To the Editor
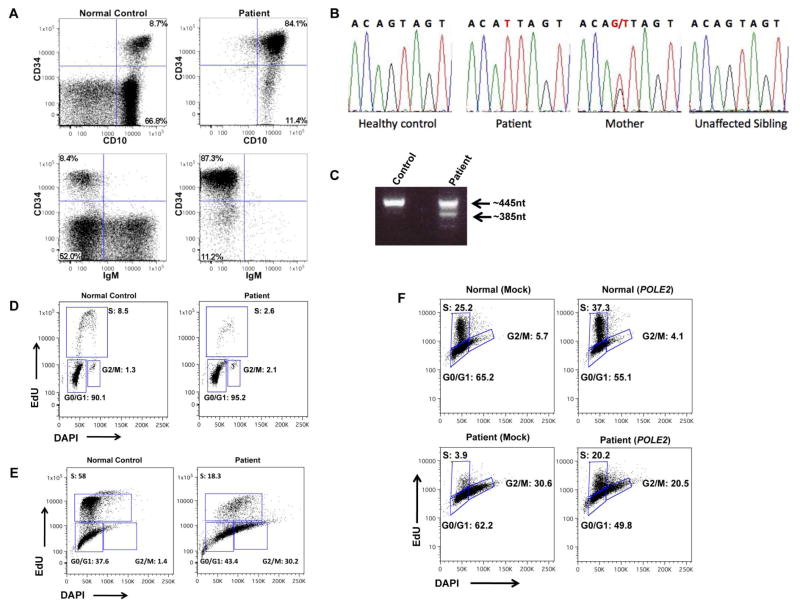
Early lymphocyte development requires the orchestrated interplay of pathways to maintain genomic integrity and accurate DNA repair during the proliferative bursts associated with antigen receptor rearrangement (1). Inborn errors in replication control or DNA repair can lead to primary immunodeficiency (2). We report the case of a patient with combined immunodeficiency, facial dysmorphisms, and autoimmunity with a novel mutation in the DNA polymerase epsilon subunit 2 (POLE2) gene. A 5-year-old male born to related parents of Saudi origin was referred to our center with a history of omphalitis and erythroderma in the neonatal period, systemic BCG infection after immunization, and subsequently, multiple respiratory infections. Diabetes mellitus was diagnosed at 5 months of age. At 8 months, he developed severe dyspnea with hypoxia, hepatomegaly and hypothyroidism. Laboratory investigation disclosed agammaglobulinemia, absence of circulating B cells, T cell lymphopenia and neutropenia. Replacement therapy with intravenous immunoglobulins (IVIG) was initiated. At 3 years, he developed generalized lymphadenopathy and a lymph node biopsy showed effaced architecture with lack of follicles, an increased number of CD163+ activated macrophages and activated (CD45R0+) T lymphocytes. He continued to have recurrent respiratory infections with pulmonary atelectasis. When evaluated at our center his height and weight were at the 3rd percentile, and head circumference was 48 cm (−2.4 s.d.). Dysmorphic features (low anterior hairline, flat supraorbital ridges, downturned corners of the mouth, and a short philtrum) were noticed. Laboratory evaluation (Table 1) confirmed lymphopenia with absence of B-lymphocytes, markedly increased proportion of effector memory T cells, undetectable TRECs, and reduced number of NK cells, 30% of which were CD56bright (our internal laboratory normal reference range: 8.4 ± 3.8%). In vitro proliferative response to mitogens was normal, but response to antigens was abrogated (Table 1). Upon stimulation of peripheral blood mononuclear cells with anti-CD3 ± anti-CD28 monoclonal antibodies, an increased proportion of cells were found to be positive for Annexin V staining by FACS, consistent with increased cell death. Flow-cytometric analysis of expression of 24 TCR Vβ families revealed normal representation in CD4+ cells, whereas some skewing was observed among CD8+ cells, with 4 families being under-represented, and 2 over-represented. Bone marrow examination disclosed a low proportion of CD19+ cells (8.2% of cells in the lymphocyte gate), most of which (89%) were CD34+. By staining for CD19, CD34, CD10 and sIgM, we observed a severe block at pre-BII stage and virtual absence of mature CD19+ IgM+ cells (Fig. 1a).
