Significance
Ubiquitylation is integral to a myriad of cellular processes, including protein destruction, cell cycle control, and regulation of gene activity. Here, we show that ubiquitylation plays a role in inactivating the expression of genes that are located close to telomeres. We present evidence that the ubiquitin ligase Asr1 associates with subtelomeric DNA and ubiquitylates RNA polymerase II to prevent it from transcribing genes at these locations. We also show that Asr1 interacts with Ubp3, an enzyme that reverses ubiquitylation, and that the two play antagonistic roles in silencing subtelomeric genes. These findings show how ubiquitylation of a core component of the transcriptional machinery impacts gene activity and reveal a mechanism for controlling the expression of telomere-proximal genes.
Keywords: ubiquitin, chromatin, gene silencing, transcription
Abstract
Ubiquitin, and components of the ubiquitin–proteasome system, feature extensively in the regulation of gene transcription. Although there are many examples of how ubiquitin controls the activity of transcriptional regulators and coregulators, there are few examples of core components of the transcriptional machinery that are directly controlled by ubiquitin-dependent processes. The budding yeast protein Asr1 is the prototypical member of the RPC (RING, PHD, CBD) family of ubiquitin-ligases, characterized by the presence of amino-terminal RING (really interesting new gene) and PHD (plant homeo domain) fingers and a carboxyl-terminal domain that directly binds the largest subunit of RNA polymerase II (pol II), Rpb1, in response to phosphorylation events tied to the initiation of transcription. Asr1-mediated oligo-ubiquitylation of pol II leads to ejection of two core subunits of the enzyme and is associated with inhibition of polymerase function. Here, we present evidence that Asr1-mediated ubiquitylation of pol II is required for silencing of subtelomeric gene transcription. We show that Asr1 associates with telomere-proximal chromatin and that disruption of the ubiquitin-ligase activity of Asr1—or mutation of ubiquitylation sites within Rpb1—induces transcription of silenced gene sequences. In addition, we report that Asr1 associates with the Ubp3 deubiquitylase and that Asr1 and Ubp3 play antagonistic roles in setting transcription levels from silenced genes. We suggest that control of pol II by nonproteolytic ubiquitylation provides a mechanism to enforce silencing by transient and reversible inhibition of pol II activity at subtelomeric chromatin.
The ubiquitin (Ub)–proteasome system features in a multitude of cellular processes, including the regulation of transcription (1). Over the past two decades, it has become clear that Ub-dependent processes impact multiple steps in gene expression, ranging from signaling histone modifications (2) to coordinating events of mRNA export from the nucleus (3). Many of the functions of Ub in transcription are directed against regulatory factors, and there are only a handful of examples in which core components of the transcriptional machinery are regulated by Ub-dependent transactions. The best understood example from this category is the Ub-mediated destruction of the largest subunit of RNA polymerase II (pol II) in response to DNA damage (4), which functions as a failsafe mechanism to remove stalled pol II complexes from damaged DNA segments. Beyond a damage response, however, it is unclear whether ubiquitylation controls the normal activity of pol II.
We previously reported that the Saccharomyces cerevisiae protein Asr1 is a RING (really interesting new gene) finger Ub-ligase that targets RNA polymerase II for nonproteolytic ubiquitylation (5). Asr1 defines the evolutionarily preserved RPC (RING, PHD, CBD) family of Ub-ligases, characterized by the presence of amino-terminal RING and PHD (plant homeo domain) fingers and a domain that binds to the carboxy-terminal domain (CTD) of the largest subunit of pol II, Rpb1 (Fig. S1A). Binding of the CTD-binding domain (CBD) of Asr1 to Rpb1 depends on phosphorylation of serine residue 5 (Ser5) within the Rpb1 CTD repeats (5), a modification that occurs commensurate with the initiation of transcription. When bound to pol II, Asr1 oligo-ubiquitylates five lysine residues adjacent to the Rpb1 CTD, as well as unknown residues within Rpb2, the result of which is the ejection of two core subunits of the enzyme—Rpb4/Rpb7 (5). Interestingly, despite association of Asr1 with the 10 subunits of pol II required for transcriptional elongation (6), the form of pol II that copurifies with Asr1 is catalytically inactive (5), suggesting that Asr1 both modifies and inactivates RNA polymerase. Whether inactivation is linked to expulsion of Rpb4/7, or polymerase ubiquitylation per se, is unknown.
Fig. S1.
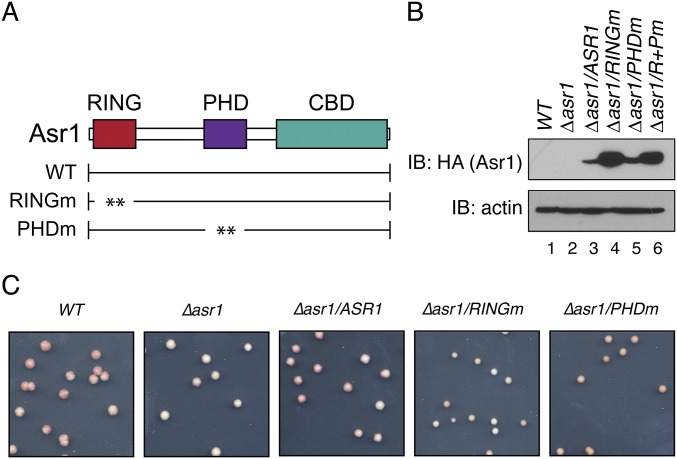
The Ub-ligase activity of Asr1 is required for silencing of a telomere-proximal ADE2 reporter. (A) Schematic of Asr1, showing the location of the RING and PHD fingers, and the CTD-binding domain (CBD). The RINGm mutation is a simultaneous substitution of cysteine residues 26, 29, 66, and 69 to alanine. The PHDm mutation is a simultaneous substitution of cysteine residues 143, 146, 186, and 189 to alanine. (B) Steady-state levels of HA-tagged Asr1 proteins assayed in Fig. 1B. Immunoblot (IB) was performed using an anti-HA antibody. An antibody against actin was used as a loading control. Note that the double RING/PHD mutant of Asr1 (R+Pm) was not included in functional assays. (C) Photograph of representative colonies used to calculate colony color ratios presented in Fig. 1B.
Despite the well-characterized biochemical actions of Asr1, a physiological role for this protein has remained enigmatic. Indeed, asr1-null cells are phenotypically normal (5), leaving open the question of what biological endpoint is mediated via interaction of Asr1 with pol II. Here, we demonstrate that the Ub-ligase activity of Asr1 is required for silencing of subtelomeric chromatin. We show that Asr1 associates with telomere-proximal genes and demonstrate that some of the prosilencing actions of Asr1 are mediated via ubiquitylation of Rpb1. We also demonstrate that Asr1 associates with the Ub-specific protease Ubp3 and recruits it to pol II and that Asr1 and Ubp3 act antagonistically to set expression levels of subtelomeric genes.
Results and Discussion
The Ub-Ligase Activity of Asr1 Is Required for Subtelomeric Gene Silencing.
We performed comparative transcriptomic analysis (RNA-seq) on RNA isolated from yeast expressing either WT Asr1 or a RING finger Asr1 mutant (Asr1RINGm) that lacks Ub-ligase activity (5). This analysis identified 27 genes that are induced, and 29 that are repressed, by a factor of two or more in Asr1RINGm mutant cells (Table S1). Genes in each category did not cluster according to ontology, but we noticed that 33% of genes in the induced set lie within 50 kb of a telomere. In contrast, for repressed genes, only 7% were telomere-proximal. The induction of subtelomeric gene expression in Asr1RINGm cells suggests that the Ub-ligase activity of Asr1 may be required for authentic patterns of subtelomeric silencing.
Table S1.
