Significance
Recent National Academies of Sciences reports have summarized evidence to understand why US health indicators lag those of other countries, in an effort to explore the fundamental question of why some populations live longer than others. However, this literature has failed to engage in the potentially more striking comparisons with countries that outperform the United States despite wielding many fewer resources. Costa Rica is one of a handful of middle-income countries with data to conduct this comparison. The finding that socioeconomic inequality in mortality is wider in the United States than in Costa Rica, and that people lower on the hierarchy in Costa Rica live longer than people in the equivalent position in the United States, is startling.
Keywords: adult mortality, socioeconomic status, inequality, Costa Rica, United States
Abstract
Mortality in the United States is 18% higher than in Costa Rica among adult men and 10% higher among middle-aged women, despite the several times higher income and health expenditures of the United States. This comparison simultaneously shows the potential for substantially lowering mortality in other middle-income countries and highlights the United States’ poor health performance. The United States’ underperformance is strongly linked to its much steeper socioeconomic (SES) gradients in health. Although the highest SES quartile in the United States has better mortality than the highest quartile in Costa Rica, US mortality in its lowest quartile is markedly worse than in Costa Rica’s lowest quartile, providing powerful evidence that the US health inequality patterns are not inevitable. High SES-mortality gradients in the United States are apparent in all broad cause-of-death groups, but Costa Rica’s overall mortality advantage can be explained largely by two causes of death: lung cancer and heart disease. Lung cancer mortality in the United States is four times higher among men and six times higher among women compared with Costa Rica. Mortality by heart disease is 54% and 12% higher in the United States than in Costa Rica for men and women, respectively. SES gradients for heart disease and diabetes mortality are also much steeper in the United States. These patterns may be partly explained by much steeper SES gradients in the United States compared with Costa Rica for behavioral and medical risk factors such as smoking, obesity, lack of health insurance, and uncontrolled dysglycemia and hypertension.
Costa Rica has higher life expectancy than the United States even though its per capita income and its health expenditure are small fractions of those in the United States (1). The purpose of this article is to contribute to explaining why Costa Rica outperforms the US in life expectancy at older adult ages, which are the ages in which Costa Rican health achievements are most impressive (2). The article focuses on economic inequality, smoking, and obesity as important explanatory factors in this comparison, following related research that has shown that these are strong factors related to the relatively poor performance of life expectancy in the United States compared with other high-income countries (3). It also addresses the relative explanatory importance of health care systems and behavioral factors in the two countries.
Comparing health and mortality in these two countries may help to identify pathways for improving health even under suboptimal economic circumstances, as well as help to improve targeting of health interventions in high-income settings. A recent series of reports by the National Academy of Sciences Panel on Understanding Divergent Trends in Longevity in High-Income Countries have summarized an impressive evidence base that help to understand why US life expectancy lags those of other wealthy countries (3–5). However, this research literature has overlooked the potentially more striking comparisons with countries that wield many fewer resources. Costa Rica is one of a handful of middle-income countries in which the availability of data allows this type of more striking comparison.
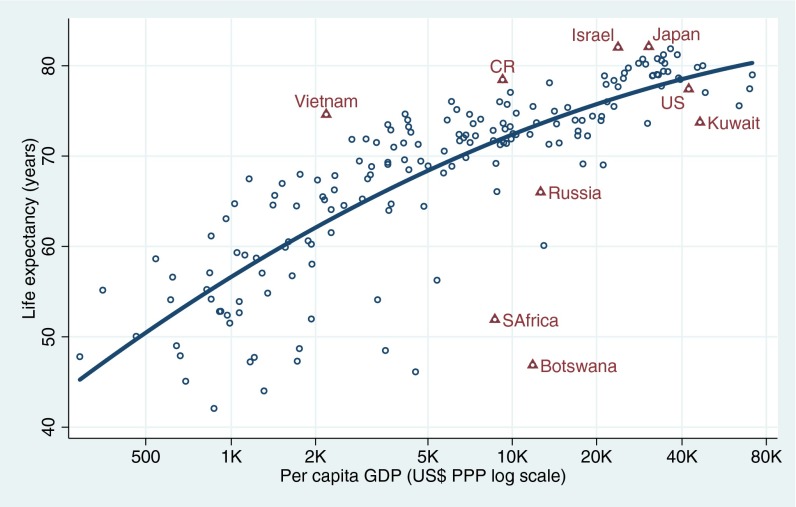
It is well established that economic development brings about higher life expectancy to countries (6). Although health technologies have allowed extraordinary health improvements independent of economic development (7), a strong relationship persists as shown in Fig. 1, which was built with World Bank data for the 5-y period before the Great Recession and with per capita gross domestic product purchasing power (GDP-PPP) as the indicator of economic well-being. Less prosperous countries with GDP of $1,000–2,000 per capita have life expectancies of about 60 y, whereas rich countries with about $40,000 GDP have life expectancies close to 80 y. The correlation is not perfect but it is high: 0.83 for the 178 countries in the figure. Particularly interesting are those countries whose health indicator substantially outperforms expectations—those above the prediction line in Fig. 1—in contrast with underachievers below the prediction line. Costa Rica, with a life expectancy at birth of 78.5 y, is a clear health overachiever given its GDP-PPP of $9,200, an income level at which the norm is a life expectancy of about 72 y according to the prediction curve and according to what is observed in countries such as Iran or Romania. The life expectancy in Costa Rica is at a level expected for economies with a GDP of about $40,000, closer to the United States, which slightly underachieves expectations with a life expectancy of 77.4 y, 1 y lower than Costa Rica (Fig. 1). Other overachievers identified in Fig. 1 are Vietnam, Israel, and Japan, whereas South Africa, the Russian Federation, and Kuwait are examples of clear underachievers.
Fig. 1.
Life expectancy by per capita GDP. World’s countries 2003–7. Data from ref. 1.
The outstanding health indicators of Costa Rica have been known for decades. The country was included, for example, as one of the four study cases in the 1985 Rockefeller Foundation report on ''Good Health at Low Cost” (8). Although historical data suggest that Costa Rica has had higher life expectancy than the Latin American average since the early 20th century, it is in the decade of the 1970s when the country essentially closed the gap in life expectancy with high-income countries (9).
Costa Rica, a small Central American country comparable to the states of South Carolina or Kentucky in territory and population size, is also known for being the oldest democracy in Latin America, with a government that invests substantially in social redistributive programs. These investments have in part been enabled by eliminating the financial burden of military expenditures after abolishing its armed forces in its 1949 Constitution (10–12). However, income distribution is not particularly egalitarian in Costa Rica. Its Gini index of 0.52 in 2012 is similar to other countries in Latin America (e.g., Mexico, 0.51; Chile, 0.52; Brazil, 0.53), which is the least egalitarian region in the world. The Costa Rican Gini is also higher than the US Gini of 0.40, which itself is higher than in most high-income countries such as Sweden (0.25), Germany (0.30), or Japan (0.32) (13).
