Abstract
Objective
Vigorous systemic exercise stimulates a cascade of molecular and cellular processes that enhance central nervous system (CNS) plasticity and performance. The influence of heat stress on CNS performance and learning is novel. We designed two experiments to determine whether passive heat stress 1) facilitated motor cortex excitability and 2) improved motor task acquisition compared to no heat stress.
Methods
Motor evoked potentials (MEPs) from the first dorsal interosseus (FDI) were collected before and after 30 minutes of heat stress at 73° C. A second cohort of subjects performed a motor learning task using the FDI either following heat or the no heat condition.
Results
Heat stress increased heart rate to 65% of age-predicted maximum. After heat, mean resting MEP amplitude increased 48% (P < 0.05). MEP stimulus-response amplitudes did not differ according to stimulus intensity. In the second experiment, heat stress caused a significant decrease in absolute and variable error (p < 0.05) during a novel movement task using the FDI.
Conclusions
Passive environmental heat stress 1) increases motor cortical excitability, and 2) enhances performance in a motor skill acquisition task.
Significance
Controlled heat stress may prime the CNS to enhance motor skill acquisition during rehabilitation.
Keywords: heat stress, cortical excitability, motor learning
Introduction
Regular physical activity plays an important role in preventing or reducing the incidence of various chronic age-related physical impairments (Kruk, 2007). Current guidelines recommend 5–7 days per week of exercise performed at an intensity that induces profuse sweating (Haskell et al., 2007). However, only 27% of the adult population in the United States engages in exercise at the recommended level that would provide protection against chronic diseases (Stewart, 2005). As the healthcare costs of age-related chronic diseases rise, it is logical to explore novel interventions that can supplement the benefits of exercise, especially for those people with physical impairments that limit vigorous activity.
In animal models, moderate intensity exercise facilitates availability of brain metabolic enzymes (Tong et al., 2001, Ding et al., 2006), long-term potentiation of hippocampal neurons (Farmer et al., 2004), the expression of brain-derived neurotrophic factor (BDNF) (Berchtold et al., 2005), and an increase in insulin-like growth factor-1 (IGF-1)(Trejo et al., 2001). Synaptic facilitation is prevalent across the primary motor cortex (M1), cerebellum, and hippocampus suggesting that systemic physiological stress in appropriate doses may have a general priming effect for CNS performance [for reviews, see: (Vaynman et al., 2005, Kramer et al., 2007, Smith et al., 2010)]. Indeed, neurochemical adaptations observed with systemic exercise are associated with greater acquisition and retention of complex learning tasks in both young and adult animals (Vaynman et al., 2004, van Praag et al., 2005, Schweitzer et al., 2006). In humans, regular aerobic exercise has been shown to improve fine motor tracking task accuracy of the upper extremity (Bakken et al., 2001), and ameliorate cognitive decline in older adults (Kramer et al., 2007, Voss et al., 2010, Erickson et al., 2011). Moreover, young highly-trained individuals showed enhanced M1 representational plasticity with exercise (Cirillo et al., 2009). Taken together, these studies support a beneficial influence of systemic cardiovascular stress on CNS plasticity, adaptation, and overall health benefits.
Aerobic exercise induces sweating in response to active hyperthermia up to or greater than 1° C in core body temperature. Similar to exercise, acute passive exposure to high ambient temperatures stimulates the sympathetic nervous system (Rowell, 1990), resulting in increased heart rate (Tei et al., 1995) and concentrations of serum catecholamines (Kukkonen-Harjula et al., 1988, Laatikainen et al., 1988). This raises the question of whether systemic passive heat stress, in the absence of muscular exertion, can enhance CNS plasticity in humans. Prior findings from our laboratory have demonstrated that 30 minutes of whole-body heat stress increased heart rate to approximately 65% of age-predicted maximum and core temperature by 0.82° C (Iguchi et al., 2011). Serum catecholamine hormones (norepinephrine and epinephrine) increased approximately 60% and prolactin, an indirect marker of serotonergic neurotransmitter activity, increased nearly three-fold (Iguchi et al., 2011, Iguchi, 2012). Functionally, acute pharmacological enhancement of norepinephrine agonists has been associated with training-dependent increases in evoked motor cortex excitability and motor skill acquisition in the upper extremity (Plewnia et al., 2002, Plewnia et al., 2004). Likewise, administration of serotonin reuptake inhibitors improved accuracy of visual tracking tasks using the hand (Loubinoux et al., 2002). From these findings, we hypothesized that the cascade of neurochemical factors observed during aerobic exercise may also be regulated during passive heat stress and therefore enhance cortical excitability and motor skill acquisition.
