Summary
Newly acquired motor skills become stabilized through consolidation [1]. However, we know from daily life that consolidated skills are modified over multiple bouts of practice and in response to newfound challenges [2]. Recent evidence has shown that memories can be modified through reconsolidation, in which previously consolidated memories can re-enter a temporary state of instability through retrieval, and in order to persist, undergo re-stabilization [3-8]. Although observed in other memory domains [5,6], it is unknown if reconsolidation leads to strengthened motor skills over multiple episodes of practice. Using a novel intervention after the retrieval of a consolidated skill, we found that skill can be modified and enhanced through exposure to increased sensorimotor variability. This improvement was greatest in those participants who could rapidly adjust their sensorimotor output in response to the relatively large fluctuations presented during the intervention. Importantly, strengthening required the reactivation of the consolidated skill and time for changes to reconsolidate. These results provide a key demonstration that consolidated motor skills continue to change as needed through the remapping of motor command to action goal, with strong implications for rehabilitation.
Keywords: Skill learning, variable practice, strategy, consolidation, memory
Results
Evidence across multiple species and memory domains has shown that reactivating a consolidated memory can renew sensitivity to memory modification interventions [3-8]. Re-stabilization of a modified memory, or its reconsolidation, updates an original memory to a strengthened [5,6] or weakened [7,8] state.
In humans, motor skills can be modified through reconsolidation, but these studies have all used interventions to weaken a consolidated skill [7,9,10]. For instance, a sequence skill is subject to interference when retrieved prior to learning a new sequence [7]. Further, offline learning, defined as additional performance improvement expressed post-practice, is blocked when a skill is retrieved during the application of disruptive repetitive transcranial magnetic stimulation over the primary motor cortex [9,10]. Although reconsolidation supports memory strengthening in episodic [5] and fear conditioning [6], it is unknown if reconsolidation is a process that leads to improvement of motor skills over multiple bouts of practice.
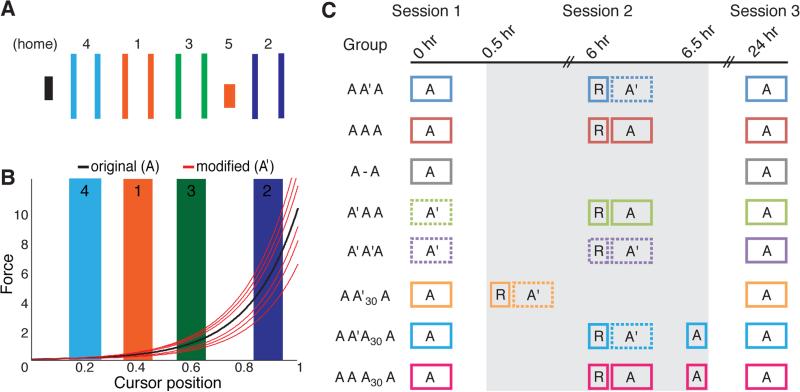
To test if reconsolidation strengthens motor skills, we presented an intervention after the reactivation of a previously consolidated skill. This consisted of a subtle trial-to-trial fluctuation of the previously learned sensorimotor mapping. Using an established protocol [11], participants (n = 86, Table S1) learned a sequential visuomotor isometric pinch task (SVIPT, Figure 1A) skill over 2 consecutive days of practice. Participants controlled a cursor to a set of visual targets through the logarithmic transduction of isometric pinch force (Figure 1B). SVIPT skill consolidates within a period of 6 hours over wake [12-14]. After 6 hours, participants reactivated the original SVIPT (A), and then either performed a modified version of the SVIPT (A') or additional practice on skill A. All participants practiced the same amount of trials. We designed the intervention A' wherein the logarithmic mapping fluctuated over consecutive trials. These mapping fluctuations were derived from a normal range of variability observed in an independent sample of participants performing skill task A (see Supplemental Experimental Procedures). Thus, we tested if increased variability in mapping between sensorimotor control and task goal would strengthen skill A through reconsolidation.