In contrast to the low financial access and fragmented health care for low socioeconomic status (SES) adults under age 65 in the United States (after age 65, essentially all US residents are covered by Medicare insurance), Costa Rica has a single national health insurance system that covers the vast majority of residents. According to the 2011 census, 86% of Costa Ricans (96% of older adults) are covered by the public health insurance and care system known as the CCSS. The few uninsured individuals (largely a self-selected healthier group) can obtain health care from the CCSS for a subsidized fee or no fee if social workers verify that a patient has no means of paying. The high health insurance and health care coverage of Costa Rica is achieved at a fraction of the cost of health care in the United States: health expenditures per capita in the United States are about 10 times as high as in Costa Rica (1). Delivery of primary health care, particularly to remote or poor populations, has been singled out as a key factor to reduce mortality in Costa Rica (14). We do not readily have data to assess the relative quality of public health care services in Costa Rica compared with the United States, but effectiveness appears high in providing preventive and basic care such as vaccinations and in controlling traditional communicable diseases such as tuberculosis and malaria. However, the role of health system differences in explaining international differences in mortality is uncertain, with at best mixed research evidence. The recent National Research Council studies of US health in international comparative perspective have noted that many of the health differences may originate from social factors outside of the medical care system (5). Furthermore, although cardiovascular mortality is comparatively elevated in the United States, cardiovascular health care is generally better than in many other countries. Nevertheless, there are well-documented disparities in access to care in the United States for low-income populations; thus, it is plausible that the fragmented health care system could contribute to elevated mortality among low SES populations in the United States.
High health inequalities by SES, race, and geography have been singled out among the factors that explain the relatively poor performance of life expectancy in the United States (15). More precisely, low-SES individuals in the United States, African Americans, and residents in some areas, such as the District of Columbia, have very low levels of life expectancy that are unusual among developed countries. Other factors identified as dragging down life expectancy in the United States are smoking and, less clearly, obesity. These other health risks also have a strong SES gradient in the United States (16).
Earlier research has shown that in Costa Rica SES disparities in adult health are small, null, or even contrary to the expected negative SES gradients (17). This article systematically compares the SES gradients in adult mortality and health risk factors in newly compiled Costa Rican data with comparable data in the United States, namely the National Longitudinal Mortality Studies (NLMS) in the two countries, the Costa Rican Longevity and Healthy Aging Study (CRELES), and the US National Health and Nutrition Examination Survey (NHANES).
Results
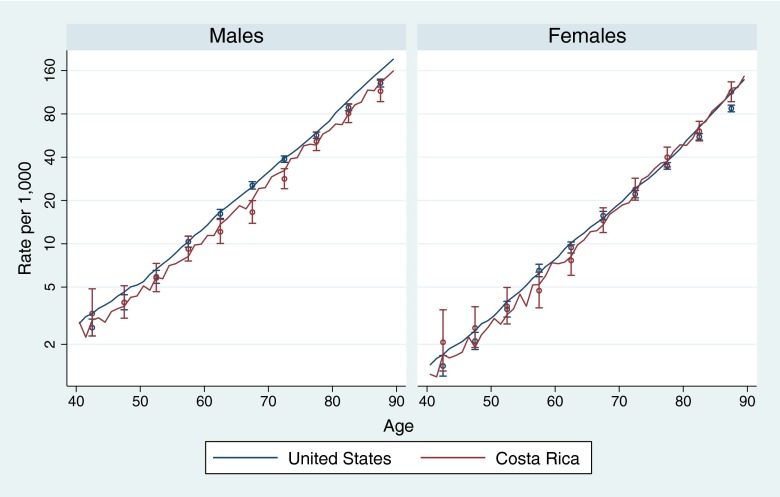
The NLMS samples estimate reasonably well the mortality of the population in both countries as shown by the overlap of the confidence intervals of the samples’ estimates with the population-based curve (18, 19) in most ages in Fig. 2. The exceptions are the US estimates after age 80, which are significantly lower than the population rates, possibly because of institutionalized individuals being excluded in the US-NLMS sampling.
Fig. 2.
Mortality by age and sex, population, and NLMS-sample estimates. United States and Costa Rica 1995–1999. Continuous lines show the rates in the population (data from refs. 18 and 19: vital statistics and population estimates). Points, with 95% CIs, show NLMS sample rates.
Fig. 2 also shows that male mortality is clearly lower in Costa Rica than in the United States at all adult ages above 55 y, according to both vital statistics and the NLMS samples. In contrast, the differences between the two countries in female mortality are less clear and vary by age and data source.
The NLMS samples show that Costa Rican male mortality is 0.85 (95% CI: 0.80–0.90) relative to the United States, or an 18% higher mortality rate in the United States (Table 1). Among females, mortality is an insignificantly 4% higher in Costa Rica. Distinguishing the rates before and after 65 y of age is of interest given that the Medicare plan in the United States begins universal health insurance only starting at this age (by contrast, Costa Ricans are eligible for universal insurance at all ages). The estimates for women are consistent with a relative mortality improvement after age 65 in the United States: women in the United States have 10% higher mortality than in Costa Rica before age 65 and 8% lower mortality after this age. However, this relative improvement in US health with age is not present (or is hidden by other factors) in the comparison of males: the mortality disadvantage of US men is larger (22% higher mortality) after age 65 than before this age (11%). Consistent with other evidence, this suggests that health insurance is not the major determinant of these patterns.
Table 1.
Death rate ratio of Costa Rica relative to United States by sex, age, and cause of death, and distribution of deaths by cause, 1990s
| Age and cause of death | Death rate ratio for Costa Rica/United States | Percent deaths | ||
| Males (95% CI) | Females (95% CI) | United States | Costa Rica | |
| All (40–89 y) | 0.85 (0.80–0.90) | 1.04 (0.97–1.10) | 100 | 100 |
| 40–64 y | 0.91 (0.82–1.01) | 0.91 (0.80–1.04) | ||
| 65–89 y | 0.82 (0.77–0.88) | 1.09 (1.01–1.17) | ||
| Cause of death | ||||
| Communicable | 0.80 (0.61–1.04) | 1.34 (1.01–1.78) | 5 | 5 |
| Lung cancer | 0.29 (0.22–0.38) | 0.17 (0.10–0.28) | 10 | 3 |
| Other cancer | 1.08 (0.96–1.22) | 1.09 (0.95–1.24) | 19 | 23 |
| Heart diseases | 0.65 (0.58–0.72) | 0.89 (0.79–1.00) | 36 | 28 |
| Cerebrovascular | 1.43 (1.17–1.75) | 1.42 (1.16–1.75) | 6 | 9 |
| Chro. respiratory | 1.06 (0.85–1.33) | 1.76 (1.39–2.22) | 5 | 7 |
| Diabetes | 1.35 (0.99–1.84) | 1.28 (0.93–1.77) | 3 | 4 |
| External injuries | 1.44 (1.16–1.79) | 1.65 (1.21–2.26) | 4 | 6 |
| Other | 1.09 (0.93–1.27) | 1.18 (1.00–1.40) | 12 | 14 |
Causes of Death.