To test these hypotheses, we performed two separate experiments, the first investigating cortical excitability measured via transcranial magnetic stimulation after a 30 minute dose of passive whole-body heat stress, and the second, investigating motor skill acquisition on a precision tracking task after a similar dose of heat stress. We specifically hypothesized that 30 minutes of passive whole-body heat stress would increase motor cortical excitability and reduce error during a precision upper extremity tracking task.
Methods
Subjects
For Experiment 1, eleven healthy right-handed individuals (5 female) with no known cardiovascular or neurological disorders, history of seizures, implanted electrodes or pacemaker, or non-dental metal in the head were recruited for participation. Prior to experimental sessions, subjects completed a TMS safety inventory to screen for potential contraindications to TMS (Keel et al., 2001). Subjects completed a control condition (ambient temperature) and an experimental condition (heat stress) with the order being random. For Experiment 2, twenty healthy right-handed male subjects were recruited to assess motor learning after heat stress. Subjects were randomly assigned to a Heat (n=10) or a Control (n=10) group. Pilot data from our laboratory showed that the learning effect of the upper extremity tracking task was partially retained after the initial learning session, necessitating separate subject cohorts, novel to the task, for each test condition (heat and control). Heat and Control group subjects did not differ in activity levels as assessed by the Marx Activity scale (Marx et al., 2001) and the Baecke physical activity questionnaire (Baecke et al., 1982). No subjects in either experiment reported regular participation in heat stress, or long-term skilled use of the hands such as playing of a musical instrument (Rosenkranz et al., 2007). Subjects were instructed to refrain from exhaustive exercise and consumption of caffeine and alcohol during the 24 hours prior to testing. All subjects gave written informed consent in accordance with the University of Iowa Human Subjects Institutional Review Board. Descriptive statistics of patient characteristics are presented in Table 1.
Table 1.
Descriptive statistics of subjects who participated in Experiment 1 and Experiment 2. Note that Experiment 2 involved two separate cohorts for the heat and control groups. The p values are presented for variables measured between the Heat and Control subjects in Experiment 2.
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
|
| ||||
| Variable | (n=11, 5 female) | Heat (n = 10) | Control (n=10) | P value |
| Age | 25.2 ± 5.8 | 26.90 ± 6.17 | 31.00 ± 7.26 | 0.19 |
| Weight (kg) | 75.6 ± 19.2 | 86.02 ± 11.6 | 88.50 ± 13.0 | 0.66 |
| BMI | 24.6 ± 3.2 | 26.88 ± 4.89 | 27.75 ± 5.37 | 0.71 |
| %Body fat | 21.8 ± 5.6 | 21.32 ± 7.16 | 21.07 ± 8.42 | 0.94 |
| Baecke Total | – | 8.09 ± 0.96 | 8.43 ± 1.20 | 0.50 |
| Work | – | 2.11 ± 0.25 | 2.38 ± 0.88 | 0.35 |
| Sport | – | 3.03 ± 0.61 | 2.90 ± 0.91 | 0.72 |
| Leisure | – | 2.95 ± 0.70 | 3.15 ± 0.47 | 0.46 |
| Marx | – | 7.40 ± 5.00 | 7.1 ± 5.60 | 0.88 |
Data expressed as mean ± standard deviation.
Heat Stress Intervention
Whole-body heat stress was induced in a custom-designed environmental heat chamber (Cokato, MN). The temperature of the chamber was thermostatically regulated to 73°C at face level (relative humidity 10%). Subjects’ body weight was recorded 5 minutes prior to entering the chamber to quantify fluid mass lost through dehydration. Subjects sat in the chamber for 30 minutes, though they were permitted to exit the chamber prior to the targeted 30-minute dose if unable to tolerate the entire duration. After exiting the chamber, subjects sat for 15 minutes at ambient room temperature prior to cortical excitability measures or motor task performance. All subjects were given water and drank an amount equal to their fluid loss (by weight) prior to post-heat measurements.
