP1flox/flox BMDMs significantly increased miR-155, without regulating miR-124, miR-9, and miR-223. In chromatin immunoprecipitation (ChiP) assays, association of the promoter region of the miR-155 parent gene with RNA polymerase II was increased when LRP1 was deleted (Fig. 6B), suggesting transcriptional activation. The miR-9 promoter was not precipitated at significant levels from LRP1-expressing or -deficient cells.
Fig. 6.
miR-155 sustains the proinflammatory activity of LRP1 antagonists. (A) BMDMs from Cre-ERT-positive-LRP1flox/flox mice were treated with TAM (+TAM) or vehicle (−TAM). miR-155, miR-124, miR-9, and miR-223 were determined by qPCR (mean ± SEM; n = 5; ***P < 0.001). (B) ChIP was performed to detect the miR-155 parent gene promoter in association with RNA polymerase II in BMDMs from Cre-ERT-positive-LRP1flox/flox mice treated with TAM (+) or vehicle (−). The miR-9 promoter was analyzed as a control. (C) BMDMs from C57BL/6J mice were treated for 8 h with LPS (0.1 µg/mL), LF (150 nM), RAP (150 nM), GST (150 nM), or vehicle (C). miR-155 was determined (n = 5; *P < 0.05, ***P < 0.001). (D) BMDMs were treated with 150 nM RAP for the indicated times. TNFα mRNA and miR-155 were determined. miR-155 also was determined in cells treated with RAP and 10 µM JSH-23 (+JSH-23). (E) BMDMs from C57BL/6J mice were transfected with 10 nM miR-155 inhibitor (Inh) (+) or with miRNA inhibitor negative control (−). After 48 h, the cells were treated with 150 nM RAP (+) or vehicle (−) for 1 h or 8 h. TNFα mRNA was determined (n = 4, ns, not statistically significant; *P < 0.05). (F) BMDMs were pretreated with TNFα-neutralizing antibody (+) or isotype-matched IgG (−) and then with RAP for 0, 30, or 60 min. Immunoblot analysis was performed. (G) BMDMs were treated with RAP (150 nM) or vehicle (C) alone or in the presence of TNFα-neutralizing antibody (1 µg/mL) or isotype-matched IgG for 8 h. miR-155 was determined. (H) BMDMs were treated with LPS (0.1 µg/mL) alone or together with El-tPA (12 nM) or α2M* (10 nM) for 8 h. miR-155 was determined (n = 5; **P < 0.01, ***P < 0.001).
Treatment of LRP1-expressing BMDMs with LF or RAP for 8 h significantly increased miR-155 (Fig. 6C); however, as shown in Fig. 6D, there was a lag phase in this response. Whereas TNFα mRNA was elevated 1 h after adding RAP, miR-155 was not significantly increased until 4–8 h. The increase in miR-155 was inhibited by JSH-23, indicating an essential role for NFκB.