Two cause-of-death groups explain all of the mortality advantage of Costa Rican men compared with the United States: lung cancer and heart disease. The death rate ratios (DRRs) in Table 1 indicate that US men have four times higher risk of dying by lung cancer and 54% higher risk of dying by heart diseases than Costa Rican men. If mortality by these two causes of death was the same in the two countries, Costa Rican men would have 13% higher general mortality than US men. Lung cancer is important as a marker of the mortality effect of smoking (20), and heart diseases are important due to causing about one-third of deaths at these ages. The large Costa Rican advantage in these two cause-of-death groups is in part counterbalanced by excess mortality of Costa Rican men by cerebrovascular conditions or stroke (DRR of 1.38), diabetes (1.30), and external injuries (1.34).
Costa Rican men also have a significant 28% lower mortality by communicable diseases (mostly influenza), possibly because of the more favorable weather of the tropics. However, this advantage has only a minor impact on life expectancy given that communicable diseases now represent only about 5% of deaths; the fact that Costa Rica has been able to essentially control its mortality by infectious diseases is a notable public health achievement. Not long ago, near three-fourths of Costa Rican deaths were caused by communicable diseases such as diarrhea, malaria, and tuberculosis (21).
Women in Costa Rican have a large advantage—larger than men—in lung cancer mortality, which is one-sixth that of the United States. However, women’s advantage in heart disease mortality (DRR of 0.85) is not as large as that of men (0.60). The advantage of Costa Rican women in these two causes of death is more than counterbalanced by significantly higher mortality by stroke (32% higher death rate), external injuries (59%), and chronic respiratory diseases (59%). The higher Costa Rican mortality by stroke among both men and women is a topic for further research. The higher mortality by external injuries comes from a poorer physical and institutional infrastructure to prevent and treat accidents. The higher female mortality by respiratory conditions is possibly a consequence of firewood cooking until recent times in rural areas.
There are no significant differences between Costa Rica and the United States in the mortality of the residual group of other causes of death, which includes ill-defined diseases. This result suggests that any concerns regarding differential quality across the two countries in coding cause of death is not driving the overall results.
Prevalence of Health Risk Factors.
The comparison of prevalence of selected health risk factors with data from CRELES and NHANES surveys (Table 2) yield clues about the proximate determinants of mortality and health in the two countries.
Table 2.
Prevalence of health risk factors. United States and Costa Rica circa 2010, ages 55–79 y by sex (age-adjusted percentages)
| Risk factors | Males | Females | ||
| United States | Costa Rica | United States | Costa Rica | |
| N | 2,072 | 1,580 | 2,119 | 2,214 |
| Total | 100 | 100 | 100 | 100 |
| Living arrangements | ||||
| Living alone | 14 | 8 | 23 | 8 |
| Couple | 60 | 23 | 55 | 28 |
| 3+ members | 26 | 68 | 22 | 65 |
| Uninsured* | 13 | 13 | 14 | 5 |
| Former smoker | 36 | 37 | 22 | 10 |
| Current smoker | 18 | 23 | 14 | 8 |
| BMI, kg/m2 | ||||
| Underweight <18.5 | 1 | 2 | 1 | 2 |
| Normal 18.5 – <25 | 21 | 36 | 26 | 25 |
| Overweight 25–29 | 41 | 43 | 31 | 39 |
| Obese 30+ | 38 | 20 | 41 | 34 |
| Central obesity† | 58 | 27 | 76 | 77 |
| Sedentary | 31 | 15 | 28 | 9 |
| High blood pressure | ||||
| No | 39 | 34 | 36 | 26 |
| Controlled | 39 | 21 | 40 | 26 |
| Uncontrolled | 23 | 45 | 24 | 48 |
| (Ratio)‡ | (1.7) | (0.5) | (1.6) | (0.5) |
| Dysglycemia | ||||
| No | 76 | 73 | 80 | 68 |
| Controlled | 7 | 9 | 7 | 9 |
| Uncontrolled | 17 | 18 | 13 | 23 |
| (Ratio)‡ | (0.4) | (0.5) | (0.5) | (0.4) |
| Dyslipidemia | ||||
| No | 38 | 53 | 35 | 33 |
| Controlled | 51 | 34 | 44 | 42 |
| Uncontrolled | 11 | 12 | 21 | 24 |
| (Ratio)‡ | (4.5) | (2.8) | (2.0) | (1.7) |
| Short telomeres | 29 | 24 | 25 | 17 |
| High CRP | 7 | 7 | 11 | 11 |
The lack of access to health care as measured by the proportion uninsured at ages 55–64 is similar for men in the two countries (13%) and lower for women in Costa Rica (5%) than in the United States (14%). The ratio of people with controlled to uncontrolled high blood pressure or dyslipidemia, which is higher in the United States than in Costa Rica, suggests that the high-coverage Costa Rican system is less effective in controlling these chronic conditions. However, the degree of control of diabetes is similar in the two countries, suggesting Costa Rican chronic disease management success in some domains. The proportion with very high levels of C-reactive protein (CRP), an objective marker of body inflammation in response to infections and other diseases, is also identical in the two counties: 7% for men and 11% for women.
Family support, in the form of living arrangements, appears higher in Costa Rica than in the United States. Although 23% of women ages 55–79 y live alone in the United States, only 8% live alone in Costa Rica. Moreover, in Costa Rica, most older adults (near 70% in this sample) live in households of three or more members, primarily with adult children, compared with only about 25% in the United States. Additionally, if the proportion with short telomeres in blood cells is taken as partially reflecting stress, the data suggest that Costa Ricans may live under less stressful circumstances than their US counterparts, especially women (17% vs. 25% short telomeres).
The lower Costa Rican prevalence of obesity, sedentariness, and dyslipidemia suggests healthier lifestyles, especially among men in this country. This lifestyle advantage is not present among Costa Rican women, who additionally show higher prevalence of high blood pressure and diabetes, which in part could originate in the high fertility levels that were common until recently in Costa Rica.
A key lifestyle factor that appears favorable in Costa Rica is smoking. Prevalence of past and current smoking is clearly lower among women in Costa Rica than in the United States (which also means lower secondhand smoking for men). Although there are not significant differences in the reported prevalence of smoking by men of the two countries, the substantially lower lung cancer mortality of Costa Ricans pointed out before suggests that smoking lifestyles (possibly including intensity of smoking and secondhand smoke consumption) are less harmful among men in Costa Rica than in the United States.