Genes differentially expressed more than twofold between WT and Asr1(RINGm) cells
| Systematic name | Standard name | Log2 (fold change) | Chromosome | Median distance from telomere, bp |
| YML123C | PHO84 | 3.4533 | chrXIII | 24,919 |
| YHR215W | PHO12 | 2.60504 | chrVIII | 9,843 |
| YDL039C | PRM7 | 2.37292 | chrIV | 383,033 |
| YHR136C | SPL2 | 2.27773 | chrVIII | 187,766 |
| YBR056W-A | YBR056W-A | 2.1083 | chrII | 461,829 |
| YKR075W-A | YKR075W-A | 1.95347 | chrXI | 86,470 |
| YBR296C | PHO89 | 1.9054 | chrII | 15,524 |
| YJR150C | DAN1 | 1.68879 | chrX | 36,492 |
| YBR056C-B | YBR056C-B | 1.68571 | chrII | 461,812 |
| YPL019C | VTC3 | 1.52209 | chrXVI | 515,765 |
| YOL016C | CMK2 | 1.49027 | chrXV | 295,449 |
| YMR316W | DIA1 | 1.46835 | chrXIII | 19,101 |
| YOR385W | YOR385W | 1.4338 | chrXV | 25,812 |
| YKR075C | YKR075C | 1.38742 | chrXI | 86,528 |
| YAR071W | PHO11 | 1.31843 | chrI | 4,057 |
| YBR005W | RCR1 | 1.28636 | chrII | 566,958 |
| YOL152W | FRE7 | 1.2059 | chrXV | 41,679 |
| YBL043W | ECM13 | 1.20173 | chrII | 137,075 |
| YGR146C | ECL1 | 1.19767 | chrVII | 306,400 |
| YKR091W | SRL3 | 1.16936 | chrXI | 54,921 |
| YDR277C | MTH1 | 1.16894 | chrIV | 516,882 |
| YOR062C | YOR062C | 1.12585 | chrXV | 648,163 |
| YOR338W | YOR338W | 1.12126 | chrXV | 133,848 |
| YDR281C | PHM6 | 1.10192 | chrIV | 509,769 |
| YMR011W | HXT2 | 1.05848 | chrXIII | 635,540 |
| YFR053C | HXK1 | 1.04324 | chrVI | 15,841 |
| YGR161C | RTS3 | 1.01338 | chrVII | 281,916 |
| snR68 | SNR68 | −1.03417 | chrIX | 97,179 |
| snR60 | SNR60 | −1.03626 | chrX | 349,182 |
| snR75 | SNR75 | −1.03683 | chrXIII | 626,469 |
| YLR046C | YLR046C | −1.05447 | chrXII | 839,770 |
| snR58 | SNR58 | −1.09758 | chrXV | 136,136 |
| snR161 | SNR161 | −1.11256 | chrII | 505,919 |
| snR73 | SNR73 | −1.12074 | chrXIII | 626,072 |
| snR55 | SNR55 | −1.14246 | chrXII | 283,432 |
| snR69 | SNR69 | −1.15528 | chrXI | 364,826 |
| snR79 | SNR79 | −1.17201 | chrXII | 729,709 |
| YOR314W | YOR314W | −1.17324 | chrXV | 188,084 |
| YLL056C | YLL056C | −1.21083 | chrXII | 27,857 |
| tL(GAG)G | tL(GAG)G | −1.22698 | chrVII | 390,225 |
| snR39B | SNR39B | −1.23423 | chrVII | 366,422 |
| YPR108W-A | YPR108W-A | −1.24543 | chrXVI | 203,785 |
| YNL042W-B | YNL042W-B | −1.27892 | chrXIV | 547,242 |
| snR66 | SNR66 | −1.32312 | chrXIV | 586,133 |
| snR52 | SNR52 | −1.3265 | chrV | 145,700 |
| YOR072W-B | YOR072W-B | −1.32751 | chrXV | 626,742 |
| snR71 | SNR71 | −1.33714 | chrVIII | 151,371 |
| snR74 | SNR74 | −1.37259 | chrXIII | 626,250 |
| snR78 | SNR78 | −1.44653 | chrXIII | 627,110 |
| YDR210C-D | YDR210C-D | −1.49455 | chrIV | 650,640 |
| YGL263W | COS12 | −1.52426 | chrVII | 3,361 |
| tR(ACG)J | tR(ACG)J | −1.64223 | chrX | 233,975 |
| YLR365W | YLR365W | −1.65871 | chrXII | 222,812 |
| snR72 | SNR72 | −1.87679 | chrXIII | 625,829 |
| YGR224W | AZR1 | −2.04648 | chrVII | 147,214 |
| tQ(CUG)M | CDC65 | −2.0612 | chrXIII | 116,150 |
The systematic and standard names for each gene are listed, along with the log2 fold change, as measured by RNA-seq. The chromosome carrying each gene is also listed, along with the median distance of each gene to its nearest telomere (in kb). Bold in the last column indicates a median distance from the telomere of less than 50 kb. Strains BY4742 (WT) and YTM27 (Asr1RINGm) were used in this analysis. See Table S2 for strain details.
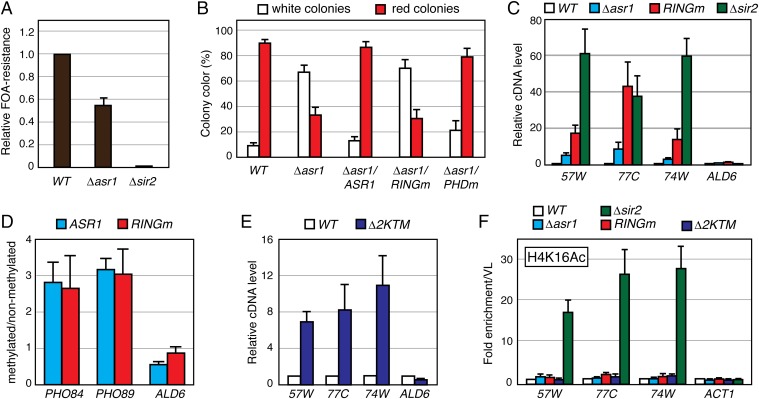
To determine whether Asr1 is involved in silencing, we monitored the effect of mutations in Asr1 on expression of one of two reporters, URA3 (7) or ADE2 (8), integrated at telomere-proximal DNA on the right arm of chromosome V. Deletion of the silent information regulator SIR2 was included as a positive control. Silencing of URA3 renders yeast resistant to 5-fluoroorotic acid (FOA); silencing of ADE2 produces red colonies. In both assays, deletion of ASR1—or mutation of its RING finger—reduced the extent of silencing, as measured by a decrease in the number of FOA-resistant (Fig. 1A) and red (Fig. 1B and Fig. S1 B and C) colonies. For a separate assessment of the extent to which silencing is perturbed in Asr1 mutant cells, we used reverse transcription quantitative PCR (RT–QPCR) to measure transcript levels from three telomere-proximal genes we have shown to be silenced (9) but were not detected in our RNA-seq analysis: YFR057W, YNR077C, and YCL074W. Here (Fig. 1C), deletion of ASR1 resulted in a two- to fivefold increase in the expression of all three genes, whereas mutation of the Asr1 RING increased telomere-proximal gene expression by a factor of 15- to 40-fold. In contrast, expression of the euchromatic ALD6 gene was unaffected by mutation of Asr1. Together, these data demonstrate that the Ub-ligase activity of Asr1 is required for subtelomeric gene silencing.
Fig. 1.
The ubiquitin ligase activity of Asr1 is required for subtelomeric gene silencing. (A) Asr1 is required for full silencing of a telomere-proximal URA3 reporter. Equal amounts of yeast (WT, LPY4819; ∆asr1, LPY4819 ∆Asr1; ∆sir2, LPY4977) were plated on media with or without 5-FOA, and the relative number of FOA-resistant colonies was calculated. Error bars represent SEM (n = 3). (B) The Ub-ligase activity of Asr1 is required for silencing of a telomere-proximal ADE2 reporter. Yeasts (WT, UTAT TM4; ∆asr1, UTAT TM5; ∆asr1/ASR1, UTAT TM6; ∆asr1/asr1RINGm, UTAT TM7; ∆asr1/ASR1PHDm, UTAT TM8) were plated on nonselective media, and the ratio of white and red colonies was calculated. Error bars represent SEM (n = 3). (C) The Ub-ligase activity of Asr1 is required for silencing telomere-proximal genes. Total RNA was extracted from yeast (WT, BY4741; ∆asr1, YTM5; asr1RINGm, YTM27; ∆sir2, ∆Sir2), and RT–QPCR was used to measure transcript levels from the indicated loci (57W, YFR057W; 77C, YNR077C; 74W, YCL074W; ALD6). Error bars represent SEM (n = 6–8). (D) Asr1 associates with subtelomeric chromatin. DNA was isolated from congenic strains expressing either an Asr1–DAM (YTM38) or Asr1RINGm–DAM (YTM39) fusion, or unfused DAM expressed from the ASR1 promoter (YTM40). DNA was then either untreated, or cleaved with DpnII. QPCR was performed using primers flanking a DpnII site within each gene to determine the extent of cutting. Signals for each fusion strain were normalized to that from the strain expressing unfused DAM protein, and data were plotted as the ratio of methylated/nonmethylated DNA. Error bars represent SEM (n = 5). (E) Asr1-dependent ubiquitylation sites on Rpb1 are required for silencing. The experiment was performed as in C, except on RNA isolated from WT yeast (BY4742) or yeast expressing the Rpb1–∆2KTM mutant (YTM29). Error bars represent SEM (n = 4–5). (F) Loss of silencing in Asr1-mutant cells is not accompanied by an increase in H4K16 acetylation. ChIP was performed on chromatin isolated from the indicated strains (WT, BY4741; ∆asr1, YTM5; asr1RINGm, YTM27; Rpb1–∆2KTM, YTM29; ∆sir2, ∆Sir2), using an anti-H4K16 acetylation (H4K16Ac)-specific antibody. Coprecipitating DNAs were quantified by QPCR and normalized to the signal from a primer set that amplifies an intergenic portion on the left arm of chromosome V. Error bars represent SEM (n = 4).
Next, we asked whether Asr1 associates with subtelomeric chromatin. For this purpose, we performed DNA adenine methyltransferase identification (DamID) (10). Here, Asr1 is expressed in yeast as a fusion to the Escherichia coli DNA adenine methyltransferase. If Asr1 binds chromatin, the DNA at that site will be ectopically methylated and resist cleavage by a methylation-sensitive restriction enzyme. Using this approach (Fig. 1D), we observed that both WT Asr1 and the Asr1RINGm mutant associate with the telomere-proximal PHO84 and PHO89 genes, two of the most strongly-induced genes in Asr1RINGm mutant cells (Table S1). The euchromatic ALD6 gene, in contrast, was not appreciably methylated. This result suggests that Asr1 physically interacts with telomere-proximal chromatin and does so in a RING finger-independent manner.
To determine whether ubiquitylation of Rpb1 is relevant to the involvement of Asr1 in subtelomeric gene silencing, we asked whether silencing is perturbed in cells that express a mutant of Rpb1 in which sites of Asr1-mediated ubiquitylation are mutated (∆2KTM) (5). Indeed, we observed increased expression of YFR057W, YNR077C, and YCL074W—but not ALD6—in the presence of the ∆2KTM Rpb1 mutant (Fig. 1E). Notably, induction of these genes was three- to fivefold lower than we observed in the presence of the Asr1RINGm mutant (Fig. 1C), revealing that loss of ubiquitylation of Rpb1 only partially contributes to the loss of gene silencing that we observed in the presence of inactive Asr1. We conclude that Asr1-mediated ubiquitylation of Rpb1 is involved in repression of telomere-proximal gene expression and that Asr1 ubiquitylates other substrates to silence gene activity at these sites.
Finally, we asked whether induction of subtelomeric gene activity in Asr1 mutant cells is accompanied by an increase in acetylation of histone H4 at lysine 16 (H4K16) at telomeres, as observed upon deletion of SIR2 (11). Interestingly, however, despite levels of gene induction comparable with SIR2 deletion (Fig. 1C), neither ASR1 deletion nor the RING finger mutation nor the ∆2KTM mutation in Rpb1 elicited any significant change in the H4K16 acetylation state at YFR057W, YNR077C, and YCL074W (Fig. 1F). This result demonstrates that the mechanism through which silenced genes are activated in Asr1 mutant cells differs from those connected to loss of classic Sir2-dependent silencing processes.
Ubp3 Associates with RNA Polymerase II via Asr1.