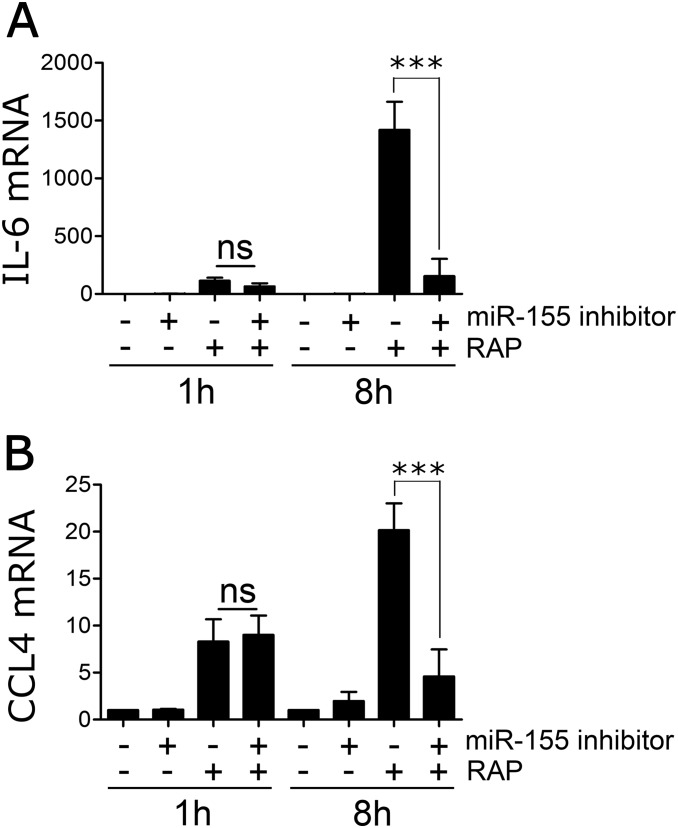
Because of the lag phase in miR-155 expression, we examined the effects of inhibiting miR-155 on cytokine expression, 1 and 8 h after adding RAP. BMDMs were transfected with 10 nM mirVANA RNA oligonucleotide miR-155 inhibitor, which decreased miR-155 by >70%, or with control oligonucleotide. Fig. 6E shows that inhibiting miR-155 had no effect on TNFα mRNA expression at 1 h. By contrast, TNFα expression was significantly decreased at 8 h. Similarly, miR-155 inhibition had no effect on expression CCL4 or IL-6 at 1 h, but substantially decreased expression of these mRNAs at 8 h (Fig. S6). These results suggest that miR-155 plays an important role in sustaining the proinflammatory response to LRP1 antagonists.
Fig. S6.
miR-155 sustains the antiinflammatory activity of LRP1 antagonists. BMDMs from C57BL/6J mice were transfected with miR-155 inhibitor (10 nM) (+) or with miRNA inhibitor negative control (−). After 48 h, the cells were treated with 150 nM RAP (+) or with vehicle (−) for 1 h or 8 h. mRNA levels for (A) IL-6 and (B) CCL4 were determined by RT-qPCR (mean ± SEM; n = 4; ***P < 0.001; ns, not statistically significant; one-way ANOVA followed by Tukey’s post hoc analysis).
We considered the possibility that miR-155 was induced as a secondary effect, downstream of cytokines such as TNFα that were expressed at increased levels by RAP-treated BMDMs. Fig. 6F shows that TNFα-neutralizing antibody did not affect the rapid phosphorylation of IκB in response to RAP; however, TNFα-neutralizing antibody substantially inhibited the increase in miR-155 observed 8 h after adding RAP (Fig. 6G).
Finally, we examined the effects of LRP1 agonists on miR-155. Fig. 6H shows that EI-tPA and α2M* blocked the increase in miR-155 induced by LPS. We conclude that LRP1 functions in a ligand-specific manner to regulate miR-155.
Discussion
Despite extensive work and the availability of multiple mouse model systems, the function of LRP1 in adult mammals remains incompletely understood (9, 32). We have demonstrated a role for macrophage LRP1 in regulating innate immunity in vivo using a model system, LPS challenge, which directly tests the response of the mouse to systemic activation of the PRR, TLR4. When LRP1 was deleted in myeloid cells, the toxicity of LPS was exacerbated and morbidity was significantly increased. Deletion of LRP1 in vitro in BMDMs isolated from Cre-ERT-LRP1flox/flox mice substantially increased expression of multiple proinflammatory mediators. The results obtained, using this second model of LRP1 deficiency, were consistent with those obtained using BMDMs isolated from mLRP1−/− mice (9–12). We conclude that LRP1 deficiency in myeloid cells in vivo and in macrophages in vitro is proinflammatory.