Inequality.
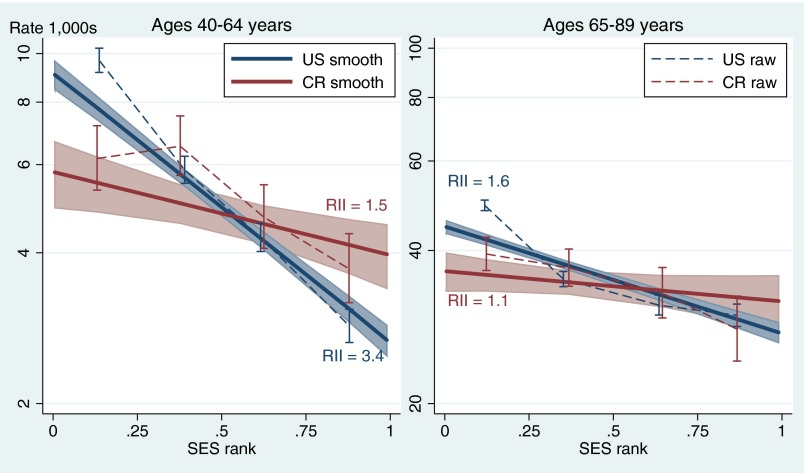
We rank individuals by sorting them out in each country according to their years of education and, as a second criteria, by their household income level (wealth in Costa Rica). Costa Ricans in the lowest SES rank quartile have significantly lower age-adjusted mortality than their counterparts in the United States (Fig. 3). By contrast, in the highest SES quartile the United States has lower mortality at ages below 65 y. In the two intermediate SES quartiles, there is no significant mortality difference between the two countries, nor is there in the highest quartile above age 65.
Fig. 3.
Mortality rates by SES quartiles and regression-estimated SES gradients. United States (US) and Costa Rica (CR) in the 1990s. RII, relative inequality index. Dashed lines connect the age-adjusted rates per SES quartile and their 95% CIs. Straight, continuous lines smooth out the SES-rank effect and the corresponding shadow areas show their CI. Data from refs. 40 and 43.
From a life expectancy standpoint, it is thus better to live in Costa Rica for low-SES individuals, whereas it is better to live in the United States for high-SES people younger than 65 y. This Costa Rican advantage is a striking result given the large differences in living standards between the two countries for individuals at the same relative SES ranks, especially at low-SES levels. Taking, for example, the most easily comparable indicator across countries—human capital as measured by educational attainment—the lowest SES quartile in the United States includes mostly high school dropouts, whereas in Costa Rica, it is composed of people with only 0–3 y of primary school. In the highest SES quartile, US adults are predominantly college graduates, whereas few of the corresponding Costa Ricans have education above high school. It is important to note, however, that this association does not imply that it is the education or income—the two components of our SES measure—itself that is exerting a direct causal influence on mortality; instead, the mortality risk could well be driven indirectly by other policy, environmental, or lifestyle risk factors that disproportionately affect low-SES populations.
The smoothed regression lines in Fig. 3 show that linear trends adequately represent the SES gradients on mortality, and thus we can use a single index—the relative inequality index (RII)—to describe the level of inequality in mortality in each population. The slope of the regression-adjusted lines in Fig. 3 measures the RII, which shows that mortality inequality is substantially higher in the United States than in Costa Rica, especially before age 65. Adults younger than 65 who are at the bottom of the SES rank die at a rate 3.4 times higher than those at the upper ranks of SES in the United States as measured by the RII. In contrast, the corresponding RII in Costa Rica is only 1.5. The US SES gradient in mortality falls substantially to an RII of 1.6 in ages 65 and over, whereas in Costa Rica, there is practically no gradient (RII of 1.1) at these ages.
Racial and geographical differences in such a big and diverse country as the United States could be confounding our estimates of SES-driven mortality inequality. We check this possibility by estimating the RII in the United States by ethnic groups and states and by controlling in regression models the effect of state of residence. The original RII of 3.4 estimated for adults below age 65 is similar when estimated just for the white non-Hispanic population (RII = 3.0). Similarly, the estimate is essentially unchanged after controlling for 49 state of residence dummy variables in the regression model (RII = 3.3). In the state of Hawaii, which has the highest life expectancy of the United States and some similarities with Costa Rica’s climate, the RII is still a very high 3.0. In Florida, another state with high life expectancy and some similarities with Costa Rica, the RII is 3.8. The one US subgroup within which a steep mortality gradient is not observed is the Hispanic population, which has an RII of 1.5, identical to Costa Rica.
Taking SES as a health risk factor and estimating its population attributable fraction (PAF) provides further information for public policy purposes. If mortality inequalities by SES quartiles were eliminated and the entire population had the mortality of the highest SES quartile, the national mortality rate would fall by 25% in the United States and 9% in Costa Rica, which would erase the entire adult mortality advantage of Costa Rica over the United States.
Table 3 shows the RII in the two countries disaggregated by sex and nine groups of cause of death. The RII falls to about half for older US adults ages 65–84 compared with ages 40–64, which is consistent with past studies showing that at older ages SES gradients in health are less steep (22, 23). Among Costa Ricans, both male and female, the RII before and after age 65 does not differ significantly; the reason for the differential RII effects by age in the two countries remains a topic for future research. Among Costa Rican men, the data show no significant SES gradient (the confidence interval overlaps 1), especially at older ages.
Table 3.
RII in mortality by age, sex, and cause of death for United States and Costa Rica in the 1990s
| Variable | United States (95% CI) | Costa Rica (95% CI) |
| Age 40–64 y | ||
| Males | 3.5 (3.0–4.0) | 1.3 (1.0–1.8) |
| Females | 3.8 (3.1–4.6) | 1.7 (1.1–2.6) |
| Age 65–89 y | ||
| Males | 1.8 (1.7–2.0) | 0.8 (0.7–1.1) |
| Females | 1.6 (1.4–1.8) | 1.5 (1.2–2.0) |
| Causes of death (age 40–89 y) | ||
| All causes | 2.1 (1.9–2.2) | 1.3 (1.1–1.5) |
| Communicable | 1.9 (1.5–2.5) | 1.5 (0.8–3.0) |
| Lung cancer | 2.8 (2.4–3.4) | 1.7 (0.7–3.7) |
| Other cancer | 1.5 (1.3–1.7) | 1.2 (0.9–1.6) |
| Heart diseases | 2.1 (1.9–2.4) | 1.0 (0.8–1.3) |
| Cerebrovascular | 1.7 (1.4–2.2) | 1.5 (1.0–2.4) |
| Chr. respiratory | 2.5 (2.0–3.3) | 2.5 (1.5–4.3) |
| Diabetes | 4.0 (2.7–6.0) | 1.5 (0.7–3.1) |
| External injuries | 1.8 (1.3–2.4) | 1.9 (1.1–3.4) |
| Other | 2.3 (1.9–2.7) | 1.0 (0.7–1.5) |
The SES gradients by cause of death, as measured by the RII, show that substantial inequality is present in the United States in all nine cause-of-death groups. By contrast, in Costa Rica, the RII is significantly higher than one only for three groups: cerebrovascular, external injuries, and, especially, chronic respiratory mortality. RII is significantly steeper in the United States than in Costa Rica only in mortality caused by heart diseases and diabetes. Lung cancer mortality inequality is also substantially higher in the United States, but the overlapping confidence intervals suggest that this could be result of chance alone.