Tympanic temperature, heart rate, and thermal sensation
Tympanic temperature was measured using a tympanic membrane infra-red sensor (ThermoScan IRT 4520 Braun, Kronberg, Germany). The tympanic membrane shares the same blood supply with the hypothalamus (Gray, 1977). As such, tympanic temperature may be representative of brain temperature in the thermoregulation centers, particularly when it is expected to change rapidly during or after the heat intervention. Temperatures were recorded prior to entering the chamber, immediately upon exiting, and at 5 minute intervals for 15 minutes thereafter. Heart rate was measured continuously via a thoracic Polar heart rate monitor (Polar Electro Inc., Woodbury NY) transmitted to a wireless data recorder (MSR Electronic GmbH, Winterthur, Switzerland) beginning 5 minutes prior to entering the heat chamber until 15 minutes after exiting. Subjects rated subjective thermal sensation on a 13-point scale (1=So Cold I am Helpless; 7 = Comfortable; 13 = So Hot I am Sick and Nauseated) during the heat intervention at 5 minute intervals, starting 5 minutes prior to heating until 15 minutes after exiting the chamber (Hollies, 1977).
Transcranial Magnetic Stimulation
In Experiment 1, stimulation of the non-dominant right motor cortex was delivered by a Magstim 2002 stimulator (Magstim Company Ltd., Whitland, Dyfed, UK) equipped with a 70 mm diameter figure-of-eight coil. The coil was positioned tangentially to the skull surface at a 45° angle to the sagittal plane with the handle oriented posterolaterally creating posterior-to-anterior current flow over the cortical surface (Brasil-Neto et al., 1992, Werhahn et al., 1994). The coil was moved in 1 cm increments systematically across the scalp surface to locate the site eliciting the largest MEPs (motor “hotspot”) in response to a supra-threshold intensity pulse (~50–60% maximal stimulator output). The experimental coil position was referenced to a 3-D head marker affixed to the subject’s forehead that was digitized to four anatomical landmarks (ear tragi, tip of nose, and skull vertex) and recorded by a Polaris infrared 3-D positional tracking camera (Northern Digital, Inc., Waterloo, Ontario, Canada). Coil position during stimulation was maintained within maximum error tolerance of 2 mm of tangential translation and 2° of planar deviation (pitch/roll) and coil rotation (yaw).
The resting motor threshold (RMT) was determined by stimulating over the hotspot with a supra-threshold magnetic pulse. Intensity was decreased in 1–2% increments of maximal stimulator output (MSO) until the stimulus became sub-threshold (Rossini et al., 1994). RMT was defined as the minimum intensity sufficient to elicit MEPs with amplitude ≥ 50 μV on at least 4 of 8 consecutive pulses. All experimental stimulus intensities were normalized as a percentage of each subject’s respective RMT.
Motor evoked potentials were recorded from the first dorsal interosseus (FDI) muscle of the left hand with bipolar Ag-AgCl electrodes (8mm diameter with 20 mm inter-electrode distance). The common ground electrode was affixed to the anterior tibia of the ipsilateral leg. EMG signals were pre-amplified on-site by a factor of 35 before being differentially amplified. The differential amplifier had an input impedance of 15 MΩ at 100Hz, a frequency response of 15–1000 Hz, a common mode rejection ratio of 87 dB at 60 Hz and gain of 500–10K times. EMG data were amplified (1–5k), filtered (20–400Hz), digitally sampled at 2 KHz, and stored on a microcomputer. Analog EMG signals were digitized for offline analysis using custom software. MEPs were quantified as the peak-to-peak MEP amplitude taken from a time window 20–50ms after the magnetic stimulus pulse onset.
MEPs from the FDI were collected over the cortical representation surrounding the motor hotspot using the 15-point motor map previously described (Littmann et al., 2013). Five MEPs were recorded at each map locus at a stimulus intensity equal to 120% RMT (75 MEPs total). MEP intensity curves were obtained at the motor hotspot by delivering 5 pulses at 80, 100, 120, 140, and 160% of RMT intensity (25 MEPs total). Upon completion of the pre-heat mapping and recruitment procedures, the FDI surface electrode, ground, and head reference marker were removed for the heat stress intervention (or control). The electrodes and reference marker were replaced according to skin site markings approximately 20 minutes after the subject had exited the chamber. Pilot data showed that skin temperature of the hand decreased quickly taking less than 10 min to return to baseline temperature. Error in head marker position relative to the digitized anatomical landmarks after replacement averaged less than 1.01 ± 0.48 mm and 1.09 ± 0.61 mm for the Control and Heat conditions, respectively. The timeline of the experimental protocol is shown in Figure 1.
Figure 1.