In LRP1-expressing macrophages, different LRP1 ligands had opposing effects on the activity of LRP1 as a regulator of the macrophage inflammatory response. RAP and LF induced rapid NFκB activation, increasing expression of the same proinflammatory mediators that were expressed by LRP1-deficient BMDMs. EI-tPA and α2M* had the entirely opposite effect on BMDM physiology, suppressing NFκB activation in response to LPS and decreasing expression of proinflammatory mediators. In previous studies with different cell types, RAP and LF have been shown to competitively antagonize LRP1-dependent cellular responses triggered by ligands like α2M* or tPA (15–17, 33–35). We hypothesized that, under basal conditions, macrophages express one or more agonistic ligands that bind to LRP1 in an autocrine pathway, suppressing NFκB activation. RAP and LF would then function by inhibiting binding of these endogenously produced agonists to LRP1. In support of this hypothesis, we demonstrated that LRP1-specific antibody, which blocks ligand-binding to LRP1, induces expression of inflammatory mediators in BMDMs, similarly to LF and RAP. Because the total number of LRP1 ligands is high (7, 9, 14), identifying LRP1 agonists that are secreted by macrophages represents an important proteomics problem for future investigation.
The antiinflammatory activity of EI-tPA and α2M* was sufficiently potent to substantially inhibit and, in some cases, entirely block cytokine expression in response to LPS. Using BMDMs from Cre-ERT-LRP1flox/flox mice, we showed that the antiinflammatory activity of EI-tPA and α2M* requires LRP1. We conclude that the antiinflammatory activity of LRP1 may be stimulated beyond the level observed in resting macrophages by exogenously added agonists. tPA and α2M* emerge as potentially important antiinflammatory agents. Although α2M is abundant in plasma, α2M circulates almost exclusively in a “nonactivated” conformation, which does not bind to LRP1 (36). The circulating level of tPA is about 0.1–0.2 nM (37), near the activity threshold demonstrated in Fig. 4.
An important consequence of LRP1 gene deletion in BMDMs was significant and selective up-regulation of miR-155. Increased miR-155 also was observed in RAP- and LF-treated cells. In a previous study, Baltimore and colleagues (19) applied array technology and identified miR-155 as the major microRNA up-regulated in macrophages in response to diverse proinflammatory stimuli. miR-155 controls inflammation in mouse models of atherosclerosis (38) and is up-regulated in synovial fluid from patients with rheumatoid arthritis (39). When we treated BMDMs with RAP, TNFα mRNA was increased by 1 h, whereas miR-155 was not significantly increased until 4–8 h. TNFα-neutralizing antibody significantly inhibited the increase in miR-155 observed 8 h after adding RAP. These results suggest that miR-155 expression may be increased, at least in part, as a secondary response downstream of the cytokines that are expressed at increased levels when macrophages are treated with LRP1 antagonists.
miR-155 sustained the proinflammatory activity of LRP1 antagonists. Inhibiting miR-155 failed to attenuate the significant increase in TNFα expression observed 1 h after adding RAP. By contrast, 8 h after adding RAP, miR-155 inhibition substantially decreased expression of TNFα, CCL4, and IL-6. These results suggest that proinflammatory LRP1 ligands may trigger a positive feedback loop by increasing expression of cytokines such as TNFα, which induce expression of miR-155. In turn, miR-155 promotes expression of proinflammatory mediators such as TNFα. This type of positive feedback loop may be important in chronic inflammation.
JSH-23 blocked the increase in miR-155 induced by RAP in BMDMs, consistent with previous studies demonstrating that NFκB activation promotes miR-155 expression (40, 41). In our model system, NFκB inhibition may have inhibited transcription of the miR-155 parent gene directly downstream of LRP1, or expression of the cytokines that increase miR-155, or the cell-signaling pathways activated by these cytokines. In any case, LRP1, NFκB, and miR-155 emerge as members of a system that may be modulated to either promote or inhibit macrophage inflammatory responses, depending on the continuum of LRP1 ligands present in the macrophage microenvironment.