Inequality in Health Risk Factors.
Table 4 compares inequality in the health risk factors whose prevalence we described before. The table shows the RII for the odds of being affected by each health risk taken as a binary condition. Being uninsured (ages 55–64) has by far the highest SES gradients in the two countries. Especially in the United States, those at the bottom of the SES rank have 26.3 times higher odds of being uninsured compared with those at the top of the SES rank. This OR is 3.9 in Costa Rica. The uninsured prevalence in the lowest SES quartile is 35% in the United States compared with 15% in Costa Rica, whereas it is about the same (about 5%) in the fourth SES quartile in the two countries.
Table 4.
RII in the odds of prevalence of selected health risk factors for United States and Costa Rica circa 2010, ages 55–79 y
| Health risk factor | United States (95% CI) | Costa Rica (95% CI) |
| Living alone | 1.4 (1.0–2.0) | 1.6 (1.0–2.8) |
| Uninsured | 26.3 (16-44) | 3.9 (2.2–7.1) |
| Current smoker | 6.0 (4.1–8.8) | 1.2 (0.8–1.8) |
| Obese (BMI 30+ kg/m2) | 1.7 (1.3–2.2) | 0.8 (0.6–1.7) |
| Centrally obese | 2.0 (1.4–2.8) | 0.8 (0.5–1.1) |
| Sedentary | 0.3 (0.2–0.4) | 0.2 (0.1–0.4) |
| Uncontrolled | ||
| Hypertension | 2.6 (1.9–3.5) | 1.3 (1.0–1.7) |
| Dysglycemia | 3.7 (2.5–5.4) | 0.9 (0.6–1.3) |
| Dyslipidemia | 0.9 (0.6–1.5) | 1.0 (0.7–1.4) |
| High CRP | 1.9 (1.2–3.0) | 1.6 (0.9–2.6) |
| Short telomeres | 1.4 (0.9–2.0) | 1.5 (0.9–2.4) |
Additionally, current smoking and uncontrolled high levels of blood sugar also show dramatic differences in the SES gradient in the two countries: no SES gradients in Costa Rica compared with steep gradients in the United States. Obesity and uncontrolled hypertension also show steeper SES gradients in the United States compared with Costa Rica, although the divergence is less severe than that observed for the proportions uninsured, smoking, and being dysglycemic. The different SES gradients usually are in a direction of relatively more favorable health of low-SES Costa Ricans compared with low-SES US adults. The exception is the proportion with uncontrolled high levels of blood sugar (dysglycemia), which is slightly lower in Costa Rica at low-SES levels, and it is substantially unfavorable in Costa Rica compared with the United States at high-SES levels (21% vs. 9% in the higher SES quartile).
Sedentariness is the only health risk in which low-SES individuals have a clear advantage over high-SES adults in the two countries. The RII of sedentariness is 0.29 in the United States and 0.23 in Costa Rica. If sedentariness is bad for health, it would be attenuating the health advantage that high-SES individuals have in many other health risk factors in the two countries.
The SES gradients in 5 of the 11 factors in Table 4 do not differ between the two countries and thus do not contribute to the higher mortality inequality observed in the United States; these are living alone, sedentariness, uncontrolled dyslipidemia, high inflammation, and short telomeres.
Discussion
We found that adult mortality of men is 18% higher in the United States than in Costa Rica, and this difference is larger among men older than 65 y. Among women, US mortality is 10% higher than in Costa Rica before age 65, but 8% lower after age 65. The simple fact that Costa Rica has achieved similar or lower adult mortality than the United States is a singular achievement considering the vastly higher living standards and health expenditures in the United States. This fact also provides a striking contradiction to expected cross-national SES gradients in mortality and strong evidence that the substantially lower life expectancy in many middle-income countries is not inevitable.
From the many factors associated with mortality levels in the two countries, SES gradients in mortality stand out as particularly different. At the highest SES quartile, adult mortality is higher in Costa Rica than in the United States, as expected considering the large advantages in income and health infrastructure of the United States. However, at the lowest SES quartile, Costa Rican mortality is substantially superior to that of the United States: a relatively poor person in Costa Rica has lower mortality than a relatively poor person in the United States. Our estimates of the PAF for SES suggest that as much as 40% of deaths of middle-aged adults in the United States and 20% of deaths at older ages are statistically attributable to SES gradients. The corresponding PAFs in Costa Rican men are 10% and 0%, respectively.
Why is health inequality lower in Costa Rica? It is not because of a more equal income distribution (i.e., shorter economic distances between SES extremes). Costa Rica’s Gini index of income inequality, for example, is 0.52 in 2012, which compares unfavorably to the US Gini of 0.40 (13).
One hypothesis is that it is related to the lifetime universal health insurance with excellent primary care access in Costa Rica, which provides a strong safety net for the poor in contrast to the high uninsurance rates among the poor before age 65 in the United States (24). The US reduction in health inequalities after age 65 is consistent with this hypothesis, although there are many competing clues beyond the scope of this paper that suggest that United States uninsurance is likely to be at most a limited factor (5). Furthermore, the Costa Rican system lacks capabilities to provide highly specialized health care, and it cannot even meet some basic standards as shown by the high prevalence of uncontrolled hypertension in Costa Rica compared with the United States. Nevertheless, the Costa Rican system has erased the typical disadvantage of developing societies regarding communicable disease mortality through widespread availability of cost-effective primary care and public health interventions, and this has helped raise overall Costa Rican life expectancy into the range of high income countries.
The story of health inequality is, however, more than just about access to health care. Other populations with universal health care systems still show high SES gradients in health outcomes (24). Furthermore, the Hispanic population in the United States shows modest SES gradients under the same health care system that produces high health inequality in the rest of society (25, 26). An important literature (27) postulates that psychosocial pathways link social hierarchy and health above and beyond material resources and access to health care. Those pathways involve concepts such as stress, control over life, insecurity, anxiety, social isolation, self-image, happiness, and depression. The magnitude of such factors in explaining broad mortality patterns is still poorly understood, however, and future work is needed to investigate the importance of these factors in explaining Costa Rican vs. US outcomes.
