The B-cell lymphoma 2 (BCL-2) ovarian killer (BOK) is an intriguing BCL-2 family protein with highest homology to the multidomain executioner proteins BAX and BAK, yet its role in apoptosis regulation has been questionable because of the reported absence of phenotypic findings in Bok−/− mice (1). In an independent Bok−/− model, we identify a selective apoptotic defect in response to endoplasmic reticulum (ER) stress (2).
Differences in mouse model phenotypes can arise from alternate mouse strains, backgrounds, and targeting strategies. Our Bok−/− mice were backcrossed to C57BL/6 mice for eight generations, and although they contain a short transcript, notably lacking key functional domains, no BOK protein was detected by Western analyses. Ultimately, such differences in modeling approach may or may not impact the functional insights gleaned, but do necessitate rigorous mechanistic validation of the generated hypotheses.
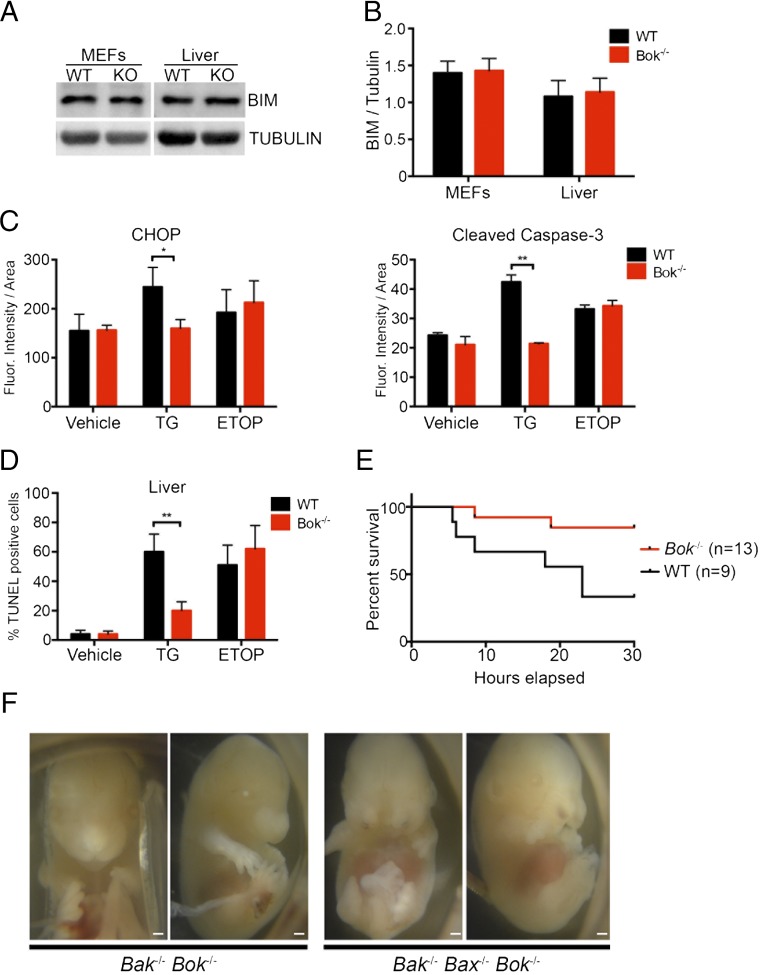
Fernandez-Marrero et al. (3) query if: (i) the selective apoptotic defect we attribute to Bok deletion derives instead from a reduction in BCL-2 interacting mediator of cell death (Bim) transcript, (ii) reversal of the apoptotic phenotype upon transient BOK reconstitution is sufficient evidence for its selective role, and (iii) quantitation of our in vivo findings would corroborate the conclusions drawn. First, we confirmed that our immortalized Bok−/− mouse embryonic fibroblasts (MEFs) and Bok−/− livers exhibit equivalent levels of BIM protein compared with the corresponding wild-type specimens (Fig. 1 A and B). Thus, differences in baseline BIM protein levels do not account for the observed phenotype. Importantly, any change in BIM level should similarly affect the apoptotic response to staurosporine, but instead we observed a selective defect in the ER stress response (2). Second, we previously probed the specificity of our Bok−/− phenotype by reconstituting MEFs with BOK protein, which reversed the apoptotic defect. Importantly, we conducted these studies using stable, not transient, BOK expression, thus avoiding any confounding effects of direct apoptosis induction by BOK itself. Intriguingly, transient overexpression of BOK has been shown to induce BIM (4), consistent with our mechanistic hypothesis that BOK functions upstream of ATF4, CHOP, and BIM, but we did not observe differences in BIM levels in the context of stable BOK expression. Finally, quantitation of our in vivo results confirmed that our Bok−/− mice are protected from thapsigargin-induced liver damage. CHOP, cleaved-caspase 3, and TUNEL all exhibit statistically significant decreases in thapsigargin-treated Bok−/− mice compared with wild-type controls (Fig. 1 C and D). To reinforce the physiologic impact of these findings, we further demonstrate a statistically significant survival advantage for Bok−/− mice challenged with thapsigargin (Fig. 1E).