The pathway by which LRP1 ligands regulate NFκB remains to be completely elucidated. Although we previously demonstrated that TNF receptor-1 (TNFR1) is increased in LRP1-deficient fibroblasts (12, 42), TNFR1 regulation does not provide a straightforward explanation for the activity of LRP1 ligands. LRP1 demonstrates other important activities in macrophages that may be critical in tissue injury and the inflammatory response, including its function in phagocytosis of large particles (12), efferocytosis (15, 43), and in regulating transforming growth factor-β (44). LRP1 also is involved in antigen presentation in support of adaptive immunity (45, 46). Understanding the integrated activity of macrophage LRP1 in the regulation of injury and inflammation remains an important future goal.
Materials and Methods
Proteins and Reagents.
α2M was purified from plasma (47) and activated for binding to LRP1 (α2M*) by reaction with 200 mM methylamine-HCl. α2M modification was confirmed by nondenaturing PAGE (36). EI-tPA was from Molecular Innovations.
Mouse Model Systems.
LRP1flox/flox mice were bred with mice that express Cre recombinase under the control of the lysozyme-M promoter (LysM-Cre), in the C57BL/6J background, to generate mLRP1−/− mice. Littermate controls (mLRP1+/+) were LRP1flox/flox and LysM-Cre-negative. LRP1flox/flox mice also were bred with CreERT mice (23), which express Tam-activated Cre in all cells (Jackson Laboratories). BMDMs were isolated from littermates that were LRP1flox/flox and either Cre-ERT-positive or -negative. Littermates were born at a 50:50 ratio. All experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee.
For additional details on proteins and reagents, LPS challenge studies, analyzing BMDM responses to LRP1 ligands, immunoblot analysis, miRNA extraction and RT-PCR, gene expression analysis, Transwell cell migration assays, ChIP assays, miRNA inhibitor studies, and statistical methods, please see SI Materials and Methods.
SI Materials and Methods
Proteins and Reagents.
LF and Escherichia coli LPS, serotype 055.B5, were from Sigma-Aldrich. RAP was expressed as a GST-fusion protein and purified as previously described (48). Free GST was expressed using empty vector. GST-fusion proteins were subjected to chromatography on Detoxi-Gel endotoxin-removing columns (Pierce). The compound 4-hydroxytamoxifen (TAM) was from Enzo Life Sciences. JSH-23 and LY294002 were from Calbiochem. Rabbit TNFα-neutralizing antibody and anti-CD11b were purchased from Abcam. Specific antibodies directed against the C-terminal 85-kDa and the N-terminal 515-kDa subunits of LRP1 and β-actin were from Sigma. Antibodies that detect phosphorylated IκBα and total IκBα were from Cell Signaling Technologies.
LPS Challenge.
mLRP1−/− and mLRP1+/+ mice were treated by i.p. injection with LPS (1 mg/kg). Control mice received an equivalent volume of normal saline. The mice were observed for changes in neurologic status, altered respiratory rate, and/or hemostasis abnormalities. Those entering a moribund condition were killed immediately by cervical dislocation under anesthesia. Kaplan–Meier survival curves were analyzed by log-rank test (Prism). In separate experiments, blood samples were recovered from the retroorbital venous plexus. Plasma was isolated and TNFα and CCL3 were measured using the mouse TNFα and CCL3/MIP-1α Quantikine ELISA kits (R&D Systems). Lungs and kidneys were recovered 24 h after LPS injection. RNA was isolated and RT-qPCR was performed.
Immunohistochemistry.
Lung tissue was harvested from mice, formalin fixed, processed, and paraffin embedded. Four-micrometer-thick tissue sections were cut and mounted on positively charged microscope slides. Sections were stained with antibody against CD11b (1:1,200) from Abcam using a Ventana Discovery Ultra (Ventana Medical Systems). Antigen retrieval was performed using CC1 for 40 min at 95 °C. The primary antibody was incubated on the sections for 1 h at 37 °C and visualized using 3,3′-diaminobenzidine as a chromagen and the UltraMap System (Ventana Medical Systems). Slides were counterstained with hematoxylin, rinsed, dehydrated through alcohol and xylene, and cover slipped.