Table 1.
Hematological and Immunological Features
| Laboratory Parameter | Patient (age)
|
Normal controls | |||
|---|---|---|---|---|---|
| (10m) | (4y 6m) | (5y 8m) | (6y 10m) | ||
| ANC (×109/L) | 0.15 | 3.27 | 2.28 | 3.88 | 2.49–5.96 |
| ALC (×109/L) | 1.90 | 2.47 | 0.70 | 0.76 | 1.33–3.47 |
| CD3+ (cells/μL) | 2030 | 2341 | 763 | 809 | 1400–6200 |
| CD3+ TCRαβ+ (%) | 97.3 | 88–99.4 | |||
| CD3+ TCRγδ+ (%) | 2.7 | 0.6–12.0 | |||
| CD4+ (cells/μL) | 1370 | 1114 | 403 | 290 | 700–2200 |
| Naïve CD4 (CD45RA+ CCR7+) (% of CD4+) | 21.9a | 25.7 | 21.6 | 57.1–84.9 | |
| Central memory CD4 (CD45RA− CCR7+) (% of CD4+) | 0.9 | 9.9 | 11.3–26.7 | ||
| Effector memory CD4 (CD45RA− CCR7−) (% of CD4+) | 72.1 | 67.7 | 3.3–15.2 | ||
| CD4+ CD45RA+ CCR7− (% of CD4+) | 1.3 | 0.8 | 0.4–2.6 | ||
| Recent thymic emigrants (CD45RA+ CD31+) (% of CD4+) | 16.2 | 19.4–60.9 | |||
| CD8+ (cells/μL) | 543 | 1262 | 328 | 505 | 490–1300 |
| Naïve CD8 (CD45RA+ CCR7+) (% of CD8+) | 2.8a | 6.6 | 2.1 | 28.4–80.6 | |
| Central memory CD8 (CD45RA− CCR7+) (% of CD8+) | 0.7 | 4.1 | 1.0–4.5 | ||
| Effector memory CD8 (CD45RA− CCR7−) (% of CD8+) | 86.0 | 85.1 | 6.2–29.3 | ||
| TEMRA (CD8+ CD45RA+ CCR7− (% of CD8+) | 6.8 | 8.6 | 9.1–49.1 | ||
| CD3− CD16/56+ (cells/μL) | 60 | 1 | 30 | 130–720 | |
| CD19+ (cells/μL) | 17 | 0 | 3 | 390–1400 | |
| IgG (mg/dL) | <150 | 516* | 556* | 740* | 639–1344 |
| IgA (mg/dL) | <25 | <7 | <7 | <7 | 50–150 |
| IgM (mg/dL) | <18 | <5 | <5 | <5 | 22–100 |
| IgE (IU/mL) | <1 | <1 | <1 | 0–200 | |
| In vitro proliferation to mitogens (cpm) | |||||
| Medium | 938 | 779 | 461 | 321–2510 | |
| PHA | 179226 | 74035 | 96355 | 104415–319780 | |
| In vitro proliferation to antigens (cpm) | |||||
| Medium | 1721 | 2605 | 2398 | ||
| Tetanus toxoid | 1157 | 7166 | 3422 | ||
| Diphteria toxoid | 1071 | 1908 | |||
| Candida albicans | 1993 | 2735 | |||
At this time point, naïve CD4 and CD8 lymphocytes were defined as CD45RA+ CD62L+
On intravenous immunoglobulin replacement therapy
Figure 1.