Fig. 1.
(A and B) Loss of Bok does not affect BIM protein levels. Representative Western blots (A) and imageJ quantification (B) for BIM in SV40-transformed MEFs and livers isolated from wild-type and Bok−/− mice (mean ± SEM, n = 3). Tubulin was used as a reference protein. (C) Quantitation of observed decreases in CHOP (Left) and cleaved caspase 3 (Right) in Bok−/− mouse livers treated with thapsigargin (TG), compared with wild-type controls. No differences were observed for vehicle or etoposide (ETOP) treatments. Mean fluorescence intensity normalized to β-actin for CHOP and GAPDH for cleaved-caspase 3, as measured by Automated Quantitative Analysis (AQUA) of tissues from wild-type and Bok−/− mice treated with thapsigargin (mean ± SEM). **P < 0.005; *P < 0.03. The analyses were performed on three animals per genotype with similar results. (D) Quantitation of observed decrease in TUNEL positivity in Bok−/− mice treated with thapsigargin, compared with wild-type controls. No differences were observed for vehicle or ETOP treatments. TUNEL+ cells were counted and normalized to propidium iodide-positive cells using ImageJ for wild-type and Bok−/− mice treated with thapsigargin (mean ± SEM, n = 3). **P < 0.005. (E) Survival advantage of Bok−/− vs. wild-type mice treated with 1 mg/kg IP thapsigargin and monitored for 30 h. P < 0.03. (F) Rare Bax−/−Bak−/−Bok−/− mice display craniofacial defects, including the facial cleft seen here, which was not observed in Bak−/−Bok−/− or other combinatorial mice. (Scale bars: 1 mm.)
Our finding of a role for BOK at the intersection of apoptosis and ER signaling is consistent with BOK’s ER localization and the observed decrease in the IRE1α branch of the unfolded protein response, as reported for independently derived Bok−/− cells (4). Importantly, we observed similar abnormalities in our Bok−/− combinatorial knock-outs as those previously reported for independently derived animals,* including rare craniofacial defects in Bax−/−Bak−/−Bok−/− mice (Fig. 1E), suggesting that the two models may have more similarities than differences. Additional links between BOK, its splice-forms, and ER signaling pathways are the subject of ongoing investigations that may reinforce a unique role for BOK in the ER stress response.
Acknowledgments
This work was supported by National Institutes of Health Grant 5K08HL103847, and awards from the Connecticut Regenerative Medicine Fund and March of Dimes (to S.G.K.).
Footnotes
The authors declare no conflict of interest.
*Grabow S, et al., The Cold Spring Harbor Laboratory Cell Death Meeting, October 8–12, 2013, Cold Spring Harbor, NY, p 157 (abstr).
References
- 1.Ke F, et al. BCL-2 family member BOK is widely expressed but its loss has only minimal impact in mice. Cell Death Differ. 2012;19(6):915–925. doi: 10.1038/cdd.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpio MA, et al. BCL-2 family member BOK promotes apoptosis in response to endoplasmic reticulum stress. Proc Natl Acad Sci USA. 2015;112(23):7201–7206. doi: 10.1073/pnas.1421063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Marrero Y, et al. Is BOK required for apoptosis induced by endoplasmic reticulum stress? Proc Natl Acad Sci USA. 2016;113:E492–E493. doi: 10.1073/pnas.1516347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echeverry N, et al. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 2013;20(6):785–799. doi: 10.1038/cdd.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]