Isolation of BMDMs.
The 12- to 16-wk-old mice were killed. Femurs and tibiae were recovered from both rear legs. Bone marrow cells were flushed from the long bones in DMEM (Invitrogen). Cell suspensions were filtered through a 100-µm nylon cell strainer and plated in 100-mm dishes at a density of 2 × 106 cells per dish in DMEM containing 10% (vol/vol) FBS (HyClone Laboratories) and 20% (vol/vol) L929 cell-conditioned medium. After 10 d, nonadherent cells were removed. Adherent cells, which constituted a nearly pure population of BMDMs (2, 3), were used in experiments.
BMDM Response to LRP1 Ligands.
BMDMs from LRP1flox/flox-Cre-ERT-positive mice and LRP1flox/flox-Cre-ERT-negative mice were pretreated with TAM (5 µM) or with vehicle for 7 d. These cells and cells from wild-type C57BL/6J mice were transferred to SFM for 30 min and then treated for 8 h with α2M*, EI-tPA, LF, or RAP. Cells were treated with LPS (0.1 µg/mL) as a control. Treatments with vehicle or GST served as negative controls. In another set of experiments, BMDMs from wild-type C57BL/6J mice were transferred to SFM for 30 min and treated with rabbit IgG1 polyclonal antibody directed against LRP1 heavy chain.
Immunoblot Analysis.
Cellular extracts were prepared in RIPA buffer (PBS with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) containing Complete Protease Inhibitor Mixture (Roche Diagnostics) and sodium orthovanadate. The protein concentration in tissue extracts was determined by bicinchoninic acid (BCA) assay. An equivalent amount of cellular protein (50 µg per lane) was subjected to SDS/PAGE and electrotransferred to methanol-activated PVDF membranes. Membranes were blocked with 5% (wt/vol) nonfat dry milk in 10 mM Tris⋅HCl, 150 mM NaCl, pH 7.4, with 0.1% Tween 20 and subsequently incubated with primary polyclonal antibody that recognizes: the C-terminal 85-kDa subunit of LRP1 (1:1,000; Sigma); phosphorylated IκB (1:1,000; Cell Signaling Technologies); total IκB (1:1,000; Cell Signaling Technologies); or monoclonal antibody that detects actin (1:1,000; Sigma). Primary antibody binding was detected by HRP-conjugated species-specific secondary antibodies (1:2,000; Cell Signaling Technologies). Immunoblots were developed using enhanced chemiluminescence ECL Plus (GE Healthcare) and HyBlot CL Autoradiography film (Denville Scientific).
ELISAs.
TNFα protein in conditioned medium was determined using the mouse TNFα Quantikine ELISA kit (R&D Systems). Apoptotic cell death was assessed using the Cell Death Detection Plus ELISA kit (Roche Diagnostics), which quantifies apoptotic cells by detecting histone-associated DNA fragments (mono- and oligonucleosomes).
Gene Expression Analysis.
Total RNA was isolated from 40-µm-thick sections of mouse kidneys and lungs and from BMDMs using the acid guanidinium-thiocyanate-phenol-chloroform extraction method (49) and TRIzol Reagent (Life Technologies). RNA was purified using the NucleoSpin RNA kit (Macherey-Nagel) according to the manufacturer’s instructions. cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad). RT-qPCR was performed using TaqMan gene expression assay products on an AB Step One Plus Real-Time PCR system (Applied Biosystems). The relative change in gene expression was calculated using the 2ΔΔCt method and GAPDH gene as a standardizer. Primer-probe sets were as follow: GAPDH (Mm99999915_g1); TNFα (Mm00443258_m1); IL-6 (Mm00446190_m1); IL1-β (Mm00434228_m1); CCL2 (Mm00441242_m1); CCL3 (Mm00441259_g1); and CCL4 (Mm00443111_m1).