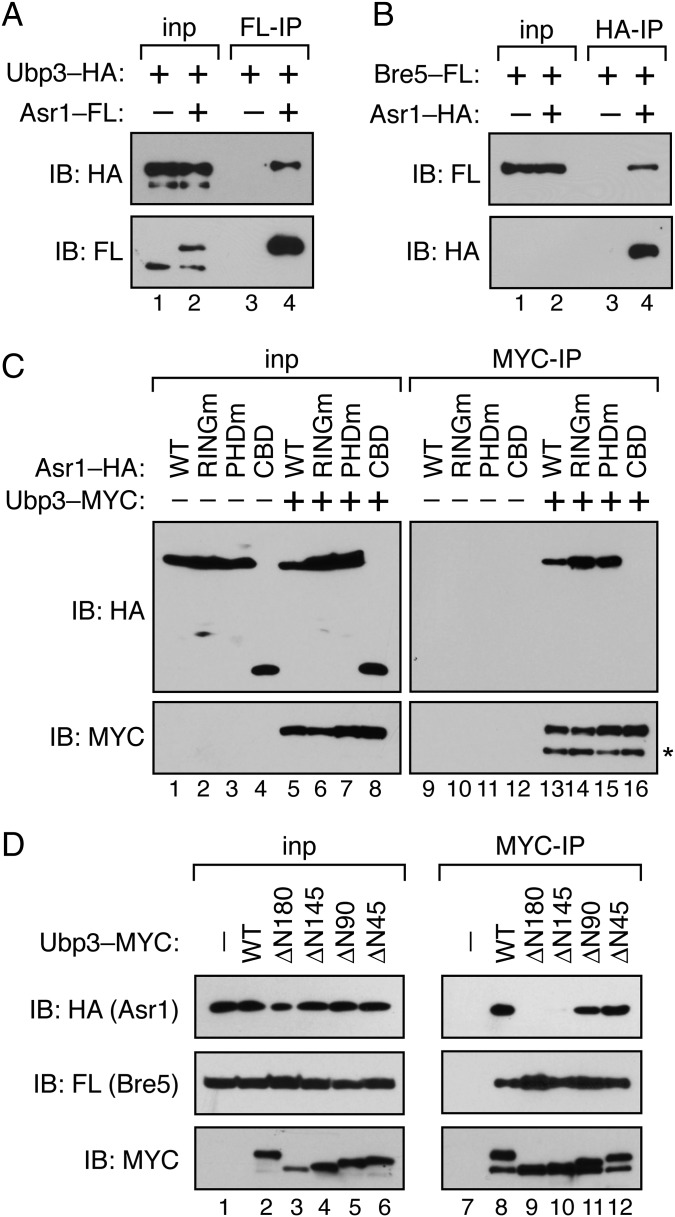
Compared with deletion of ASR1, mutation of the Asr1 RING finger has a much more substantial effect on activation of telomere-proximal genes (Fig. 1C). This differential effect could be due to a dominant-negative action by the Asr1RINGm protein, but it is also possible that Asr1 interacts with proteins that antagonize its role in repression of telomere-proximal genes. Previously, we reported that TAP-tagged Asr1 isolated from yeast cells associates with 10/12 core subunits of pol II, excluding Rpb4 and -7 (5). To identify additional proteins that associate with Asr1, we subjected an Asr1–TAP purification to further proteomic screening, comparing Asr1–TAP with a parallel purification of Rpb1–TAP (Table 1). Two proteins that were overrepresented in the Asr1–TAP purification were the Ub-specific protease Ubp3 and its essential cofactor Bre5. The selective enrichment of Ubp3 in the Asr1–TAP preparation is intriguing because Ubp3 is known to interact with pol II and to deubiquitylate Rpb1 (12), and because Ubp3 has been shown to function as an antisilencing factor in S. cerevisiae (13), albeit through an unknown mechanism. We confirmed that endogenous Asr1 associates with both Ubp3 (Fig. 2A) and Bre5 (Fig. 2B), demonstrating that Ubp3 and Bre5 are bona fide Asr1-interaction partners.
Table 1.
Validated proteins associated with Asr1-TAP
| Protein | Rpb1–TAP | Asr1–TAP | Asr1/Rpb1 |
| Rpb1 | 2,418 | 70 | 0.03 |
| Rpb2 | 2,066 | 58 | 0.03 |
| Rpb3 | 1,007 | 26 | 0.03 |
| Rpb4 | 526 | 4 | 0.01 |
| Rpb5 | 377 | 10 | 0.03 |
| Rpb6 | 202 | 3 | 0.01 |
| Rpb7 | 684 | 0 | 0.00 |
| Rpb8 | 88 | 2 | 0.02 |
| Rpb9 | 120 | 4 | 0.03 |
| Rpb10 | 338 | 2 | 0.01 |
| Rpb11 | 256 | 5 | 0.02 |
| Rpb12 | 34 | 0 | 0 |
| Asr1 | 1 | 20 | 20 |
| Ubp3 | 13 | 62 | 4.8 |
| Bre5 | 6 | 17 | 2.8 |
The table lists the total spectrum counts for each protein detected in the Asr1–TAP or Rpb1–TAP preparations, and the ratio of counts between them (Asr1/Rpb1). Only validated Asr1-associated proteins are presented in this list.
Fig. 2.
Asr1 associates with the Ubp3/Bre5 deubiquitylase. (A) Asr1 coprecipitates with Ubp3. Extract was prepared from cells expressing HA-tagged Ubp3, either alone (YTM1) or in conjunction with FLAG (FL)-tagged Asr1 (YTM2). Immunoprecipitation (IP) was performed with an anti-FLAG antibody (F-IP), and products were subjected to immunoblotting (IB) with anti-HA and -FLAG antibodies. For Ubp3–HA, 1% of the input (inp) to the IP was also analyzed by IB; for Asr1–FLAG, 7.5% of the input was analyzed. (B) Asr1 coprecipitates with Bre5. Extract was prepared from cells expressing FLAG (FL)-tagged Bre5, either alone (YTM3) or in conjunction with HA-tagged Asr1 (YTM4). IP was performed with an anti-HA antibody (H-IP), and products were subjected to IB with anti-HA and -FLAG antibodies. For Bre5–FL, 1% of the input (inp) to the IP was analyzed by IB; for Asr1–HA, 7.5% of the input was analyzed. (C) The amino terminus of Asr1 is required for association with Ubp3. Extract was prepared from yeast cells expressing galactose-inducible HA-tagged Asr1 proteins, either alone (WT, YTM9; Asr1RINGm, YTM10; Asr1PHDm, YTM11; CBD, YTM12) or in the presence of a plasmid expressing MYC-tagged Ubp3 (WT, YTM13; Asr1RINGm, YTM14; Asr1PHDm,YTM15; CBD, YTM16). IP was performed with an anti-MYC antibody (MYC-IP), and products were subjected to IB with anti-HA and -MYC antibodies. The * indicates a low molecular weight MYC-reactive species that we assume is a degradation product of Ubp3 that forms during the IP. (D) The amino terminus of Ubp3 mediates interaction with Asr1. Extract was prepared from yeast cells expressing galactose-inducible HA-tagged Asr1 and FLAG-tagged Bre5, either alone (–, YTM9) or in the presence of a plasmid expressing MYC–tagged Ubp3 proteins (WT, YTM13; ∆N180, YTM17; ∆N145, YTM18; ∆N90, YTM19; ∆N45, YTM20). IP was performed with an anti-MYC antibody (MYC-IP), and products were subjected to IB with anti-HA, -FLAG, and -MYC antibodies.
Next, we mapped the regions in Asr1 and Ubp3 that mediate their association (Fig. 2C). We found that interaction of Asr1 with Ubp3 does not depend on the integrity of the Asr1 RING or PHD fingers but does require the amino terminus of Asr1, because expression of the CBD alone is insufficient to support interaction with Ubp3. Within Ubp3, we showed that deletion of the amino-terminal 145 or 180 residues of Ubp3 (Fig. S2) disrupts interaction with Asr1 (Fig. 2D). Taking these data together, we conclude that the interaction between Asr1 and Ubp3 is likely mediated via amino-terminal sequences in both proteins.
Fig. S2.
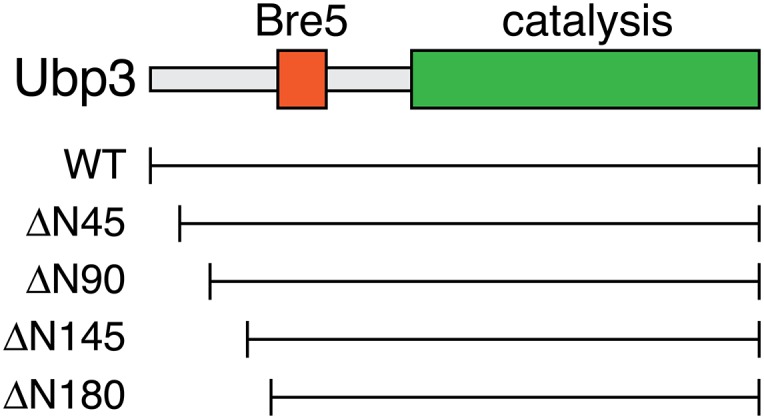
Schematic of Ubp3. Shown are the location of the domains required for interaction with Bre5 and for catalysis. Beneath the schematic is a scaled representation of the location of amino-terminal truncation mutants characterized for interaction with Asr1 in Fig. 2D.