Figure 1.
Experiment protocol. (A) Depiction of the visual stimuli used to learn the SVIPT. Black bar on left is the cursor. Participants learned to control the lateral movement of the cursor from the “home” position (not shown during experiment) to a target using the order home-1-home-2-home-3-home-4-home-5. (B) Logarithmic mapping between pinch force and cursor displacement. Black trace represents the mapping for the original SVIPT (A). During the modified SVIPT (A'), participants controlled the cursor with a new set of mappings (red curves) along with the original mapping (black curve), which were presented in a pseudorandom order. (C) SVIPT skill was acquired over 3 sessions (4 blocks of 30 trials/session) so that Session 3 was performed 24 hours after Session 1. Blocks with a solid border represent training with skill A, and those with a dashed border represent training with A'. All groups with the exception of A'AA and A'A'A began training with skill A. Session 2, serving as the reconsolidation intervention, began with a 15-trial reactivation block (R) of skill A. The AA'A group (n = 11) received the A' intervention 6 hours after learning A, whereas AAA (n = 11) continued to train on A, and A-A (n = 10) did not receive any intervention. To test for a general enhancement of performance due to variable practice, A'AA (n = 11) trained on A' during Session 1, and then A during Sessions 2 and 3. To test if repetition of A' led to increased performance on A during Session 3, A'A'A (n = 11) trained on A' during Session 1 and Session 2. For this group, A' was reactivated prior to Session 2. To test if skill A must first be consolidated prior to skill strengthening, AA'30A (n = 11) were exposed to A' just 30 minutes after learning A. To test for the immediacy of the A' intervention, AA'A30A (n = 11) were tested on A just 30 minutes after exposure to A'. To test for skill strengthening using this procedure, an additional group, AAA30A (n = 11), trained repeatedly on skill A.
Contextual variability can strengthen retention [15] and generalization of skills [16]. Here, however, we chose to increase sensorimotor variability while maintaining constant the original learning context. This is noteworthy because context [17] and size of errors [18] experienced during increased variability can affect learning. Given that attributing errors to internal sources can strengthen learning [19,20] and generalization [21] of motor behavior, we chose an intervention that required remapping for accurate performance, but without a salient change in context as typically done in contextual interference studies [15]. Participants were unaware of this manipulation, as reported by post-test assessment (see Supplemental Experimental Procedures).
Participants practiced skill A during Session 1 (4 blocks of 30 trials). They returned 6 hours later during Session 2, were exposed to a brief reactivation block (15 trials; R, Figure 1C) and then by a post-reactivation intervention (A or A': 4 blocks of 30 trials). They were finally tested on skill A during Session 3, 24 hours after Session 1 (A: 4 blocks of 30 trials, Figure 1C). Participants were randomly assigned to 1 of 3 intervention groups, so that one group trained on the modified SVIPT (AA'A), one group continued to train on the original SVIPT (AAA), and another did not train (A-A) (Figure 1C).