A) FACS analysis of bone marrow from a healthy control and the patient. Upon gating on CD19+/CD45+ positive cells, an increased proportion of B cell precursors (CD10+/CD34+), and decreased proportion of mature IgM+ B cells was demonstrated in the patient. B) Sequence chromatogram depicting sequence variance at position-1 of intron 13 in a healthy control, the patient, his mother and one unaffected sibling. C) RT-PCR spanning from exon 12 through exon 16 of POLE2. A single band of 445 nt is detected in the normal control, whereas two bands are observed in the patient: a 442nt product (resulting from use of a cryptic splice site at position +3 of exon 14, and a 385nt product with skipping of exon 14). D) Peripheral blood lymphocytes from a healthy control (left) and patient (right) stimulated for 96 hours with PHA, then stained with BrdU and DAPI. A decreased proportion of cells in S phase are observed in the patient. E) Cell cycle analysis of fibroblasts grown asynchronously, 24 hours after pulsing with EdU and DAPI. Patient’s fibroblasts show accumulation of cells in G2/M, and decreased proportion of cells in S phase. F) Cell cycle analysis of fibroblasts transfected with either empty- or wild-type POLE2-containing vector, 6 hours after release from growth synchronization. Partial rescue of cell cycle progression is observed in patient’s POLE2-transduced fibroblasts. These data are representative of two experiments.
In the attempt to identify the underlying cause of the disease, we performed whole exome sequencing (see: Online Repository). A homozygous splice-site mutation (g>t) at position-1 of intron 13 of the POLE2 gene was identified, within a region of chromosome 14 where SNP analysis demonstrated homozygosity. This splice-site mutation was confirmed by Sanger sequencing (Fig. 1b), and has not been previously reported in dbSNP or 1000Genome. Both parents were heterozygous for the mutation, and none of the 4 healthy siblings were homozygous for the mutation. RT-PCR analysis yield two transcripts in the patient: a normal-sized transcript with a deletion of 3 nucleotides resulting from usage of a cryptic acceptor splice site at position +3 in exon 14, and a shorter transcript due to skipping of exon 14 (Fig 1c). The product with the 3-nt deletion is predicted to result in loss of a single highly-conserved amino acid residue, Ser 359 (NP_001184260.1). Immunoblot analysis for POLE2 revealed similar expression in patient and control samples (data not shown).
DNA polymerases help achieve replication of DNA with remarkable accuracy through conserved pathways that repair DNA damage and correct nucleotide misincorporation during DNA replication (3) Polε is a large, multi-subunit polymerase that is conserved throughout evolution in eukaryotes. Of the four genes that encode for the POLε complex, POLE1 encodes for a 261kDa protein that contains the catalytic activity and is complexed with the POLE2 (59kDa) subunit. POLE3 (17kDa) and POLE4 (12kDa) represent two additional subunits in the complex (12). The functional role of the POLE holoenzyme includes leading strand synthesis required for DNA replication and proofreading activity required for the repair of DNA damage and for maintenance of sequence (epigenetic) inheritance (4,5). The exact function of the POLE 2 subunit is not known, but is thought to involve protein-protein interactions, including dimerization with POLE1 and influencing the C-terminal part of the catalytic subunit. Given the role of POLε in DNA replication, we investigated cell cycle progression after stimulation of the patient’s PBMC with PHA and cells with failed DNA replication in S phase were demonstrated (Fig 1d). Similarly, asynchronous culture of skin-derived primary fibroblasts from the patient showed reduced numbers of cells in S phase and increased proportion of cells in G2/M (Fig. 1e) as compared to control fibroblasts. Furthermore, patient’s fibroblasts had stunted growth, and karyotype analysis demonstrated that 66% of the cells in culture were tetraploid. Upon transduction of patient and control fibroblasts with a lentiviral vector expressing wild-type POLE2 (wtPOLE2), followed by growth synchronization, a partial rescue of cell cycle progression in wtPOLE2-transduced patient fibroblasts, with a significant increase in the number of cells in S phase (Fig 1f).