Further clues can be gleaned from examining relative gradients for different causes of death, as well as for key risk factors. We estimated substantial SES gradients in the United States in all cause-of-death groups and in many risk factors such as lack of insurance, smoking, obesity, and uncontrolled dysglycemia and hypertension. The existence of these multiple gradients is consistent with the “theory of fundamental causes” that postulates that SES acts through multiple pathways and on multiple disease outcomes because higher-SES individuals have more of the multiple resources (money, knowledge, power, prestige, connections, etc.) that allow them to avoid health risks or to obtain effective health care (28). However, this still begs the question as to why SES is less important in Costa Rica, where SES-driven inequality is substantially lower than in the United States in almost all of the studied dimensions.
A particularly informative finding is that the adult mortality advantage of Costa Rica over the United States concentrates primarily in two causes of death: lung cancer and heart disease. Lung cancer mortality is four times higher among men and six times higher among women in the United States compared with Costa Rica. Mortality by heart disease is 54% and 12% higher in the United States than in Costa Rica for men and women, respectively. These results point to smoking as an important explanatory factor of low adult mortality of Costa Rica, as it has been in other international comparisons of mortality (20, 29). Using the epidemiological method proposed by Peto et al. (30) to estimate smoking-related mortality under the assumption that lung cancer mortality is a reliable marker of the cumulative damage of smoking exposure, we estimate that if smoking was eradicated, the advantage in cardiovascular mortality of Costa Rica over the United States would disappear for men, and it would reverse into a 34% disadvantage for Costa Rican women. In turn, chronic respiratory mortality would be six times higher among Costa Rican women (instead of the observed 1.76 rate ratio) if smoking was eliminated in the two countries. In terms of remaining life expectancy at age 40, eradicating smoking would completely erase the observed advantage of almost 2 y of Costa Rican men, and it would amplify to 3 y the observed slight advantage of US women.
The data on self-reported smoking show, however, a paradox in the smoking effect: current and past reported exposure to smoking is significantly higher in the United States only among women. Reported exposure to smoking is about the same among men in the two countries. A similar paradox has been observed for the Hispanic population compared with other ethnic groups in the United States: substantially lower lung cancer mortality among Hispanic men but about the same reported past and present prevalence of smoking. This apparent contradiction has been explained by a duration and intensity of smoking that is lower among Hispanics (31, 32) and by a second-hand smoking exposure of Hispanic men that is substantially lower than non-Hispanics given the very low smoking prevalence of Hispanic women. The levels of cotinine (a marker of smoking exposure) in blood are substantially lower among Hispanic smokers than smokers of other ethnic groups in the United States: one-sixth of the level in whites and one-seventh of blacks (33). We do not have cotinine information for Costa Rica, but, given the similarity of outcomes, it is plausible to hypothesize that the low level of exposure of US Hispanic smokers could also occur among Costa Rican smokers.
Both the prevalence and SES gradient of obesity are also substantially lower in Costa Rica than in the US male population, suggesting that obesity might be another important part of the explanation of the favorable health outcomes of Costa Rica. Important international differences in life expectancy of high-income countries, and its time trend, have been linked to obesity (34). It must be noted, however, that the magnitude of obesity effects on mortality, especially at older ages, is a matter of controversy (35–37). To illustrate the magnitude of the possible effect of obesity, we estimate that mortality of US men would be 16% lower if they had the distribution by waist circumference observed in Costa Rica and if the all-cause mortality associated with central obesity was of the magnitude estimated in a meta-analysis of 18 longitudinal studies in white adults (38). Under those assumptions, the differences in central obesity would be sufficient by itself to statistically explain all of the Costa Rican male mortality advantage.
Thus, overall, an important part of the higher mortality of low-SES individuals existing in the US appears to be linked to unhealthy lifestyle factors, including smoking and obesity.
A known limitation of estimates of SES effects on health is reverse causation: some individuals fall into low-SES ranks because of their poor health. If that was the case, then the high negative SES association with poor health in the United States might be spurious. However, substantial research suggests that the effects are not spurious, but rather may reflect long-term effects of low SES that operate via myriad pathways (39).
Using relative SES rank scales in each country may also lead to different inferences than when using an absolute socioeconomic indicator. To check the sensitivity of our results to this choice, we estimated the effect of education as an absolute scale for the two countries. Fourteen fewer years of education—the approximate entire rank of this indicator—would increase US mortality by a factor of 2.9 and 1.6 in ages 40–64 and 65–89, respectively. These figures are similar to the RII estimated with the relative SES scale: 3.4 and 1.6. The effects of 14 y of education in the two age groups in Costa Rica mortality are 1.5 and 1.2, identical to those estimated with the SES-rank relative scale. This coincidence suggests that differential distributions of the population with very high or very low SES have not skewed overall inferences from the relative inequality measure used in our main analysis.
Known limitations of adult mortality data in developing countries are age exaggeration of older individuals and underregistration of deaths. The data used in this article for Costa Rica should be virtually free of those limitations because (i) age was established from the date of birth in the national registry and (ii) the follow-up of deaths double checked survival with independent sources: the voting lists in the NLMS and household visits in the CRELES (40, 41). Two limitations in the databases used in this article are (i) the exclusion of institutionalized individuals in the US samples, which would slightly underestimate US mortality, and (ii) the exclusion of foreigners (about 4% of the population) in the Costa Rica follow-up, which might slightly overestimate mortality if we believe that immigrants are a select group with better than average health (42). The effect of these two limitations would be, however, to understate the overperformance of Costa Rican mortality.
Although this article compares individuals in the same age range in the two countries, it is worth noting that they may have had substantially different life course experiences, especially considering that there was a huge gap in life expectancy and health between the two countries until the 1970s. Survival selection of Costa Rican adults is much higher than that of US adults. If this is a factor that has given an advantage to elderly Costa Ricans, such advantage would disappear in the future when cohorts with similar health experiences in the two countries reach older ages.
Data and Methods
Data Used.
Mortality estimates and its SES gradients in this article are based on parallel NLMS conducted in the United States and Costa Rica by linking large samples of individual-level census data (including SES variables) to the death registries. For the United States, we use version 4 of the US-NLMS public use file (43), which consists of a 6-y follow-up, starting in 1992, of a sample of noninstitutionalized individuals in the Current Population Survey (CPS). For Costa Rica, we use a CR-NLMS that we created from a probabilistic sample of adults in the 1984 census (40). We restrict Costa Rican observations to the 12-y follow-up period starting in January 1990, so the observation period in both countries is centered in 1996. We refer to the follow-up window as the 1990s period. To minimize the possibility of death underregistration errors, the follow-up of deaths in Costa Rica included survival checks against the voting lists for the presidential elections conducted every 4 y starting in 1990. Only Costa Rican nationals (96% at the study’s ages) were included in the Costa Rican data.