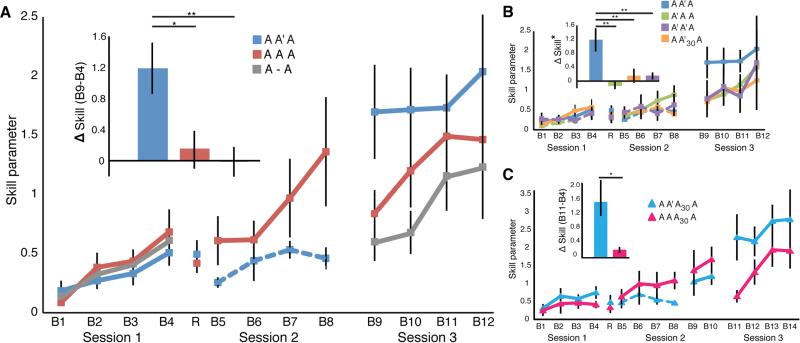
To measure skill, we quantified changes in the speed-accuracy tradeoff function (SAF) separately for each training block as previously done [11-14] (see Supplemental Experimental Procedures). We predicted a greater change in learning of A for those exposed to the A' intervention (AA'A). We found an interaction between intervention group and training session [F2,203 = 7.29; P < 0.001, Figure 2A], which was driven by a larger between-session difference in the skill parameter for AA'A relative to A-A (t(16.73) = 2.93; p < 0.01) and AAA (trending: t(19.72) = 1.54; p < 0.14). This comparison averaged block over session, combining retention and within-session learning. To more closely examine how the intervention influenced skill retention, we compared the last training block of Session 1 to the first training block from Session 3. This post-pre intervention effect, or Δ skill, revealed greater change for AA'A relative to AAA (t(18.39) = 2.52; p = 0.02) and A-A (t(15.86) = 3.15; p = 0.01) (Figure 2A, inset). This demonstrates that exposure to A' strengthened A. We did not find any difference in Δ skill between AAA and A-A (t(15.67) = 0.89; p = 0.39). This suggests that additional repetitive practice after reactivation is not an efficient method for strengthening skill during early learning. Notably, there was no difference in skill retention during the delay between Session 1 and Session 2 (B4 and R: AA'A t(13.05) = 0.6; p = 0.56, AAA t(12.29) = 1.33; p = 0.20), suggesting similar retention at the start of the intervention.
Figure 2.
Change in skill performance during multiple SVIPT training sessions. Increasing values on the y-axis (skill parameter) represent improved performance through change in the speed-accuracy tradeoff function. Epochs on the x-axis show data in 30 trial increments for each of the training sessions completed over a period of 24 hours. The reactivation block (R) at the start of Session 2 is an average of 15 trials. Large rectangles represent training sessions that contain 4 blocks of 30 trials. Small rectangles shown for groups AA'A30A, AAA30A represent training sessions that contain 2 blocks of 30 trials. Dashed line reflects training with the variable intervention and solid line reflects training with the original skill task. (A) Exposure to A' (AA'A) during reconsolidation strengthened skill A in Session 3. Strengthening was not present for participants that continued to practice A during Session 2 (AAA) or for participants that did not receive an intervention (A-A). Bar graph inset shows the effect of the intervention on strengthening the performance of A (Δ skill B9-B4). Skill strengthening was greater for AA'A than AAA and A-A. (B) Skill strengthening was not found when A' preceded learning A (A'AA), when A' preceded consolidation of A (AA'30A), or when A' was repeated during Sessions 1 and 2 (A'A'A). These controls are shown with respect to a re-plotting of the AA'A data. Note (*): Δ skill for A'AA (B9-B8) differed from AA'A and AA'30A (both B9-B4) in order to match the training exposure for skill A. (C) Strengthening was not found just 30 minutes after exposure to A' (AA'A30A, Session 2, 6.5hr), but was found after enough time was given for reconsolidation to occur (Session 4, 24hr). Replication of the strengthening effect is shown in bar graph inset. Data are means +/− SEM.
Could skill strengthening be a non-specific effect related to variable training? Previous investigations show retention and generalization are strengthened by increased variability at the level of the task goal [15,22,23]. To test if memory strengthening was due to a general effect of practicing a variable task, we compared a new group that performed A' during Session 1 (A'AA) to the AA'A group. Skill strengthening was greater for AA'A with respect to A'AA (Δ skill, AA'A vs A'AA: t(12.98) = 3.72, p = 0.003, Figure 2B inset), suggesting that strengthening requires the initial exposure to skill A. In addition, we tested if strengthening required switching from A to A'. We therefore tested a group that trained on A' in both Session 1 and Session 2 (A'A'A). We did not observe skill strengthening for A'A'A (Δ skill, AA'A vs. A'A'A: t(11.06) = 3.02, p = 0.01, Figure 2B inset), which, similar to AAA, suggests that strengthening requires more than repetition.