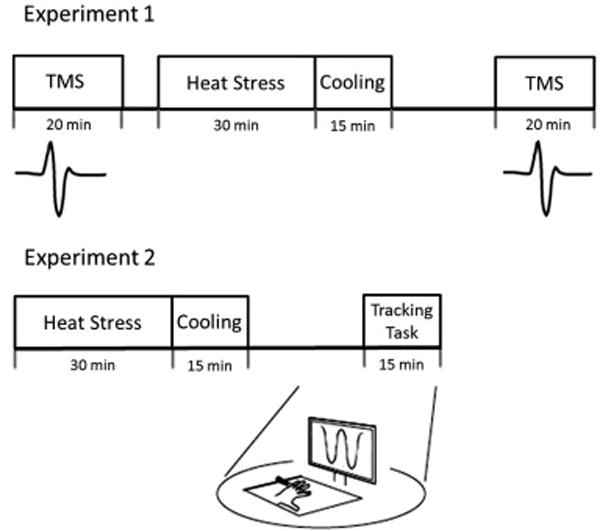
Schematic timeline of the group receiving heat stress for experiment 1 (upper figure) and experiment 2 (lower figure). In both experiments there were control conditions consisting of ambient room temperature exposure.
Motor Acquisition Task
Subjects performed a visually-guided fine motor tracking task. Briefly, subjects sat facing a computer screen with the left hand stabilized with palm down on a custom metal frame. The index finger was attached by a cuff to a pulley housing a potentiometer. A weighted lanyard resisted abduction at approximately 5% of isometric maximum voluntary contraction force (MVC). Custom computer software (Shields et al., 2005, Madhavan et al., 2011) generated a progressive sine wave trace across the screen that served as the tracking target. Subjects controlled the vertical position of a cursor using the first dorsal interossei for abduction (downward cursor displacement) and adduction (upward displacement) of the index finger, attempting to overlay the cursor trace over the target trace. End points of the sinusoidal target reflected approximately 15 degrees of abduction/adduction of the metacarpal phalangeal joint (MCP). Both traces were simultaneously generated in real time, such that subjects did not have prior visual feedback of the target position. A numerical absolute error score was displayed on the computer screen after each trial, providing the subject with knowledge of results. Change in absolute and variable tracking error over 10 blocks constituted the magnitude of motor skill acquisition.
Experimental Protocol
Schematic timelines for both experiments are shown in Figure 1. In Experiment 1, 11 subjects participated in 2 sessions, separated by at least 14 days and performed at a similar time of the day for each subject. Testing on each day began with acquisition of MVC EMG for the FDI muscle in the motor task apparatus. After 2–3 warm-up contractions, subjects performed three 5-second abduction MVCs with the index finger. Subjects were given visual feedback of the exerted torque and were verbally encouraged. Subjects rested approximately one minute between MVCs. The MVC producing the greatest peak torque was used as the normalizing factor for EMG measurements (mid 200 milliseconds centered on peak). The MVC EMG was repeated after 15 minutes of cooling (or 15 minutes of control condition) and occurred at least 10 minutes before the testing of the MEPs.
The mean peak MVC torque was 1.51 ± 0.21 and 1.46 ± 0.19 Nm before and after the heat stress condition (p = 0.41), respectively; while the mean peak EMG (RMS) was 2.1 ± 0.28 and 2.26 mV ± 0.23 before and after the heat stress condition (p = 0.32). The mean peak MVC torque was 1.49 ± 0.19 and 1.53 ± 0.22 Nm before and after the control condition (p = 0.32), respectively; while the mean peak EMG (RMS) was 1.9 ± 0.26 and 2.15 mV ± 0.21 after the control condition (p = 0.28). These findings are consistent with our previous reports (Iguchi et al., 2011). Extensive preliminary data also support that three MVC’s followed by 10 minutes rest do not alter MEPs (Iguchi et al., 2011). In addition, pilot data from our lab support that M waves are highly reproducible before versus after heat stress (8.12 mV versus 7.8 mV; p = 0.6). For these reasons we were confident that normalizing the MEP’s to both the pre heat/post heat MVC EMG fully accounted for any fluctuations in skin impedance. Subjects next underwent cortical excitability testing procedures at least 10 minutes after the MVC testing and prior to and following a 30-minute heat stress intervention (Heat), or in the chamber at ambient room temperature (Figure 1). Procedures were identical between the Heat and Control condition sessions except for the temperature of the environmental heat chamber.
In Experiment 2, twenty subjects received 30 minutes of passive heat stress (n=10) or control conditions (n=10) and then performed 10 training blocks of the tracking task, each comprising 5 sinusoidal movement cycles at 0.4 Hz, with one minute rest given between trials.