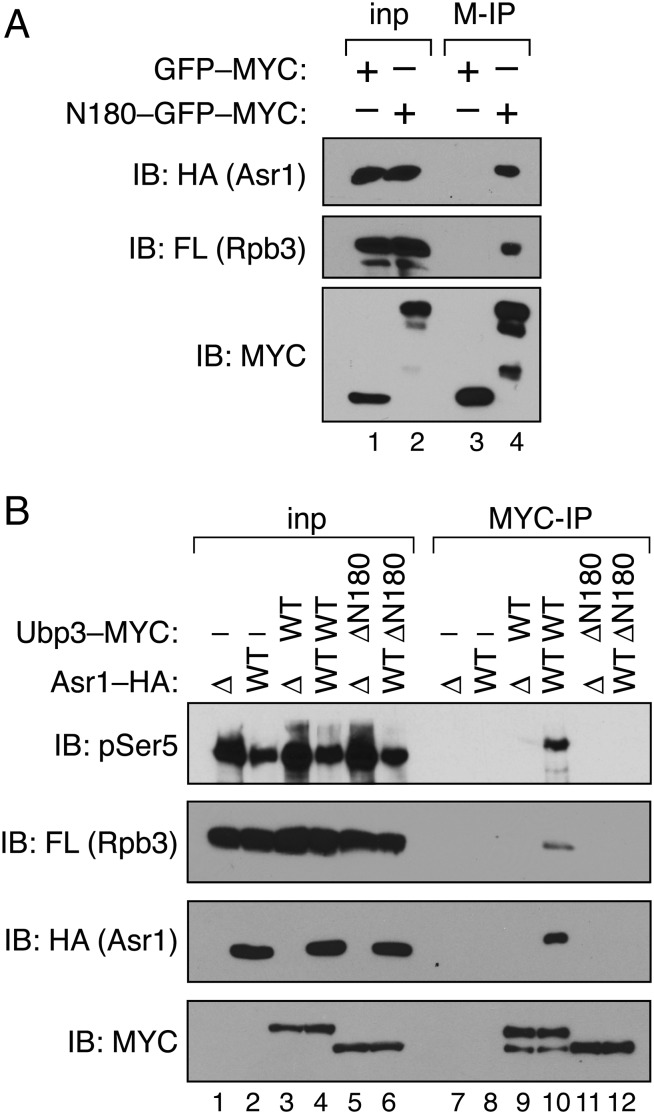
Because Asr1 and Ubp3 are both pol II-interaction partners, we considered that association of Asr1 and Ubp3 results from the simultaneous interaction of each protein with RNA polymerase II. This possibility, however, is not correct because the CBD of Asr1—which suffices for interaction with Ser5-phosphorylated Rpb1—does not associate with Ubp3 (Fig. 2C). We therefore considered an alternative model, in which association of Ubp3 with pol II is indirect and mediated via its interaction with Asr1. Three observations support this concept. First, the amino-terminal 180 residues of Ubp3, when fused to the green fluorescent protein (GFP), suffice for interaction with both Asr1 and the pol II subunit Rpb3 (Fig. 3A). Second, deletion of ASR1 blocks the ability of Ubp3 to interact with Rpb3 and S5-phosphorylated-Rpb1 (Fig. 3B, lane 9). And third, the ∆N180 mutant of Ubp3, which is defective for interaction with Asr1, is also defective for interaction with Rpb1 and Rpb3 (Fig. 3B, lane 12). The overlapping determinants within Ubp3 for Asr1 and pol II association, and the dependence of the Ubp3-pol II interaction on Asr1, reveal that interaction of Ubp3 with RNA polymerase II is mediated via Asr1.
Fig. 3.
Interaction of Ubp3 with RNA polymerase II is mediated via Asr1. (A) The amino terminus of Ubp3 suffices for interaction with Asr1 and pol II. Extract was prepared from cells expressing HA-tagged Asr1, FLAG-tagged Rpb3, and either MYC-tagged GFP alone (YTM43), or MYC-tagged GFP fused to the amino-terminal 180 residues of Ubp3 (YTM42). Immunoprecipitation (IP) was performed with an anti-MYC antibody (M-IP), and products were subjected to immunoblotting (IB) with anti-HA, -FLAG, and -MYC antibodies. For Rpb3–FLAG and Asr1–HA, 2.5% of the input was analyzed; for Ubp3–MYC, 0.1% of the input was analyzed. (B) Ubp3 requires Asr1 to interact with pol II. Extracts were prepared from yeast expressing FLAG-tagged Rpb3, and carrying combinations of (i) an ASR1 gene deletion (∆) or expression of WT HA-tagged Asr1, and (ii) WT MYC-tagged Ubp3 (WT) or the ∆N180 MYC-tagged Ubp3 mutant (lanes 1 and 7, YTM21; lanes 2 and 8, YTM22; lanes 3 and 9, YTM23; lanes 4 and 10, YTM24; lanes 5 and 11, YTM25; lanes 6 and 12, YTM26). IP was performed with an anti-MYC antibody (MYC-IP), and products were subjected to IB with anti-pSer5, -FLAG, -HA, and -MYC antibodies. For pSer5, Rpb3–FLAG, and Asr1–HA, 2.5% of the input was analyzed; for Ubp3–MYC, 0.1% of the input was analyzed.
Antagonistic Role of Asr1 and Ubp3 in Silencing.
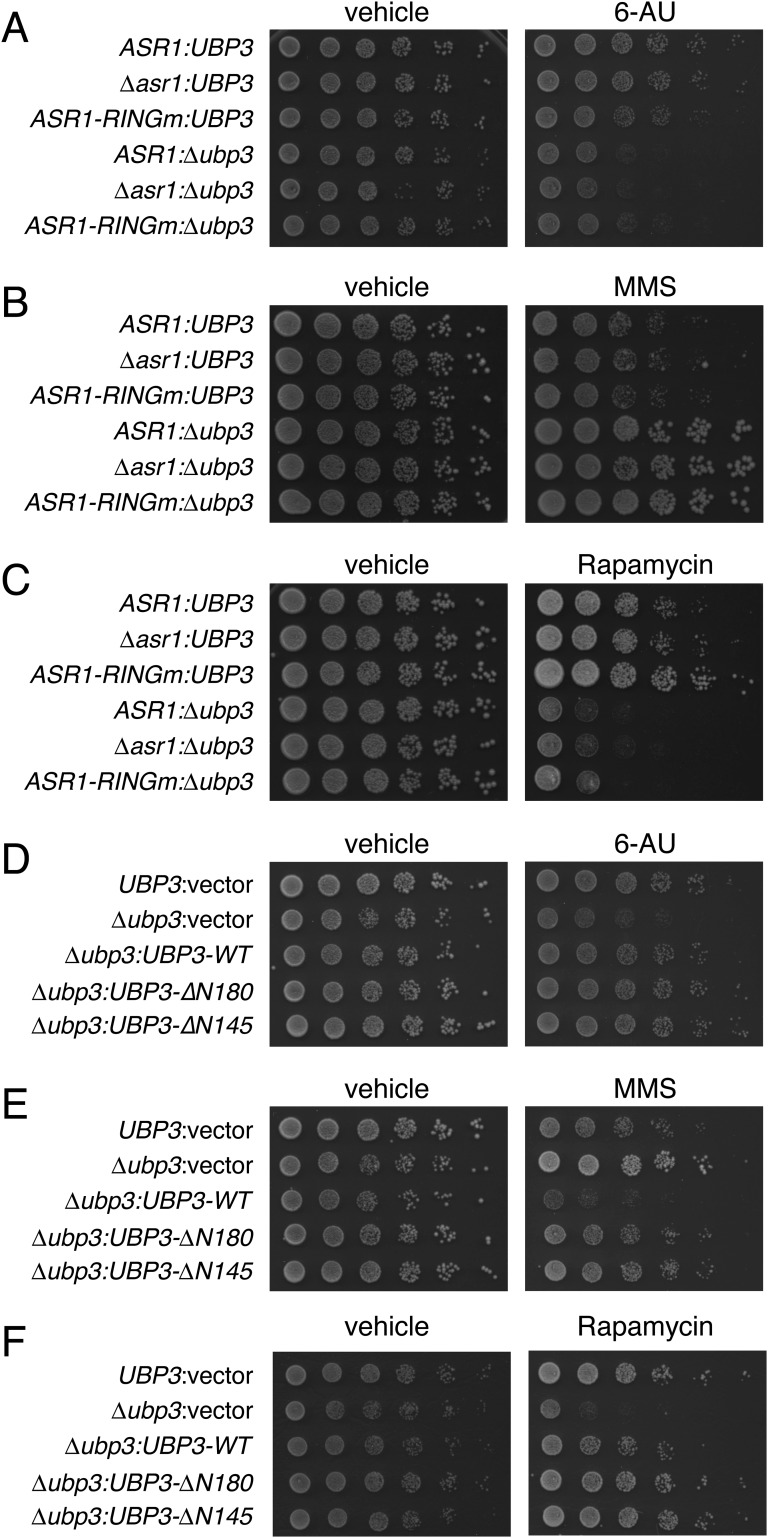
The opposing molecular functions of Asr1 and Ubp3 prompted us to ask whether these proteins play antagonistic roles in silencing. As controls, we asked whether other phenotypes associated with loss of UBP3 are impacted by mutations in Asr1, focusing on (i) increased sensitivity to 6-azauracil (6-AU) (12), (ii) increased resistance to the DNA-methylating agent methyl methanesulfonate (MMS) (14), and (iii) increased sensitivity to rapamycin (15). For all three agents, we reproduced the effects of UBP3 deletion but saw no synthetic interactions with an ASR1 deletion or RING finger mutation (Fig. S3 A–C). We also found that the ∆N145 and ∆N180 mutants of Ubp3 had WT activity in these assays (Fig. S3 D–F), revealing that interaction with Asr1 is not connected to the known functions of Ubp3 in these phenotypes.
Fig. S3.
Lack of genetic interaction between ASR1 and UBP3 in modulating sensitivity to chemical agents. (A–C) Analysis of interactions between ∆ubp3 and the ASR1 null or RING finger mutations. Fivefold serial dilutions of the indicated yeast strains (ASR1:UBP3, BY4741; ∆asr1:UBP3, YTM5; ASR1-RINGm:UBP3, YTM27; ASR1:∆ubp3, ∆Ubp3; ∆asr1:∆ubp3, YTM6; ASR1-RINGm:∆ubp3, YTM28) were spotted on plates containing (A) 150 μg/mL 6-AU, (B) 0.03% MMS, or (C) 5 nM Rapamycin, or relevant vehicle control, and grown for 2–3 d before being photographed. (D–F) Analysis of the impact of mutations in Ubp3 that disrupt interaction with Asr1. Fivefold serial dilutions of the indicated yeast strains (UBP3:vector, YTM44; ∆ubp3:vector, YTM45; ∆ubp3:UBP3-WT, YTM46; ∆ubp3:UBP3-∆N180, YTM47; ∆ubp3:UBP3-∆N145, YTM48) were spotted on plates containing (D) 100 μg/mL 6-AU, (E) 0.02% MMS, or (F) 1 nM Rapamycin, or relevant vehicle control, and grown for 2–3 d before being photographed. See Table S2 for strain details.