Is skill strengthening a reconsolidation process? Reconsolidation involves (1) the retrieval of a previously consolidated memory, and (2) time to re-stabilize newly modified memories [3,4]. Our results confirm that both of these components were necessary for skill strengthening. Firstly, there was no evidence of strengthening when participants were exposed to A' just 30 minutes after having learned A (AA'30A), thus prior to consolidation of A (Δ skill, AA'A vs AA'30A: t(14.94) = 2.74; p = 0.015, Figure 2B inset). Secondly, exposure to A' did not lead to immediate strengthening on A when tested 30 minutes after the intervention (AA'A30A, Session 2, 6.5hr, block 9 vs. Session 2 original SVIPT trials, 6hr, blocks 5-8: t(10) = 0.28; p=0.79), but did when tested the next day (Session 3, block 11 vs. Session 2, 6.5hr, block 9: t(10) = 2.58; p = 0.03). This is consistent with evidence that reconsolidation is not an immediate process [24-26]. Moreover, the lack of immediate improvement confirms that strengthening was not simply the product of learning a different skill when performing A'. Importantly, we replicated the A' strengthening effect (Δ skill, AA'A30A vs AAA30A: t(10.52) = 2.2; P = 0.05, Figure 2D inset).
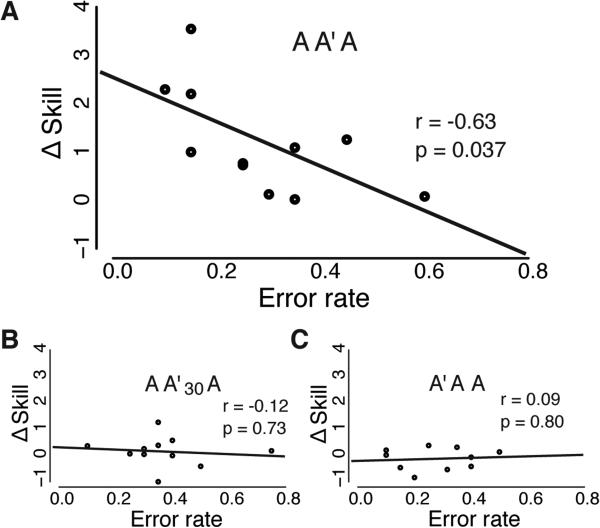
Why is it that skill strengthening happened after exposure to A'? During A', the sensorimotor mapping slightly changed from one trial to the next. Because of this, participants needed to rapidly adjust their motor plan in order to achieve the same task goal. We performed a trial history analysis to test how the response to mapping fluctuations during A' might influence skill strengthening. Each trial was assigned a grouping label according to its mapping, which were derived from the variance step size (+/− 3SD, 1SD increments) used to fit variants of the original mapping (see Supplemental Experimental Procedures). Trial history reflected the absolute value difference of the grouping label from the previous 2 trials (e.g., [3SD, trialN-2 ] - [−2SD, trialN-1] = 5SD). Greater trial history differences corresponded to greater fluctuation in the mapping over consecutive trials. We formed trial history groups that ranged from no fluctuation (0, same function on consecutive trials) to relatively large fluctuations (>3SD). Rapid adjustment in response to change in a learning environment requires higher-level processing or strategy [27,28]. We thus expected participants with high accuracy on trials following large fluctuations to have greater skill strengthening, indicative of higher-level strategic processing. Indeed, those who were more accurate on trials immediately following the largest mapping fluctuations also experienced the largest Δ skill (r = −0.63; p = 0.037, Figure 3A). Although sequence movement time was moderately inversely proportional to error rate on these trials (r = −0.52; p = 0.1), it was not related to Δ skill (r = 0.02; p = 0.94). In other words, participants that could rapidly adjust performance to maintain accuracy, experienced the greatest benefit from A'. We did not find a similar effect for other groups that failed to show skill strengthening (A'AA, AA'30A, Figure 3b,c, respectively). No other correlation between Δ skill and the other trial history groups reached significance for AA'A, A'AA, or AA'30A. Overall, this suggests that even though subjects were unaware of A', the rapid modification of motor control policy, potentially through the implementation of increased strategic processing, was a critical factor in the overall effect of skill strengthening.