miRNA Detection.
miRNA was isolated using the mirVANA miRNA isolation kit (Applied Biosystems). miRNA (10 ng) was retrotranscribed into cDNA using the Taqman miRNA Reverse transcription kit (Applied Biosystems) and RT primers specific for: miR-155, miR-9, miR-223, and miR-124. qPCR was performed in duplicate using Taqman Universal PCR Master Mix, No AmpErase UNG, and the following miRNA-specific probe mixes (Applied Biosystems): mmu-miR-155 (002571); mmu-miR-223–5 (007896_mat); hsa-miR-9 (000583); and mmu-miR-124a (001182). The relative change in gene expression was calculated using the 2ΔΔCt method and snoRNA202 (001232) as a control.
miRNA Inhibitor Studies.
BMDMs isolated from C57BL/6J mice were transfected with 10 nM miR-155 inhibitor (mmu-miR-155–5p, Applied Biosystems) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer's instructions. As positive and negative controls, cells were transfected with the mirVana miRNA Inhibitor let-7c Positive Control and mirVana miRNA Inhibitor Negative Control 1 (Ambion). miR-155 expression was determined by qPCR to confirm depletion. Subsequently, BMDMs were treated with LF or RAP, beginning 48 h after transfection with miR-155 inhibitor.
Transwell Cell Migration Assays.
BMDMs (5 × 105) were added to the top chamber of 24-well Transwell units with 8.0-µm pores. The underside of each membrane was coated with 10 μg/mL fibronectin (Millipore). When BMDMs were pretreated with LRP1 ligands, including RAP (150 nM) or LF (150 nM), the same ligands were added to the medium in the Transwells. The bottom chamber contained 10% (vol/vol) FBS. Cells were allowed to migrate for 16 h. Nonmigrating cells were removed from the upper surface. The lower surface was stained with Hema 3 (Fisher Scientific). Stained membranes were imaged using a Leica DMIRE2 microscope. The number of migrated cells was determined in four representative fields, selected by a blinded investigator, using ImageJ software. Three separate membranes were analyzed for each condition in three separate experiments.
ChIP.
BMDMs (1 × 107) were treated with 0.75% formaldehyde. Cross-linking was terminated with 0.12 M glycine. Nuclei were isolated from cell extracts by centrifugation at 600 × g. Chromatin was sheared into 200- to 1,000-bp fragments by sonication. Cell debris was cleared by centrifugation and the chromatin filtered using a 0.2-µm filter. For immunoprecipitation (IP), sheared chromatin was incubated with anti-RNApol II antibody (Abcam), which recognizes the phospho-S-5 epitope in the C-terminal domain of activated RNA polymerase II, for 2 h at 4 °C and Protein G-Sepharose. DNA was eluted in 1% SDS and treated with RNase A and Proteinase K. DNA was purified using the minElute gel extraction kit (Qiagen). PCR was performed using Phusion polymerase (NEB). The primers (5′-GTTTCCTTTAAGAATAAAGCA-3′ and 5′-GTTCTTGGAACCTCCGGC-TCGA-3′) amplified a region near the TATA box of the BIC gene, which encodes miR-155.
Statistics.
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software). All results are expressed as mean ± SEM. Survival studies were analyzed by log-rank test. ELISA results, qPCR data, and cell migration studies were analyzed by one-way ANOVA followed by Tukey’s or Dunnett’s post hoc analysis. Differences between two experimental groups were evaluated using a Mann–Whitney test. In the SI figures, statistical significance is indicated as follows: *P < 0.05; **P < 0.01; ***P < 0.001.