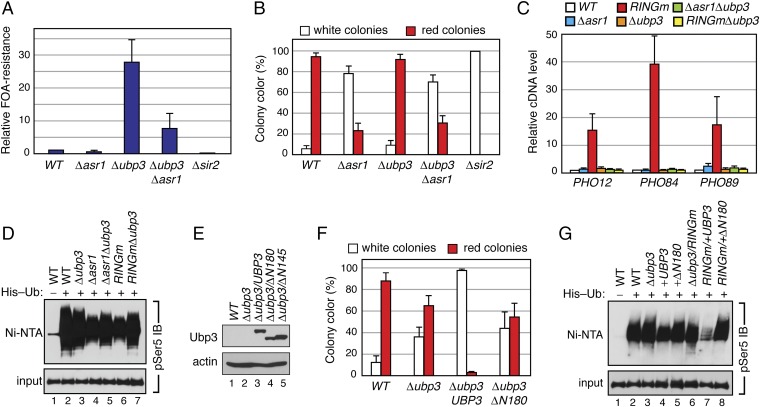
When we probed for silencing, however, we observed strong genetic interactions between UBP3 and ASR1. In the URA3 reporter assay, deletion of UBP3 resulted in a striking increase in the number of FOA-resistant colonies (Fig. 4A), consistent with reports that loss of Ubp3 increases telomere-proximal silencing (13). Importantly, simultaneous deletion of ASR1 reversed this increase, demonstrating that Asr1 opposes the function of Ubp3 in this context. Interestingly, the level of silencing of the URA3 reporter in ∆ubp3∆asr1 cells is still higher than in ∆asr1 cells, revealing that Ubp3 must have Asr1-independent functions that repress the silent chromatin state. In the ADE2 reporter strain, the majority of colonies were red in WT cells (Fig. 4B), making it impossible to observe any increase in silencing, but here we also found the extent of silencing to be significantly reduced in ∆ubp3∆asr1 cells, compared with the ∆ubp3 mutation alone. Direct analysis of transcript levels from the telomere-proximal PHO12, PHO84, and PHO89 genes revealed a similar pattern of behavior, with the induction of expression of all three genes observed in the presence of the Asr1RINGm mutant blocked by deletion of UBP3 (Fig. 4C). These data demonstrate that Asr1 and Ubp3 antagonistically control subtelomeric gene silencing, and that the association of these two proteins in this context is distinct from the function of Ubp3 in mediating sensitivity to 6-AU, MMS, and rapamycin.
Fig. 4.
Asr1 and Ubp3 act antagonistically in silencing of subtelomeric chromatin. (A) Deletion of ASR1 attenuates the impact of UBP3 deletion on silencing. Equal amounts of yeast (WT, LPY4819; ∆asr1, LPY4819 ∆Asr1; ∆ubp3, LPY4819 ∆Ubp3; ∆ubp3∆asr1, LPY4819 ∆Asr1 ∆Ubp3; ∆sir2, LPY4977) were plated on media with or without 5-FOA, and colonies were counted. Error bars represent SEM (n = 3). (B) Loss of ASR1 is epistatic to loss of UBP3. Yeast (WT, YPH499UTAT; ∆asr1, UTAT TM1; ∆ubp3, UTAT TM2; ∆asr1∆ubp3, UTAT TM3; ∆sir2, UTAT TM12) were plated on nonselective media (CSM), and the ratio of white and red colonies for each plate was calculated. Error bars represent SEM (n = 3). (C) Deletion of UBP3 reverses the impact of the Asr1RING mutation deletion on silencing. Total RNA was extracted from yeast (WT, BY4741; ∆asr1, YTM5; asr1RINGm, YTM27; ∆ubp3, ∆Ubp3; ∆asr1∆ubp3, YTM6; asr1RINGm∆ubp3, YTM28), and RT–QPCR was used to measure transcript levels from the indicated loci. The expression level of each gene in WT (WT) congenic cells was set to one. Error bars represent SEM (n = 8). (D) Asr1 and Ubp3 oppositely impact ubiquitylation of serine 5-phosphorylated Rpb1. Denaturing extracts were prepared from a WT yeast strain (lane 1, YTM31), or yeast expressing polyhistidine-tagged ubiquitin (His–Ub) and carrying the indicated genotype (WT, YTM32; ∆ubp3, YTM35; ∆asr1, YTM33; ∆asr1∆ubp3, YTM36; asr1RINGm, YTM34; asr1RINGm∆ubp3, YTM37). Ubiquitylated proteins were recovered on Ni-NTA resin. Levels of serine 5-phosphorylated Rpb1 in the Ni-NTA–bound material and a sample of the input were detected by immunoblotting with a pSer5-specific antibody (pSer5 IB). (E) Steady-state levels of Ubp3 deletion mutants assayed in F. Ubp3 was MYC-tagged and visualized by an anti-MYC IB. An antibody against actin was used as a loading control. (F). The amino terminus of Ubp3 is required for its prosilencing function. Yeast cells (WT, UTAT TM4; ∆ubp3, UTAT TM9; ∆ubp3UBP3, UTAT TM10; ∆ubp3∆N180, UTAT TM11) were plated to single colony density on leucine drop-out media, and the ratio of white and red colonies for each plate was calculated. Error bars represent SEM (n = 3). (G) The amino terminus of Ubp3 is required for its effects on ubiquitylation of serine 5-phosphorylated Rpb1. A His–Ub assay, as in D, was performed on a WT yeast strain (lane 1, YTM49) or yeast expressing polyhistidine-tagged ubiquitin (His–Ub) and carrying the indicated genotype (WT, YTM50; ∆ubp3, YTM51; +UBP3, YTM52; +∆N180, YTM53; ∆ubp3/RINGm, YTM54; RINGm/+UBP3, YTM55; RINGm/+N180∆, YTM56).
Consistent with the idea that at least part of the antagonistic actions of Asr1 and Ubp3 is mediated via Rpb1 ubiquitylation, we found that deletion of UBP3 partially reverses the decrease in the extent of ubiquitylation of S5-phosphorylated-Rpb1 observed in both ∆asr1 and asr1RINGm cells (Fig. 4D). The links between Ubp3 and Asr1 in this process are strengthened by the observation that overexpression of WT Ubp3, but not the Asr1 interaction-defective ∆N180 Ubp3 mutant (Fig. 4E), results in an almost complete loss of silencing in this context (Fig. 4F). Moreover, we found that overexpression of Ubp3 reduces the extent of ubiquitylation of S5-phosphorylated Rpb1 (Fig. 4G), a phenomenon that is more prominent in the presence of the catalytically inactive Asr1RINGm protein. Notably, in both cases, this action of Ubp3 is blocked by the ∆N180 mutation. Together, these data demonstrate that Asr1 and Ubp3 antagonistically control Rpb1 ubiquitylation and that the ability of Ubp3 to target S5-phosphorylated Rpb1 for deubiquitylation depends on its interaction with Asr1.
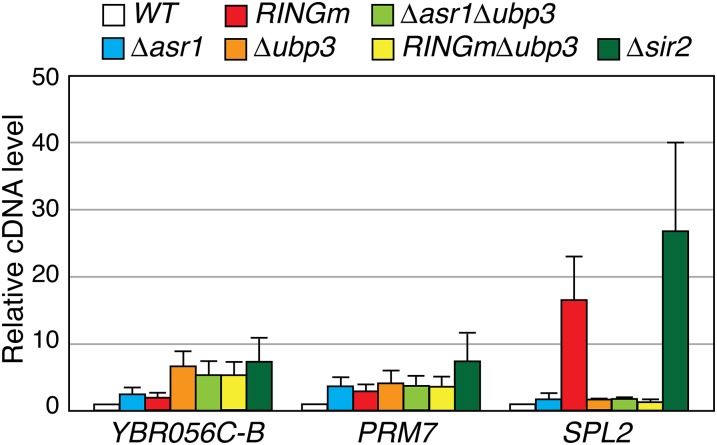
Many genes induced in response to the Asr1RING mutation are located far from telomeres. To determine whether the connection between Asr1 and Ubp3 extends beyond telomere-proximal genes, we selected three robustly induced euchromatic genes from Table S1 and asked how their expression is changed in response to mutations in both proteins (Fig. S4). At YBR056C–B and PRM7, we observed modest induction in the ∆asr1 and asr1RINGm strains, but these levels were not impacted by additional disruption of UBP3. At SPL2, however, we observed induction in the presence of the Asr1RING mutation, which was blocked upon subsequent deletion of UBP3. Interestingly, and unlike the other two loci, SPL2 expression was also induced by deletion of SIR2. Thus, despite lying distal to a telomere, SPL2 is under the control of the silent information regulators. The finding that the ostensibly euchromatic SPL2 gene is regulated by Asr1, Ubp3, and Sir2 in a manner similar to subtelomeric loci further strengthens the notion that Asr1 and Ubp3 act antagonistically to control the expression of silent chromatin.
Fig. S4.
Asr1 and Ubp3 control expression of a euchromatic gene also regulated by Sir2. Total RNA was extracted from yeast cells of the indicated genotype (WT, BY4741; ∆asr1, YTM5; asr1RINGm, YTM27; ∆ubp3, ∆Ubp3; ∆asr1∆ubp3, YTM6; asr1RINGm∆ubp3, YTM28; ∆sir2; ∆Sir2), and RT–QPCR was used to measure transcript levels from the indicated loci. ACT1 was used as control locus. The expression level of each gene in WT (WT) congenic cells was set to one. Error bars represent SEM (n = 7). See Table S2 for strain details.