Figure 3.
Correlation between Δ skill and accuracy (error rate) on those trials during A' intervention that followed a large mapping change over previous consecutive trials. Y-axis represents Δ skill. This is the difference in skill from the last block of Session 1 from the first block of Session 3 (B9-B4) in groups AA'A, AA'30A; or the last block of Session 2 from the first of Session 3 (B9-B8) in group A'AA. X-Axis depicts error rate, which represents the proportion of SVIPT trials with a target miss for those trials that are preceded by a large fluctuation in mapping step size > 3SD (see Supplementary Experimental Procedures). Fluctuation in mapping size refers to the difference in cursor sensitivity found between previous consecutive trials. Each data point on the plots represent a participant's error rate following the largest fluctuation in step size during the A' intervention (i.e. error rate for trialN after a change in map of >3SD between trialN-2 and trialN-1). (A) Error rate was proportional to skill strengthening. Participants in AA'A with the lowest error rate on trials that followed a large change in mapping experienced the greatest benefit in skill strengthening (Δ skill). We did not observe this relationship for other groups that received A' but showed no evidence for skill strengthening: (B) A'AA, and (C) AA'30A.
Discussion
For skills to improve, we must update an existing memory with new information. Until recently, it was accepted that consolidated memory was maintained permanently [3,4], thus raising a conceptual challenge for how a motor skill could change over multiple learning episodes. Our results confirm the involvement of reconsolidation in skill learning by demonstrating that manipulation after reactivation can lead to the strengthening of an existing skill.
We found that when participants were unknowingly exposed to an intervention that increased sensorimotor variability after they retrieved a consolidated memory, that their performance on the previously learned skill was strengthened when tested the next day (AA'A). Participants that practiced the same skill multiple times (AAA or A'A'A) failed to show a similar increase. Moreover, the magnitude of skill strengthening depended on flexibility during the variable intervention.
These results are consistent with the hypothesis that reconsolidation mediates memory updating to maintain relevance [5,29]. Reconsolidation can be used to account for new information experienced at retrieval, which might be activated by prediction error [5,30]. Through the variable intervention, or A', we introduced a discrepancy between skill A and the command needed to complete the task. This is reminiscent of evidence showing that reconsolidation is driven by a mismatch between a learned contingency and what is experienced during intervention [30]. This suggests that repeating A (or A') failed to promote skill strengthening because reactivation occurred without a sufficient mismatch to promote reconsolidation updating.
Similar to previous investigations [7,9,10], we tested for motor reconsolidation after initial consolidation. In contrast to these investigations showing that manipulations can weaken skills [7,9,10], our variable intervention strengthened skill. This is consistent with findings in other memory domains, which show that consolidation processes continue throughout learning [31,32]. Despite specific cellular mechanisms being linked to reconsolidation [3,33], it remains difficult to isolate behavioral changes as being reconsolidation-specific. However, we observed skill strengthening only after reactivation of consolidated skill, and further, only after enough time for changes post-intervention to reconsolidate. Because we did not find evidence of strengthening after repetitive practice on A (or A'A'A), we contend that strengthening is driven by the reconsolidation of changes from A' instead of a recapitulation of skill A consolidation.
We tested early in skill acquisition and prior to a plateau in the learning curve [11]. To date, no experiments have tested reconsolidation during long-term skill learning. It is, however, well known that reconsolidation is sensitive to age and exposure of a memory, such that newer and less practiced memories are subject to greater modification [29,34]. Thus, it is of future interest to determine how this intervention, or a similar one, would affect skill when applied after performance has reached plateau.