Acknowledgments
This work was supported by Grant R01 HL60551 from the National Institutes of Health and Fondo per gli Investimenti della Ricerca di Base 2013 (RBFR13BPK9) from the Italian Ministry of Education, Universities, and Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515480113/-/DCSupplemental.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284(5418):1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Loo YM, Gale M., Jr Immune signaling by RIG-I-like receptors. Immunity. 2011;34(5):680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell. 2006;124(4):823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R, Janeway C., Jr Innate immunity. N Engl J Med. 2000;343(5):338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 7.Strickland DK, Gonias SL, Argraves WS. Diverse roles for the LDL receptor family. Trends Endocrinol Metab. 2002;13(2):66–74. doi: 10.1016/s1043-2760(01)00526-4. [DOI] [PubMed] [Google Scholar]
- 8.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71(3):411–421. doi: 10.1016/0092-8674(92)90511-a. [DOI] [PubMed] [Google Scholar]
- 9.Gonias SL, Campana WM. LDL receptor-related protein-1: A regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am J Pathol. 2014;184(1):18–27. doi: 10.1016/j.ajpath.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staudt ND, et al. Myeloid cell receptor LRP1/CD91 regulates monocyte recruitment and angiogenesis in tumors. Cancer Res. 2013;73(13):3902–3912. doi: 10.1158/0008-5472.CAN-12-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overton CD, Yancey PG, Major AS, Linton MF, Fazio S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100(5):670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 12.Gaultier A, et al. Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood. 2008;111(11):5316–5325. doi: 10.1182/blood-2007-12-127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.May P, Bock HH, Nofer J-R. Low density receptor-related protein 1 (LRP1) promotes anti-inflammatory phenotype in murine macrophages. Cell Tissue Res. 2013;354(3):887–889. doi: 10.1007/s00441-013-1699-2. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Castaneda A, et al. Identification of the low density lipoprotein (LDL) receptor-related protein-1 interactome in central nervous system myelin suggests a role in the clearance of necrotic cell debris. J Biol Chem. 2013;288(7):4538–4548. doi: 10.1074/jbc.M112.384693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stiles TL, et al. LDL receptor-related protein-1 is a sialic-acid-independent receptor for myelin-associated glycoprotein that functions in neurite outgrowth inhibition by MAG and CNS myelin. J Cell Sci. 2013;126(Pt 1):209–220. doi: 10.1242/jcs.113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantuano E, Lam MS, Gonias SL. LRP1 assembles unique co-receptor systems to initiate cell signaling in response to tissue-type plasminogen activator and myelin-associated glycoprotein. J Biol Chem. 2013;288(47):34009–34018. doi: 10.1074/jbc.M113.509133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Y, Mantuano E, Inoue G, Campana WM, Gonias SL. Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci Signal. 2009;2(68):ra18. doi: 10.1126/scisignal.2000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 21.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101(3):689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardí M, et al. Distinct patterns of urokinase receptor (uPAR) expression by leukemic cells and peripheral blood cells. Thromb Haemost. 1996;76(6):1009–1019. [PubMed] [Google Scholar]
- 23.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 24.LaMarre J, Wolf BB, Kittler EL, Quesenberry PJGS, Gonias SL. Regulation of macrophage alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein by lipopolysaccharide and interferon-gamma. J Clin Invest. 1993;91(3):1219–1224. doi: 10.1172/JCI116283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 2010;88(4):769–778. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Declerck PJ, De Mol M, Vaughan DE, Collen D. Identification of a conformationally distinct form of plasminogen activator inhibitor-1, acting as a noninhibitory substrate for tissue-type plasminogen activator. J Biol Chem. 1992;267(17):11693–11696. [PubMed] [Google Scholar]