The NLMS analysis is restricted to ages 40–89 y. The lower age limit is determined by the Costa Rican sample. The upper age limit is determined by the lack of single year of age data in the public use US-NLMS files. Because the US-NLMS sample does not include the institutionalized population, our upper age limit also reduces the bias from this exclusion that mostly affects very old individuals.
The analytical NLMS sample sizes differ substantially for the two countries: 288,000 in the United States and 17,500 in Costa Rica. The number of observed deaths in the analysis period is 22,440 in the US sample and 2,415 in Costa Rica.
We analyze all-cause mortality and mortality by nine large groups of causes, namely (i) communicable diseases (which also includes HIV and acute respiratory diseases), (ii) lung cancer, (iii) other cancer, (iv) heart diseases (mostly myocardial infarction and chronic ischemic heart disease), (v) cerebrovascular diseases (mostly stroke), (vi) chronic respiratory diseases (mostly emphysema), (vii), diabetes mellitus, (viii) external injuries (accidents, homicide, and suicide), and (ix) a residual group of other causes.
We complement the mortality analysis with a comparison of key health risk factors with data from comparable nationally representative health surveys publicly available: NHANES 2007–2010 in the United States (44) and CRELES in Costa Rica (45). We used the information from individuals aged 65 or more interviewed in the second CRELES wave of interviews conducted mostly in 2007, as well as the data from the retirement cohort of individuals (ages 55–64), interviewed mostly in 2011. The analytical sample sizes in these two datasets were ∼4,000 in each country, ages 55 (the minimal age of individuals in CRELES) to 79 y (the highest age with detailed death information in NHANES).
Definition of Variables.
Based on previous literature, a range of health risk and behavioral factors are compared using the NHANES and CRELES samples: living arrangements as indicator of family support, whether uninsured, smoking (never, former, current), body mass index (BMI) standard classification (underweight, normal, overweight, and obese), abdominal girth (if waist circumference is more than 102 cm in males and 88 cm in females), sedentariness (report of usually being seated more than 8 h/d), three categories (none, controlled, and uncontrolled if biomarker level is fine but there is medical diagnosis of the disease) of high blood pressure (cutoff: 140/90 mmHg systolic/diastolic), dysglycemia (cutoff: 6.5% HbA1c) and dyslipidemia (cutoff: 240 mg/dL total cholesterol), high CRP (cutoff at 1.0 mg/dL) levels as indicator of inflammation in response to recent infection or heart diseases, and leukocyte telomere length [cutoff at 0.8 relative telomere single copy gene (T/S) ratio] as potential marker of stress and cellular aging.
Given the large differences in the absolute values of SES indicators in the two countries such as education or income, we define a relative scale that facilitates comparisons across countries: the SES rank of individuals in each country as measured by their relative position in each sample after they are sorted by years of attained education and, within each education category, quintiles of income in the US or household wealth in Costa Rica. (An alternative approach would have been to compare mortality across the two countries at any given SES level; e.g., comparing mortality across countries for those with 9–11 y of education, then for 12 y, etc. A key drawback of such an approach is that the selection into and meaning of a given education level can also be very different in the two contexts, thus undermining the attempt to standardize absolute SES level. For example, those with 9–11 y of education in Costa Rica are among the relatively advantaged, whereas this group in the United States is highly disadvantaged.)
The age of individuals in the Costa Rica data was determined using the exact date of birth as recorded in the civil registration system (which was linked to the surveys with the unique identification number of the Costa Rican ID card). This procedure to determine age minimizes the possibility of self-reported age errors that might distort mortality rates or other indicators for elderly individuals. The US databases use self-reported ages.
Statistical Analysis.
After splitting the NLMS databases by single-year age segments during the survival follow-up periods, we compute death rates using as the denominator the exact count of the number of person-years of exposure in the surveys. We model age-adjusted death rates and death rate-ratios using Poisson regression models, assuming that mortality grows exponentially with age—i.e., a Gompertz distribution—which is a reasonable assumption for human populations in these ages (46).
To compare the prevalence of health risk factors in the two countries, we estimate age-adjusted proportions standardized using the US age distribution.
The effect of the SES rank (on a 0–1 scale) on mortality rates or in the odds of risk factors is an estimate of the so-called RII, or how many times higher the mortality is at the lowest SES rank compared with the highest SES rank (47). We estimate the RII using Poisson regression models for death rates and logistic regression models for the probability of having each health risk factor. All models control the effect of age as a continuous variable and its square to allow nonlinear age effects.
Acknowledgments
We acknowledge support from National Institute of Aging Grants P30AG012839 and R01AG031716. The US Census Bureau provided the US NLMS data used in this article.
Footnotes
The authors declare no conflict of interest.
References
- 1.World Bank 2013 World development indicators. Available at databank.worldbank.org/data/reports.aspx?source=world-development-indicators. Accessed October 16, 2013.
- 2.Rosero-Bixby L. The exceptionally high life expectancy of Costa Rican nonagenarians. Demography. 2008;45(3):673–691. doi: 10.1353/dem.0.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Research Council . Explaining Divergent Levels of Longevity in High-Income Countries. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 4.National Research Council . International Differences in Mortality at Older Ages: Dimensions and Sources. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 5.National Research Council & Institute of Medicine . U.S. Health in International Perspective: Shorter Lives, Poorer Health. National Academies Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 6.World Bank . World Development Report 1993. Investing in Health. World Bank; Washington, DC: 1993. [Google Scholar]
- 7.Preston SH. The changing relation between mortality and level of economic development. Popul Stud (Camb) 1975;29(2):231–248. [PubMed] [Google Scholar]
- 8.Halstead SB, Walsh JA, Warren KS. 1985. Good Health at Low Cost: Proceedings of a Conference Held in Bellagio, Italy (Rockfeller Foundation, New York)
- 9.Rosero-Bixby L. Socioeconomic development, health interventions and mortality decline in Costa Rica. Scand J Soc Med Suppl. 1991;46(46):33–42. [PubMed] [Google Scholar]
- 10.Seligson MA. Peasants of Costa Rica and the Development of Agrarian Capitalism. Univ of Wisconsin Press; Madison, WI: 1980. [Google Scholar]
- 11.Hanson LÅ, Köhler L. Peace, Health, and Development: A Nobel Seminar Held in Göteborg, Sweden, December 5, 1991. Univ of Göteborg; Göteborg: 1993. [Google Scholar]
- 12.Mesa-Lago C. Market, Socialist, and Mixed Economies: Comparative Policy and Performance–Chile, Cuba, and Costa Rica. Johns Hopkins Univ Press; Baltimore: 2000. [Google Scholar]
- 13.Underwood E. A world of difference. Science. 2014;344(6186):820–821. doi: 10.1126/science.344.6186.820. [DOI] [PubMed] [Google Scholar]
- 14.Rosero-Bixby L. Infant mortality in Costa Rica: Explaining the recent decline. Stud Fam Plann. 1986;17(2):57–65. [PubMed] [Google Scholar]
- 15.Dow WH, Rehkopf D. Socioeconomic gradients in health in international and historical context. Ann NY Acad Sci. 2010;1186(1):24–36. doi: 10.1111/j.1749-6632.2009.05384.x. [DOI] [PubMed] [Google Scholar]
- 16.Crimmins EM, Preston SH, Cohen B. Explaining Divergent Levels of Longevity in High-Income Countries. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 17.Rosero-Bixby L, Dow WH. Surprising SES Gradients in mortality, health, and biomarkers in a Latin American population of adults. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):105–117. doi: 10.1093/geronb/gbn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.University of California Berkeley & Max Planck Institute for Demographic Research 2014 Human mortality database. Available at www.mortality.org. Accessed June 23, 2014.