Although motor learning is commonly described as a reduction of variability, it is clear that endogenous [35,36] and exogenous [22,23] variability sources enhance learning. Here we implemented a exogenous intervention that, unbeknown to the participant, increased variability by requiring force to change from one trial to the next to perform the SVIPT. Akin to theories of reinforcement learning wherein several action alternatives are sampled prior to arriving at the best solution [37], the increased variability of the motor command needed to control the cursor led participants to a renewed exploration of potential action alternatives [35,38-40]. Thus, participants strengthened skill through the re-exploration of sensorimotor space. This is supported by the result that strengthening was highest for participants that could perform more accurately on trials that followed relatively large fluctuations in the sensorimotor mapping. Interestingly, our correlation results suggest that participants with greater stability at the point of reactivation might show reduced strengthening. This is similar to the resistance that older, more stable memories demonstrate during reconsolidation-based modification [29,34].
We chose to manipulate sensorimotor variability so that participants were unaware of the fact that there was any change in the task (see Supplemental Experimental Procedures). We intended performance fluctuations during the intervention to be attributed to the participant instead of an obvious exogenous perturbation. Error size and error context can influence error source attribution, or credit assignment [17,18,41]. Credit assignment interacts with learning rate [18], retention [41,42], and generalization [17,21]. By maintaining the original learning context but increasing sensorimotor variability, we expected that changes in control needed to match previously learned task performance would strengthen skill. This is consistent with improved retention and generalization following small rather than abrupt errors during single-session learning [17,41]. Our results might have shown a different pattern had the intervention been devised to amplify error. For instance, unexpectedly large errors can lead to reduced learning [18,43]. Exposure to an intervention that was clearly different might have weakened the memory for skill A [7,44]. It should be noted that although A' was unbeknown to participants, we cannot exclude the possibility that attention or motivation processes below conscious awareness affected strengthening. Altogether, we suggest that the variable intervention led to skill strengthening rather than degradation by maintaining credit assignment of error to internal sources.
Our results show that reconsolidation provides a crucial mechanism for the strengthening of an existing motor skill. Increased sensorimotor variability after skill retrieval promotes additional learning changes that are absent following the simple continuation of practice. This demonstrates the importance of a renewed interplay between the exploration and exploitation of sensorimotor space in order for consolidated skills to improve. This finding has important implications for the use of reconsolidation-based interventions to strengthen skill learning in healthy individuals as well as in the context of rehabilitation when it is advantageous to maximize learning within a limited window of time.
Experimental Procedures
Details for experimental procedures can be found within Results and a full description in Supplemental Experimental Materials.
Supplementary Material
Acknowledgements
This study was supported by funding from the NICHD (R01HD073147) to P.A.C. and a Ruth L. Kirschstein National Research Service Award (T32-NRSA) to N.F.W. We thank Matt Statton for supplying the dataset used to fit the functions that were included in the variable SVIPT intervention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
N.F.W., A.J.B, and P.A.C. designed the study. N.F.W. performed the research and analyzed the data. N.F.W., A.J.B, and P.A.C. wrote the manuscript.