- 27.Wang DZ, Rose JA, Honings DS, Garwacki DJ, Milbrandt JC. Treating acute stroke patients with intravenous tPA. The OSF stroke network experience. Stroke. 2000;31(1):77–81. doi: 10.1161/01.str.31.1.77. [DOI] [PubMed] [Google Scholar]
- 28.Flood EC, Hajjar KA. The annexin A2 system and vascular homeostasis. Vascul Pharmacol. 2011;54(3-6):59–67. doi: 10.1016/j.vph.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD. MicroRNAs: New regulators of immune cell development and function. Nat Immunol. 2008;9(8):839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 30.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287(26):21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33(2):170–177. doi: 10.1161/ATVBAHA.112.300068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88(3):887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonacci GR, Cáceres LC, Sánchez MC, Chiabrando GA. Activated alpha(2)-macroglobulin induces cell proliferation and mitogen-activated protein kinase activation by LRP-1 in the J774 macrophage-derived cell line. Arch Biochem Biophys. 2007;460(1):100–106. doi: 10.1016/j.abb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Mantuano E, et al. The hemopexin domain of matrix metalloproteinase-9 activates cell signaling and promotes migration of schwann cells by binding to low-density lipoprotein receptor-related protein. J Neurosci. 2008;28(45):11571–11582. doi: 10.1523/JNEUROSCI.3053-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer H, et al. α2-Macroglobulin enhances vasculogenesis/angiogenesis of mouse embryonic stem cells by stimulation of nitric oxide generation and induction of fibroblast growth factor-2 expression. Stem Cells Dev. 2013;22(9):1443–1454. doi: 10.1089/scd.2012.0640. [DOI] [PubMed] [Google Scholar]
- 36.Gonias SL, Pizzo SV. Chemical and structural modifications of alpha 2-macroglobulin: Effects on receptor binding and endocytosis studied in an in vivo model. Ann N Y Acad Sci. 1983;421(Hl 24066):457–471. doi: 10.1111/j.1749-6632.1983.tb18139.x. [DOI] [PubMed] [Google Scholar]
- 37.Thögersen AM, et al. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: Evidence for the fibrinolytic system as an independent primary risk factor. Circulation. 1998;98(21):2241–2247. doi: 10.1161/01.cir.98.21.2241. [DOI] [PubMed] [Google Scholar]
- 38.Du F, et al. MicroRNA-155 deficiency results in decreased macrophage inflammation and attenuated atherogenesis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2014;34(4):759–767. doi: 10.1161/ATVBAHA.113.302701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurowska-Stolarska M, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108(27):11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol. 2011;3(3):159–166. doi: 10.1093/jmcb/mjr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluiver J, et al. Regulation of pri-microRNA BIC transcription and processing in Burkitt lymphoma. Oncogene. 2007;26(26):3769–3776. doi: 10.1038/sj.onc.1210147. [DOI] [PubMed] [Google Scholar]
- 42.Gonias SL, Wu L, Salicioni AM. Low density lipoprotein receptor-related protein: Regulation of the plasma membrane proteome. Thromb Haemost. 2004;91(6):1056–1064. doi: 10.1160/TH04-01-0023. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian M, et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J Clin Invest. 2014;124(3):1296–1308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muratoglu SC, et al. Macrophage LRP1 suppresses neo-intima formation during vascular remodeling by modulating the TGF-β signaling pathway. PLoS One. 2011;6(12):e28846. doi: 10.1371/journal.pone.0028846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowers EV, Horvath JJ, Bond JE, Cianciolo GJ, Pizzo SV. Antigen delivery by alpha(2)-macroglobulin enhances the cytotoxic T lymphocyte response. J Leukoc Biol. 2009;86(5):1259–1268. doi: 10.1189/jlb.1008653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pawaria S, Kropp LE, Binder RJ. Immunotherapy of tumors with α2-macroglobulin-antigen complexes pre-formed in vivo. PLoS One. 2012;7(11):e50365. doi: 10.1371/journal.pone.0050365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic “fast” forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256(15):8134–8139. [PubMed] [Google Scholar]
- 48.Herz J, Goldstein JL, Strickland DK, Ho YK, Brown MS. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266(31):21232–21238. [PubMed] [Google Scholar]
- 49.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]