Conclusions
Here, we have found that the ubiquitin-ligase activity of Asr1 is required for silencing of at least a subset of subtelomeric loci and have shown that part of this activity is mediated via ubiquitylation of Rpb1. We have also found that Asr1 recruits the deubiquitylating enzyme Ubp3 to pol II and that Asr1 and Ubp3 play antagonistic roles in controlling Rpb1 ubiquitylation and expression levels from subtelomeric chromatin. Based on our observations, we conclude that Asr1 and Ubp3 associate with one another, as demonstrated for other Ub-ligase/Ub-protease pairs such as BRCA1 and BAP1 (16). We suggest that Ubp3 is recruited to pol II via the CTD-binding ability of Asr1 and that the relevant enzymatic activities of each are directed against factors involved in silencing telomeric chromatin, one of which is the serine 5-phosphorylated Rpb1.
Our model for how Asr1/Ubp3 control transcription at telomere-proximal genes is as follows. We hypothesize that pol II that has initiated transcription at these sites is phosphorylated at serine 5 within the CTD. These phosphorylation events are then recognized by the CBD of Asr1, which leads to the recruitment of Asr1/Ubp3/Bre5. At this point, we propose that Asr1 ubiquitylates Rpb1—and likely other factors—the result of which is ejection of the Rpb4/7 heterodimer and termination of subtelomeric transcription. Although Rpb4/7 are dispensable for transcriptional elongation in vitro (6), this model is compatible with our observation that Asr1-associated pol II is catalytically inactive (5) and that transcriptional elongation by pol II in vivo can be stimulated by Rpb4/7 (17–19). After transcription is terminated, we suggest that corecruitment of Ubp3 with Asr1 allows rapid reversal of Rpb1 ubiquitylation, reassembly of the pol II complex, and restoration of polymerase function. In this way, nonproteolytic ubiquitylation of Rpb1 by Asr1 acts as a transient and reversible second tier mechanism for silencing telomere-proximal gene activity, over and above control via the silent information regulators.
Materials and Methods
Yeast Strains and Plasmids.
Yeast strains and plasmids are listed in Tables S2 and S3, respectively.
Table S2.
Yeast strains used in this study
| Strain | Genotype | Source |
| BY4741 | MATa, his3∆1, leu2∆0, lys2∆0, ura3∆0 | Open Biosystems |
| BY4742 | MATα, his3∆1, leu2∆0, lys2∆0, ura3∆0 | Open Biosystems |
| YTM1 | BY4741 but UBP3-3HA::KanMX6 | This study |
| YTM2 | YTM1 but ASR1-3FLAG::HIS3MX6 | This study |
| YTM3 | BY4741 but BRE5-3FLAG::HIS3MX6 | This study |
| YTM4 | YTM3 but ASR1-3HA::KanMX6— | This study |
| YTM5 | BY4741 but asr1∆::NatMX4 | This study |
| ∆Ubp3 | BY4741 but ubp3∆::KanMX6 | Thermo Scientific MATa deletion library |
| ∆Sir2 | BY4741 but sir2∆::KanMX6 | Thermo Scientific MATa deletion library |
| YTM6 | Ubp3∆ but asr1∆::NatMX4 | This study |
| YTM7 | YTM6 but BRE5-3FLAG::HIS3MX6 | This study |
| YTM8 | YTM6 but RPB3-3FLAG::HIS3MX6 | This study |
| YTM9 | YTM7 but +[pRS415 GPD] +[pYES2 HA-Asr1] | This study |
| YTM10 | YTM7 but +[pRS415 GPD] +[pYES2 HA-Asr1 RING] | This study |
| YTM11 | YTM7 but +[pRS415 GPD] +[pYES2 HA-Asr1 PHD] | This study |
| YTM12 | YTM7 but +[pRS415 GPD] +[pYES2 HA-CBD] | This study |
| YTM13 | YTM7 but +[pRS415 Ubp3-3MYC] +[pYES2 HA-Asr1] | This study |
| YTM14 | YTM7 but +[pRS415 Ubp3-3MYC] +[pYES2 HA-Asr1 RING] | This study |
| YTM15 | YTM7 but +[pRS415 Ubp3-3MYC] +[pYES2 HA-Asr1 PHD] | This study |
| YTM16 | YTM7 but +pRS415 Ubp3-3MYC] +[pYES2 HA-CBD] | This study |
| YTM17 | YTM7 but +[pRS415 Ubp3-3MYC N180∆] +[pYES2 HA-Asr1] | This study |
| YTM18 | YTM7 but +[pRS415 Ubp3-3MYC N145∆] +[pYES2 HA-Asr1] | This study |
| YTM19 | YTM7 but +[pRS415 Ubp3-3MYC N90∆] +[pYES2 HA-Asr1] | This study |
| YTM20 | YTM7 but +[pRS415 Ubp3-3MYC N45∆] +pYES2 HA-Asr1] | This study |
| YTM21 | YTM8 but +[pRS415 GPD] +[pYES2] | This study |
| YTM22 | YTM8 but +[pRS415 GPD] +[pYES2 HA-Asr1] | This study |
| YTM23 | YTM8 but +[pRS415 Ubp3-3MYC] +[pYES2] | This study |
| YTM24 | YTM8 but +[pRS415 Ubp3-3MYC] +[pYES2 HA-Asr1] | This study |
| YTM25 | YTM8 but +[pRS415 Ubp3-3MYC N180∆] +[pYES2] | This study |
| YTM26 | YTM8 but +[pRS415 Ubp3-3MYC N180∆] +[pYES2 HA-Asr1] | This study |
| YTM27 | BY4742 but asr1::C26A/C29A | (5) |
| YTM28 | YTM27 but ubp3∆::KanMX6 | This study |
| YTM29 | BY4742 but rpb1::K1452R/K1458R/K1487R ∆1720–1734 | (5) |
| LPY4819 | MATα hmr::TRP1 rDNA::ADE2-CAN1 TELVR::URA3 | (7) |
| LPY4819 ∆Asr1 | LPY4819 but asr1∆::NatMX4 | This study |
| LPY4819 ∆Ubp3 | LPY4819 but ubp3∆::KanMX6 | This study |
| LPY4819 ∆Asr1 ∆Ubp3 | LPY4819 but asr1∆::NatMX4 ∆ubp3::KanMX6 | This study |
| LPY4977 | LPY4819 but sir2∆::HIS3MX6 | (7) |
| YPH499UTAT | MATa ura3-52, lys2-801, ade2-101, trp1- 63, his3- 200, leu2- 1, TELVR::ADE2, TELVII-L::URA3 | (8) |
| UTAT TM1 | YPH499UTAT but asr1∆::NatMX4 | This study |
| UTAT TM2 | YPH499UTAT but ubp3∆::KanMX6 | This study |
| UTAT TM3 | UTAT TM1 but ubp3∆::KanMX6 | This study |
| UTAT TM4 | YPH499UTAT +[pRS415 GPD] | This study |
| UTAT TM5 | UTAT TM1 +[pRS415 GPD] | This study |
| UTAT TM6 | UTAT TM1 +[pRS415 HA-Asr1] | This study |
| UTAT TM7 | UTAT TM1 +[pRS415 HA-Asr1 RING] | This study |
| UTAT TM8 | UTAT TM1 +[pRS415 HA-Asr1 PHD] | This study |
| UTAT TM9 | UTAT TM2 +[pRS415 GPD] | This study |
| UTAT TM10 | UTAT TM2 +[pRS415 Ubp3-3MYC] | This study |
| UTAT TM11 | UTAT TM2 +[pRS415 Ubp3-3MYC N180∆] | This study |
| UTAT TM12 | YPH499UTAT but sir2∆::KanMX6 | This study |
| YTM31 | BY4741 +[pRS316] | This study |
| YTM32 | BY4741 +[pUB221] | This study |
| YTM33 | YTM5 +[pUB221] | This study |
| YTM34 | BY4741 but asr1::C26A/C29A/C66A/C69A +[pUB221] | This study |
| YTM35 | ∆Ubp3 but +[pUB221] | This study |
| YTM36 | YTM6 but +[pUB221] | This study |
| YTM37 | YTM34 but ubp3∆::KanMX6 | This study |
| Asr1-TAP | BY4742 but ASR1-TAP::KlURA3 | (5) |
| Rpb1-TAP | BY4741 but RPB1-TAP::HIS3MX6 | Open Biosystems |
| YTM38 | BY4742 but ASR1-DAM::KanMX6 | This study |
| YTM39 | BY4742 but asr1::C26A/C29A-DAM::KanMX6 | This study |
| YTM40 | BY4742 but ASR1pr-DAM::KanMX6 | This study |
| YTM41 | BY4741 but UBP3-3MYC::HIS3MX6 | This study |
| YTM42 | YTM8 +[pYES2 HA-ASR1] +[pRS415 N180Ubp3-GFP-3MYC] | This study |
| YTM43 | YTM8 +[pYES2 HA-ASR1] +[pRS415 GFP-3MYC] | This study |
| YTM44 | BY4741 +[pRS415 GPD] | This study |
| YTM45 | ∆Ubp3 +[pRS415 GPD] | This study |
| YTM46 | ∆Ubp3 +[pRS415 Ubp3-3MYC] | This study |
| YTM47 | ∆Ubp3 +[pRS415 Ubp3-3MYC N180∆] | This study |
| YTM48 | ∆Ubp3 +[pRS415 Ubp3-3MYC N145∆] | This study |
| YTM49 | BY4741 but +[pRS316] + [pRS415 GPD] | This study |
| YTM50 | BY4741 but +[pUB221 + [pRS415 GPD] | This study |
| YTM51 | ∆Ubp3 but +[pUB221] + [pRS415 GPD] | This study |
| YTM52 | ∆Ubp3 but +[pUB221] + [pRS415 Ubp3-3MYC] | This study |
| YTM53 | ∆Ubp3 but +[pUB221] + [pRS415 Ubp3-3MYC N180∆] | This study |
| YTM54 | YTM37 but +[pRS415 GPD] | This study |
| YTM55 | YTM37 but +[pRS415 Ubp3-3MYC] | This study |
| YTM56 | YTM37 but +[pRS415 Ubp3-3MYC N180∆] | This study |
The strain name, relevant genotype, and source are listed. Plasmids referred to are described in Table S3.