References
- 1.Robertson EM. From creation to consolidation: a novel framework for memory processing. PLOS Biol. 2009;7(1):11–19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat. Rev. Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 4.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 5.Lee JLC. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandrini M, Censor N, Mishoe J, Cohen LG. Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Curr. Biol. 2013;23:2181–2184. doi: 10.1016/j.cub.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 8.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 9.Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr. Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censor N, Horovitz SG, Cohen LG. Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron. 2014;81:69–76. doi: 10.1016/j.neuron.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantarero G, Lloyd A, Celnik P. Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. J. Neurosci. 2013;33:12862–12869. doi: 10.1523/JNEUROSCI.1399-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantarero G, Tang B, O'Malley R, Salas R, Celnik P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J. Neurosci. 2013;33:4634–4641. doi: 10.1523/JNEUROSCI.4706-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B. Time-but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cereb. Cortex. 2015;25(1):109–117. doi: 10.1093/cercor/bht208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shea J, Morgan R. Contextual interference effects on the acquisition, retention, and transfer of a motor skill. J. Exp. Psychol. Hum. Learn. 1979;5:179–187. [Google Scholar]
- 16.Schmidt R. A schema theory of discrete motor skill learning. Psych. Rev. 1975;82:225–260. [Google Scholar]
- 17.Torres-Oviedo G, Bastian AJ. Natural error patterns enable transfer of motor learning to novel contexts. J. Neurophysiol. 2012;107:346–356. doi: 10.1152/jn.00570.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei K, Körding K. Relevance of error: what drives motor adaptation? J. Neurophysiol. 2009;101:655–664. doi: 10.1152/jn.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp. Brain. Res. 2005;164:250–259. doi: 10.1007/s00221-005-2247-4. [DOI] [PubMed] [Google Scholar]
- 20.Hatada Y, Rossetti Y, Miall RC. Long-lasting aftereffect of a single prism adaptation: shifts in vision and proprioception are independent. Exp. Brain. Res. 2006;173:415–424. doi: 10.1007/s00221-006-0381-2. [DOI] [PubMed] [Google Scholar]
- 21.Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J. Neurophysiol. 2008;100:1455–1464. doi: 10.1152/jn.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nat. Neurosci. 2010;13:923–925. doi: 10.1038/nn.2596. [DOI] [PubMed] [Google Scholar]
- 23.Malone LA, Vasudevan EVL, Bastian AJ. Motor adaptation training for faster relearning. J. Neurosci. 2011;31:15136–15143. doi: 10.1523/JNEUROSCI.1367-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J. Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn. Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 27.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J. Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor JA, Thoroughman KA. Divided attention impairs human adaptation but not feedback control. J. Neurophysiol. 2007;98:317–326. doi: 10.1152/jn.01070.2006. [DOI] [PubMed] [Google Scholar]
- 29.Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichelt AC, Lee JL. Ventral tegmental dopamine dysregulation prevents appetitive memory destabilization. J. Neurosci. 2013;33:14205–14210. doi: 10.1523/JNEUROSCI.1614-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tronel S, Milekic MH, Alberini CM. Linking new information to a reactivated memory requires consolidation and not reconsolidation mechanisms. PLOS Biol. 2005;3(9):1630–1638. doi: 10.1371/journal.pbio.0030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debiec J, Doyère V, Nader K, Ledoux JE. Directly reactivated, but not indirectly reactivated, memories undergo reconsolidation in the amygdala. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3428–3433. doi: 10.1073/pnas.0507168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of 'crystallized' adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- 36.Wu HG, Miyamoto YR, Gonzalez Castro LN, Ölveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat. Neurosci. 2014;17:312–321. doi: 10.1038/nn.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutton RS, Barto AG. Introduction to Reinforcement Learning. 1998 MIT Press. [Google Scholar]
- 38.Braun DA, Aertsen A, Wolpert DM, Mehring C. Motor task variation induces structural learning. Curr. Biol. 2009;19:352–357. doi: 10.1016/j.cub.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charlesworth JD, Tumer EC, Warren TL, Brainard MS. Learning the microstructure of successful behavior. Nat. Neurosci. 2011;14:373–380. doi: 10.1038/nn.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat. Rev. Neurosci. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 41.Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J. Neurosci. 2010;30:17015–17022. doi: 10.1523/JNEUROSCI.4205-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton JL, Wei YJ, Bajaj P, Scheidt RA. Visuomotor learning enhanced by augmenting instantaneous trajectory error feedback during reaching. PLoS ONE. 2013;8(1):1–6. doi: 10.1371/journal.pone.0046466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Beukelaar TT, Wooley DG, Wenderoth N. Gone for 60 seconds: reactivation length determines motor memory degradation during reconsolidation. Cortex. 2014;59:138–145. doi: 10.1016/j.cortex.2014.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.