- 19.Centro Centroamericano de Población 2014 Tablas de vida completas quinquenales. Available at ccp.ucr.ac.cr/observa/CRindicadores/TVcompletas.html. Accessed March 4, 2013.
- 20.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 2010;39(2):430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mata L, Rosero-Bixby L. National Health and Social Development in Costa Rica: A Case Study of Intersectorial Action. Pan American Health Organization; Washington, DC: 1988. [Google Scholar]
- 22.Crimmins EM. Socioeconomic differentials in mortality and health at the older ages. Genus. 2005;LXI(1):163–178. [Google Scholar]
- 23.Elo IT, Preston SH. Educational differentials in mortality: United States, 1979-85. Soc Sci Med. 1996;42(1):47–57. doi: 10.1016/0277-9536(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 24.Marmot MG, et al. Health inequalities among British civil servants: The Whitehall II study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 25.Turra CM, Goldman N. Socioeconomic differences in mortality among U.S. adults: Insights into the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. 2007;62(3):S184–S192. doi: 10.1093/geronb/62.3.s184. [DOI] [PubMed] [Google Scholar]
- 26.Lariscy JT, Hummer RA, Hayward MD. Hispanic older adult mortality in the United States: New estimates and an assessment of factors shaping the Hispanic paradox. Demography. 2015;52(1):1–14. doi: 10.1007/s13524-014-0357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marmot M, Wilkinson RG. Psychosocial and material pathways in the relation between income and health: A response to Lynch et al. BMJ. 2001;322(7296):1233–1236. doi: 10.1136/bmj.322.7296.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. J Health Soc Behav. 2010;51(1) Suppl:S28–S40. doi: 10.1177/0022146510383498. [DOI] [PubMed] [Google Scholar]
- 29.Bongaarts J. Trends in causes of death in low‐mortality countries: implications for mortality projections. Popul Dev Rev. 2014;40(2):189–212. [Google Scholar]
- 30.Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet. 1992;339(8804):1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 31.Siahpush M, Singh GK, Jones PR, Timsina LR. Racial/ethnic and socioeconomic variations in duration of smoking: Results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J Public Health (Oxf) 2010;32(2):210–218. doi: 10.1093/pubmed/fdp104. [DOI] [PubMed] [Google Scholar]
- 32.Fenelon A. Revisiting the Hispanic mortality advantage in the United States: The role of smoking. Soc Sci Med. 2013;82(1):1–9. doi: 10.1016/j.socscimed.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 34.Preston SH, Stokes A. Contribution of obesity to international differences in life expectancy. Am J Public Health. 2011;101(11):2137–2143. doi: 10.2105/AJPH.2011.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta NK, Chang VW. Secular declines in the association between obesity and mortality in the United States. Popul Dev Rev. 2011;37(3):435–451. doi: 10.1111/j.1728-4457.2011.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masters RK, Powers DA, Link BG. Obesity and US mortality risk over the adult life course. Am J Epidemiol. 2013;177(5):431–442. doi: 10.1093/aje/kws325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z. Re: “Obesity and US mortality risk over the adult life course”. Am J Epidemiol. 2014;179(4):529–530. doi: 10.1093/aje/kwt329. [DOI] [PubMed] [Google Scholar]
- 38.Carmienke S, et al. General and abdominal obesity parameters and their combination in relation to mortality: A systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67(6):573–585. doi: 10.1038/ejcn.2013.61. [DOI] [PubMed] [Google Scholar]
- 39.Kawachi I, Adler N, Dow WH. Money, schooling, and health: Causal evidence mechanism. Ann NY Acad Sci. 2010;1186(1):56–68. doi: 10.1111/j.1749-6632.2009.05340.x. [DOI] [PubMed] [Google Scholar]
- 40.Rosero-Bixby L, Antich D. Estudio longitudinal de mortalidad de adultos costarricenses 1984-2007. Población Salud Mesoamérica. 2010;7(2):1–24. [Google Scholar]
- 41.Rosero-Bixby L, Dow WH, Rehkopf DH. The Nicoya region of Costa Rica: A high longevity island for elderly males. Vienna Yearb Popul Res. 2013;11:109–136. doi: 10.1553/populationyearbook2013s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herring AA, Bonilla-Carrión RE, Borland RM, Hill KH. Differential mortality patterns between Nicaraguan immigrants and native-born residents of Costa Rica. J Immigr Minor Health. 2010;12(1):33–42. doi: 10.1007/s10903-008-9121-y. [DOI] [PubMed] [Google Scholar]
- 43.Rogot E, Sorlie PD, Johnson NJ, Schmitt C. A Mortality Study of 1.3 Million Persons by Demographic, Social, and Economic Factors: 1979-1985 Follow-up: US National Longitudinal Mortality Study. National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 1992. [Google Scholar]
- 44.Johnson CL, et al. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999-2010, Vital Health Statistics. National Center for Health Statistics; Hyattsville, MD: 2013. Series 2, No 161. [PubMed] [Google Scholar]
- 45.Rosero-Bixby L, Fernández X, Dow WH. 2010 CRELES: Costa Rican Longevity and Health Aging Study, 2005 (Costa Rica Estudio de Longevidad y Envejecimiento Saludable): Sampling and Methods No. ICPSR26681-v2. Available at www.icpsr.umich.edu/icpsrweb/NACDA/studies/26681/documentation. Accessed January 15, 2013.
- 46.Bongaarts J, Feeney G. How long do we live? Popul Dev Rev. 2002;28(1):13–29. [Google Scholar]
- 47.Pamuk ER. Social class inequality in mortality from 1921 to 1972 in England and Wales. Popul Stud (Camb) 1985;39(1):17–31. doi: 10.1080/0032472031000141256. [DOI] [PubMed] [Google Scholar]