Somatic mutations in human POLE have been demonstrated in colon cancer, and are thought to contribute to genomic instability (6, 7). Recently, a mutation in POLE1, a member of the DNA polymerase pathway has been found in a large consanguineous family leading to a syndrome with facial dysmorphism, immunodeficiency with hypogammaglobulinemia, livedo reticularis, and short stature (9). A variable decrease in the absolute numbers of B and to a lesser degree naive T cells, was detected among the affected individuals, with impaired G1- to S-phase progression in primary T cells and in lymphoblastoid cell lines generated from B cells. By contrast, our patient had a more severe block in B cells development than that POLE1-mutated patients, a difference that may reflect the distinct nature of the mutations or functions of the POLE subunits. To our knowledge, this is the first case in which germline mutations in a replicative DNA polymerase have been associated with an early block in lymphocyte development in humans (1, 8). Altogether, identification of POLE1 and POLE2 mutations in patients with immunodeficiency and other extra-immune manifestations, indicates an important role for the POLE holoenzyme in cell proliferation, and in the lymphoid lineage in particular. The relative contribution of impaired lymphocyte proliferation due to defective DNA replication, altered cellular survival and/or defective repair of lymphoid specific DNA alterations in patients with POLE defects remains to be addressed (6). Finally, longitudinal follow up studies are needed to uncover the potential role of germline POLE1 and POLE2 mutations in cancer susceptibility.
Methods
Patient
Blood samples from the patient and his parents were obtained either at Boston Children’s Hospital or were shipped from collaborators, with informed consent according to our Institutional Review Board.
Genomic DNA was extracted from whole blood by using the Gentra Puregene Blood Kit (Qiagen, Valencia, Calif).
FACS analysis
Flow cytometry was used to measure the percentages of lymphocyte populations in whole blood using the monoclonal antibody conjugates (6 Color TBNK Reagent, CD3-PerCP-Cy5.5, CD4-APC, CD8-V450, CCR7-FITC, CD45RA-PE, CD19-PerCP-Cy5.5, CD27-PE, IgD-FITC, CD24-APC and CD38V450, all from BD Biosciences (Franklin Lakes NJ). The percentage of each patient’s lymphocyte subset was compared with normal control ranges for age that have either been established independently in the Boston Children’s flow cytometry laboratory. Lymphocyte proliferation assays were performed in three independent experiments.
Acknowledgments
This work was supported by the National Institutes of Health, grant 5R01AI100887 (to LDN) and R21AI87355 (to JPM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013 Jul;13(7):519–33. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 2.Al-Herz W, Bousfiha A, Casanova J-L, Chatila T, Conley ME, Cunningham-Rundles C, Etzioni A, Franco JL, Gaspar HB, Holland SM, Klein C, Nonoyama S, Ochs HD, Oksenhendler E, Picard C, Puck JM, Sullivan K, Tang MLK. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Front Immunol. 2014 Apr 22; doi: 10.3389/fimmu.2014.00162. (ePub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callén E, Nussenzweig MC, Nussenzweig A. Breaking down cell cycle checkpoints and DNA repair during antigen receptor gene assembly. Oncogene. 2007;26(56):7759–64. doi: 10.1038/sj.onc.1210873. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel TA. Balancing eukaryotic replication asymmetry with replication fidelity. Current Opinion in Chemical Biology. 2011;15(5):620–6. doi: 10.1016/j.cbpa.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeb LA, Monnat RJ. DNA polymerases and human disease. Nature Reviews Genetics. 2008;9(8):594–604. doi: 10.1038/nrg2345. [DOI] [PubMed] [Google Scholar]
- 6.Palles C, Cazier J-B, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nature Genetics. 2012;45(2):136–44. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agbor AA, Göksenin AY, LeCompte KG, Hans SH, Pursell ZF. Human Polε-dependent replication errors and the influence of mismatch repair on their correction. DNA Repair. 2013;12(11):954–63. doi: 10.1016/j.dnarep.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmink BA, Sleckman BP. The Response to and Repair of RAG-Mediated DNA Double-Strand Breaks. Annual Review of Immunology. 2012;30(1):175–202. doi: 10.1146/annurev-immunol-030409-101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pachlopnik Schmid J, Lemoine R, Nehme N, Cormier-Daire V, Revy P, Debeurme F, et al. Polymerase epsilon1 mutation in a human syndrome with facial dysmorphism, immunodeficiency, livedo, and short stature (“FILS syndrome”) J Exp Med. 2012 Dec 17;209(13):2323–30. doi: 10.1084/jem.20121303. [DOI] [PMC free article] [PubMed] [Google Scholar]