Table S3.
Plasmids used in this study
| Plasmid | Description | Source |
| pYES2 | GAL1pr/URA3/2µ vector | ThermoFisher |
| pYES2 HA-Asr1 | pYES2 but HA-ASR1 | (5) |
| pYES2 HA-Asr1 RING | pYES2 but HA-asr1 C26A/C29A/C66A/C69A | (5) |
| pYES2 HA-Asr1 PHD | pYES2 but HA-asr1 C143A/C146A/C186A/C189A | (5) |
| pYES2 HA-CBD | pYES2 but HA-asr1 N198∆ | (5) |
| pRS415 GPD | GPD1pr/LEU2/CEN vector | (26) |
| pRS415 Ubp3-3MYC | pRS415 GPD but UBP3-3Myc | This study |
| pRS415 Ubp3-3MYC N45∆ | pRS415 GPD but upb3-3Myc N45∆ | This study |
| pRS415 Ubp3-3MYC N90∆ | pRS415 GPD but upb3-3Myc N90∆ | This study |
| pRS415 Ubp3-3MYC N145∆ | pRS415 GPD but upb3-3Myc N145∆ | This study |
| pRS415 Ubp3-3MYC N180∆ | pRS415 GPD but upb3-3Myc N180∆ | This study |
| pRS415 HA-Asr1 | pRS415 GPD but HA-ASR1 | This study |
| pRS415 HA-Asr1 RING | pRS415 GPD but HA-asr1 C26A/C29A/C66A/C69A | This study |
| pRS415 HA-Asr1 PHD | pRS415 GPD but HA-asr1 C143A/C146A/C186A/C189A | This study |
| pRS316 | URA3/CEN | ATCC 77145 |
| pUB221 | CUP1pr::6xHIS-MYC-Ubi/TRP1 and URA3/2µ vector | (32) |
| pRS415 N180Ubp3-GFP-3MYC | pRS415 GPD but GFP-N180UBP3-3Myc | This study |
| pRS415 GFP-3MYC | pRS415 GPD but GFP-3Myc | This study |
RNA Sequencing and Analysis.
RNA from strains BY4742 and YTM27 was isolated by hot acidic phenol extraction. Ribosomal RNA reduction was performed, and RNA was fragmented and converted to cDNA. Library preparation was performed using the NEBNext DNA Library Prep Master Mix Set for Illumina (NEB). Then, 50 million single-end reads were obtained for each sample on an Illumina HiSeq2500 Sequencer. RNA-seq data are deposited at GEO (accession no. GSE72740).
Tandem Affinity Purification and Proteomics.
The tandem affinity purification (TAP) procedure was performed as described (5) using yeast strains Asr1–TAP and Rpb1–TAP. After the final elution step, proteins were concentrated by TCA precipitation, and samples were subjected to trypsin digestions and analysis using an 8-step MudPIT as described (20, 21). Peptide spectral data were searched against an S. cerevisiae protein database using Sequest (22).
Reporter Gene Assays for Subtelomeric Gene Silencing.
For URA3 reporter strains, logarithmic cultures of yeast were diluted, divided in two, and spread onto complete synthetic defined media (CSM) plates, or CSM plates containing 5-FOA. Resultant colonies were counted, and relative FOA resistance was calculated by dividing the number of colonies on CSM–FOA by the number on the CSM control media. For ADE2 reporter assays, cells were diluted and spread onto CSM (or CSM-Leu plates). The percentage colony color was determined by calculating the percentage of red and white colonies for each strain.
DNA Adenine Methyltransferase Identification.
DamID was performed as described (23), with minor modifications. Genomic DNA was isolated from three yeast strains: YTM38, which expresses a WT Asr1–Dam fusion; YTM39, which expresses a RING mutant Asr1–Dam fusion; and YTM40, which expresses Dam alone under the control of the ASR1 promoter. After purification, DNA was either undigested, or digested with DpnI. The extent of cleavage was determined by using QPCR to amplify gene-specific segments that include a DpnII site. The ratio of methylated/nonmethylated DNA for each site was calculated by determining the extent of DNA cleavage in Dam fusion strains, compared with the unfused Dam strain.
SI Materials and Methods
Construction of Yeast Strains.
Yeast strains used in this study are described in Table S2. Strains LPY4819 and LPY4977 were supplied by L. Pillus, University of California, San Diego, La Jolla, CA (7). Strain YPH499UTAT was provided by V. Zakian, Princeton University, Princeton, NJ (8). Epitope tagging was performed by PCR-mediated gene modification, using gene-specific primers to amplify the indicated epitope-tag/marker cassettes (24, 25). Gene deletions were performed via a similar method, except that primers with 50 bp of homology to sequences immediately flanking the ORF of each gene were used and only the selectable marker portion of the epitope-tag/marker cassettes was amplified. In both cases, PCR products were introduced into yeast cells via high-efficiency lithium acetate-mediated transformation, and stable transformants were selected. Correct integration/deletion was confirmed by PCR using gene-specific, or a combination of gene-specific and cassette-specific, primers. Where indicated, plasmids were introduced into yeast cells by high-efficiency lithium acetate-mediated transformation.
Plasmids and DNA Manipulations.
Plasmids used in this study are described in Table S3. pRS415 Ubp3–3MYC was made by PCR amplification of coding sequences of MYC epitope-tagged UBP3 from genomic DNA of strain YTM41 (Table S2) and cloning into the XhoI and SpeI sites of pRS415 GPD (26). Amino-terminal Ubp3 deletion mutants N45∆, N90∆, N145∆, and N180∆ were made in the pRS415 Ubp3–3MYC context via whole plasmid PCR. pRS415 N180Ubp3-GFP-3MYC was constructed using Gibson assembly with eGFP (pKT0127) (27) and pRS415 Ubp3-3MYC as a template. The pRS415 GFP-3MYC plasmid was made in the pRS415 N180Ubp3-GFP-3MYC context via whole plasmid PCR using primers designed to delete Ubp3 sequences. pRS415 HA–Asr1, pRS415 HA–Asr1 RING, and pRS415 HA–Asr1 PHD plasmids were made by PCR amplification of the coding sequences of HA–Asr1 from the relevant pYES2 HA–Asr1 vectors (5) and cloning into pRS415 GPD. The integrity of all recombinant plasmids was confirmed by DNA sequencing.
Antibodies.
The following antibodies and antibody conjugates were used: α-FLAG, M2-HRP (A8592; Sigma); M2 affinity gel (A2220; Sigma); α-MYC, 9E10 (Vanderbilt Molecular Biology Core); α-MYC-HRP (11 814 150 001; Roche); α-HA, 12CA5 (Cold Spring Harbor Monoclonal Shared Resource); α-HA-HRP (3F10; Roche); α-H4K16ac (07-329; Millipore); α-RNA polymerase II subunit B1 (phospho-CTD Ser-5) (04-1572; Millipore); α-Act1 (ab8224; Abcam); rabbit α-rat IgG HRP (PA128573; Thermo Fisher Scientific); and goat α-mouse IgG HRP (32430; Thermo Fisher Scientific).
RNA Isolation and RT-QPCR.
RNA was isolated from overnight 25-mL cultures of yeast by extraction with hot acidic phenol. After purification, cDNA was synthesized with reverse transcriptase and random hexamer primers and quantified with real-time QPCR. Actin primers were used as an internal control. Primer sequences were as follows: PHO12, GGTGGTTCTGGGCCATACTA and TTCACCGTGTCTACCAACCA; PHO84, GACCGCTTTGTTCTGTGTCA and TTGGACCGAAGTTTTGGAAG; PHO89, TTGCATTTTTGGATGCCTTT and GGGTCGTTGGTAAAAATGGA; ALD6, TGACACTGCTGAACCAGTCA and TGAATAGACCGGTTGGTTGC; 57W, GCCAAGCTTCCAATATCACGA and GGAATGATCTTGGAAATCGATCA; 77C, GCGGCCCCAAATATTGTAT and TGGTGGTGATTTTGTGGGTA; 74W, CAGATGGACGTTGACACTGC and AACCCGGGTGGTTGTTTTAC; and ACT1, GACGCTCCTCGTGCTGTCTT and GTCTTTTTGACCCATACCGACC.
RNA Sequencing and Analysis.
RNA from strains BY4742 and YTM27 was isolated by hot acidic phenol extraction. Ribosomal RNA reduction was performed using the Ribo-Zero Gold (Yeast) Kit (Epicenter), followed by RNA fragmentation and conversion to cDNA using the NEBNext First Strand, and Second Strand, Synthesis Modules (NEB). Library preparation was performed using the NEBNext DNA Library Prep Master Mix Set for Illumina (NEB). Fifty million single-end reads were obtained for each sample on an Illumina HiSeq2500 Sequencer (Illumina). Ribosomal RNA reduction, library preparation, and sequencing were performed by The Genomic Services Lab at Hudson Alpha. Two distinct biological replicates were analyzed. Data were quality controlled at multiple stages (28) during processing using QC3 (29). TopHat2 (30) was used to align reads to the sacCer2 reference genome. Cufflinks (31) was used to quantify gene expression and perform differential gene expression analysis. A false discovery rate of less than 0.05 was used as a significance threshold. RNA-seq data are deposited at GEO with accession number GSE72740.
Coimmunoprecipitation Assays.
One hundred-milliliter cultures of the relevant yeast were harvested, and lysates were prepared by bead beating in yeast lysis buffer (0.1% Nonidet P-40, 10 mM phosphate buffer, pH 8.0, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 0.1 mM Na3VO4), with protease inhibitors added fresh [per 50 mL of buffer: 1 Complete tablet (Roche), 130 µL of 0.5 M benzamidine (Sigma), and 500 µL of 0.1 M PMSF (Life Technologies)]. After a 3-h incubation with the relevant antibody, immune complexes were captured on Protein G Sepharose, washed extensively in yeast lysis buffer, and eluted by boiling in SDS/PAGE loading buffer. For α-FLAG immunoprecipitations, antibody-conjugated (M2) beads were used. In both cases, eluted material was resolved by SDS/PAGE, and proteins of interest were detected by immunoblotting. Samples of input for each immunoprecipitation were analyzed in parallel. Alternatively, where indicated in the text, total cell lysates were prepared by rapid alkali treatment lysis of parallel cultures and were used to monitor total steady-state protein levels.
Tandem Affinity Purification and Proteomics.
The tandem affinity purification (TAP) procedure was performed as described (5) using 4-L cultures of yeast strains Asr1–TAP and Rpb1–TAP grown in YPAD to an OD600 of ∼2.0. After the final elution step from calmodulin beads, proteins were concentrated by TCA precipitation, and half the samples were subjected to trypsin digestions and analysis using an eight-step MudPIT as described (20, 21). Peptide MS/MS spectral data were searched against a Saccharomyces cerevisiae protein database using Sequest (22), and the resulting identifications were collated and filtered using Scaffold (Proteome Software).
Reporter Gene Assays for Subtelomeric Gene Silencing.
For URA3 reporter strains, logarithmic cultures of yeast were serially diluted, divided in two, and spread onto control complete synthetic defined media (CSM) plates, or CSM plates containing 1 g/L 5-fluoroorotic acid (FOA). After growth for 2–3 d at 30 °C, colonies were counted on plates of equal dilution, and relative FOA resistance was calculated by dividing the number of colonies on CSM–FOA plates by the number on the equivalent CSM control plate. For ADE2 reporter assays, cells were serially diluted, spread onto CSM (or CSM-Leu plates), and grown for 2–3 d at 30 °C. Plates were stored at 4 °C for 2 d before red and white colonies on each were counted. The percentage of colony color was determined by calculating the percentage of red and white colonies for each strain.
Compound Sensitivity Assays.
Fivefold serial dilutions of logarithmic cultures of the indicated yeast strains were spotted onto yeast plates containing either 6-AU (Sigma), MMS (Sigma), or rapamycin (Life Technologies), or the relevant vehicle control, as indicated in the legend to Fig. S3. Plates were incubated for 2–3 d at 30 °C before being photographed.
In Vivo Ubiquitylation Assay.
Analysis of the ubiquitylation status of Rpb1 was performed using the His-tagged Ub method, essentially as described (5). Copper-inducible His-tagged Ub was expressed from the plasmid pUB221 (32).
Chromatin Immunoprecipitation Analysis.
ChIP assays were performed on logarithmic cultures of yeast as described (5). DNAs coprecipitating with the α-H4K16ac antibody were quantified by QPCR using gene-specific primers. Signals for each gene were normalized to that from a primer pair amplifying an intergenic sequence on the left arm of chromosome five (VL). Primer sequences were as follows: 57W, GCCAAGCTTCCAATATCACGA and GGAATGATCTTGGAAATCGATCA; 77C, GCGGCCCCAAATATTGTAT and TGGTGGTGATTTTGTGGGTA; 74W, TGAAGGCGAACATGGCTTAT and TTTAGGAGAGGGAGCAGCAA; ACT1, GACGCTCCTCGTGCTGTCTT and GTCTTTTTGACCCATACCGACC; and VL, AATCTATCGGCAAGTATGGGGTAGC and TCATTTACGTGCAGAGTGCAAGAAC.
DNA Adenine Methyltransferase Identification.
DamID was performed as described (23), with minor modifications. Briefly, genomic DNA was isolated from three yeast strains: YTM38, which expresses a WT Asr1–Dam fusion; YTM39, which expresses a RING mutant Asr1–Dam fusion; and YTM40, which expresses Dam alone under the control of the ASR1 promoter. After purification, DNA was either undigested or digested overnight with DpnII (NEB), which cleaves DNA only when its recognition site (GATC) is unmethylated. The extent of cleavage was determined by using QPCR to amplify gene-specific segments that include a DpnII site. The ratio of methylated/nonmethylated DNA for each site was then calculated by determining the extent of DNA cleavage in Dam fusion strains, compared with the unfused Dam expressed from the ASR1 promoter.
Acknowledgments
We thank A. Daulny, D. Finley, M. Funk, H. Madhani, L. Pillus, and V. Zakian for reagents. For advice, support, and comments, we thank C. Howard, S. Lorey, A. Weissmiller, and S. Wenzel. This work was supported by National Institutes of Health Grant GM067728 (to W.P.T.), Cellular and Molecular Microbiology Training Program Grant 5T32AI007611, and Vanderbilt Ingram Cancer Center Support Grant P30CA68485.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: RNA-seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE72740).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1518375113/-/DCSupplemental.
References
- 1.Geng F, Wenzel S, Tansey WP. Ubiquitin and proteasomes in transcription. Annu Rev Biochem. 2012;81:177–201. doi: 10.1146/annurev-biochem-052110-120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun ZW, Allis CD. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002;418(6893):104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- 3.Gwizdek C, et al. Ubiquitin-associated domain of Mex67 synchronizes recruitment of the mRNA export machinery with transcription. Proc Natl Acad Sci USA. 2006;103(44):16376–16381. doi: 10.1073/pnas.0607941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Svejstrup JQ. Rescue of arrested RNA polymerase II complexes. J Cell Sci. 2003;116(Pt 3):447–451. doi: 10.1242/jcs.00271. [DOI] [PubMed] [Google Scholar]
- 5.Daulny A, et al. Modulation of RNA polymerase II subunit composition by ubiquitylation. Proc Natl Acad Sci USA. 2008;105(50):19649–19654. doi: 10.1073/pnas.0809372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards AM, Kane CM, Young RA, Kornberg RD. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J Biol Chem. 1991;266(1):71–75. [PubMed] [Google Scholar]
- 7.Clarke AS, Samal E, Pillus L. Distinct roles for the essential MYST family HAT Esa1p in transcriptional silencing. Mol Biol Cell. 2006;17(4):1744–1757. doi: 10.1091/mbc.E05-07-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monson EK, de Bruin D, Zakian VA. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc Natl Acad Sci USA. 1997;94(24):13081–13086. doi: 10.1073/pnas.94.24.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezhkova E, Tansey WP. Proteasomal ATPases link ubiquitylation of histone H2B to methylation of histone H3. Mol Cell. 2004;13(3):435–442. doi: 10.1016/s1097-2765(04)00026-7. [DOI] [PubMed] [Google Scholar]
- 10.van Steensel B, Henikoff S. Identification of in vivo DNA targets of chromatin proteins using tethered dam methyltransferase. Nat Biotechnol. 2000;18(4):424–428. doi: 10.1038/74487. [DOI] [PubMed] [Google Scholar]
- 11.Suka N, Luo K, Grunstein M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet. 2002;32(3):378–383. doi: 10.1038/ng1017. [DOI] [PubMed] [Google Scholar]
- 12.Kvint K, et al. Reversal of RNA polymerase II ubiquitylation by the ubiquitin protease Ubp3. Mol Cell. 2008;30(4):498–506. doi: 10.1016/j.molcel.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Moazed D, Johnson D. A deubiquitinating enzyme interacts with SIR4 and regulates silencing in S. cerevisiae. Cell. 1996;86(4):667–677. doi: 10.1016/s0092-8674(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 14.Bilsland E, Hult M, Bell SD, Sunnerhagen P, Downs JA. The Bre5/Ubp3 ubiquitin protease complex from budding yeast contributes to the cellular response to DNA damage. DNA Repair (Amst) 2007;6(10):1471–1484. doi: 10.1016/j.dnarep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10(5):602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- 16.Jensen DE, et al. BAP1: A novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16(9):1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- 17.Runner VM, Podolny V, Buratowski S. The Rpb4 subunit of RNA polymerase II contributes to cotranscriptional recruitment of 3′ processing factors. Mol Cell Biol. 2008;28(6):1883–1891. doi: 10.1128/MCB.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verma-Gaur J, Rao SN, Taya T, Sadhale P. Genomewide recruitment analysis of Rpb4, a subunit of polymerase II in Saccharomyces cerevisiae, reveals its involvement in transcription elongation. Eukaryot Cell. 2008;7(6):1009–1018. doi: 10.1128/EC.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babbarwal V, Fu J, Reese JC. The Rpb4/7 module of RNA polymerase II is required for carbon catabolite repressor protein 4-negative on TATA (Ccr4-not) complex to promote elongation. J Biol Chem. 2014;289(48):33125–33130. doi: 10.1074/jbc.C114.601088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacCoss MJ, et al. Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci USA. 2002;99(12):7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez MN, et al. Obesity and altered glucose metabolism impact HDL composition in CETP transgenic mice: A role for ovarian hormones. J Lipid Res. 2012;53(3):379–389. doi: 10.1194/jlr.M019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yates JR, 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem. 1995;67(8):1426–1436. doi: 10.1021/ac00104a020. [DOI] [PubMed] [Google Scholar]
- 23.Leung A, et al. Histone H2B ubiquitylation and H3 lysine 4 methylation prevent ectopic silencing of euchromatic loci important for the cellular response to heat. Mol Biol Cell. 2011;22(15):2741–2753. doi: 10.1091/mbc.E11-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knop M, et al. Epitope tagging of yeast genes using a PCR-based strategy: More tags and improved practical routines. Yeast. 1999;15(10B):963–972. doi: 10.1002/(SICI)1097-0061(199907)15:10B<963::AID-YEA399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Funakoshi M, Hochstrasser M. Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast. 2009;26(3):185–192. doi: 10.1002/yea.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mumberg D, Müller R, Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156(1):119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 27.Sheff MA, Thorn KS. Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast. 2004;21(8):661–670. doi: 10.1002/yea.1130. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Ye F, Sheng Q, Clark T, Samuels DC. Three-stage quality control strategies for DNA re-sequencing data. Brief Bioinform. 2014;15(6):879–889. doi: 10.1093/bib/bbt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, et al. Multi-perspective quality control of Illumina exome sequencing data using QC3. Genomics. 2014;103(5-6):323–328. doi: 10.1016/j.ygeno.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaglom JA, Goldberg AL, Finley D, Sherman MY. The molecular chaperone Ydj1 is required for the p34CDC28-dependent phosphorylation of the cyclin Cln3 that signals its degradation. Mol Cell Biol. 1996;16(7):3679–3684. doi: 10.1128/mcb.16.7.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]