Data Analysis
Three tympanic temperatures and two heart rate measures were averaged for each time interval collected. Cortical excitability was quantified by the mean MEP amplitude of the 15-point motor map. Five MEPs were collected at each of the 15 map loci and averaged by map locus. MEP map amplitude represented the mean MEP amplitudes of the 15 points. MEP amplitude was normalized to the rectified EMG signal of each subject’s maximal voluntary contraction (MVC) and expressed as a percent of MVC for analysis. For clarity, the MEP amplitudes were expressed as a ratio of the post-heat MEP amplitude divided by pre-heat MEP amplitude. MEP amplitude ratios were calculated for each intensity of the recruitment procedure.
Tracking task learning was quantified by the reduction in absolute and variable error between Block 1 and Block 10. Absolute error represented the mean absolute value of the difference in displacement between subject finger trace and the target trace every 100 ms of the task. Variable error represented the standard deviation of absolute error within a trial every 100 ms of the task. Error scores were normalized as a percentage of Block 1 error.
Statistical Analyses
One-way repeated measures analysis of variance (ANOVA) was performed for time effects relative to baseline on temperature, heart rate, and thermal comfort during the heat stress procedure. Student’s T tests were used to test for group differences in age, body weight, body fat percentage, body mass index (BMI), and activity scores. A two-way Analysis of Variance (ANOVA) with repeated measures for time and conditions was used to test for an interaction using the normalized cortical excitability data. A subsequent one-way Analysis of Variance was also used to demonstrate changes in the ratio of the post/pre MEP measures. A Split-Plot repeated measures ANOVA was used to test for differences between the Heat and Control groups for the skill acquisition tasks in experiment 2. Pearson correlations were performed to test the association of stress variables (HR, temperature, thermal sensation, fluid loss), subject demographics (body weight, body fat %) with learning success and cortical excitability. In the text, results are expressed as mean ± standard deviation (SD), whereas in the figures, the error bars represent standard error (SE). After testing for significant interaction, main effects or simple effects analyses were carried out as appropriate. Results of all analyses were considered significant at P ≤ 0.05. All statistical analyses were performed using SPSS 19 for Windows software package.
Results
Overall Responses to Heat Stress
All subjects in the Heat condition completed 30 minutes of passive heat stress. Heart rate, tympanic temperature, and thermal sensation responses are presented in Table 2. Tympanic temperature increased rapidly by approximately 2° C by the end of the 30 minutes of heat stress. Mean tympanic temperature using this protocol showed subjects return near baseline (< 0.25° C difference) by 25–30 minutes after leaving the chamber (Iguchi et al., 2011), the approximate time of the cortical mapping and MEP recruitment procedures. HR increased to 126–128 beats/min after 30 minutes of heat, or approximately 65% of age-predicted maximum. HR did not differ from the pre-heat baseline 15 minutes after exiting the chamber prior to the TMS study. After sitting in the heat chamber for 30 minutes, subjects felt “hot” to “very hot”, with mean thermal sensation rating reaching 10.8 ± 0.75 (11 = very hot). Fifteen minutes after exiting the chamber, thermal sensation did not differ from baseline, decreasing to 7.1 ± 0.38 (7 = comfortable). Males and females did not differ in maximum tympanic temperature (p=0.37), heart rate (p=0.08), or thermal comfort (p=0.43) during the heat stress protocol.
Table 2.
Physiological responses to heat stress. Measurements were taken immediately prior to entering the chamber (baseline), immediately after exiting (Post0), and 15 minutes after the heat stress (Post15).
| Experiment 1 | Experiment 2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Baseline | Post0 | Post15 | Baseline | Post0 | Post15 |
| HR (beats/min) | 69.4 ± 12.3 | 128.8 ± 15.1** | 81.7 ± 12.2 | 67.0 ± 7.4 | 126.1 ± 16.0** | 75.9 ± 9.3 |
| Temp (° C) | 36.7 ± 0.26 | 38.7 ± 0.41** | 37.2 ± 0.59* | 36.7 ± 0.18 | 38.6 ± 0.51** | 36.8 ± 0.24 |
| Thermal Sensation | 6.8 ± 0.87 | 10.8 ± 0.75** | 7.1 ± 0.38 | 6.8 ± 0.42 | 10.7 ± 1.3** | 7.1 ± 0.33 |
| Body Weight (kg) | 75.6 ± 19.2 | 75.1 ± 19.1 | – | 86.0 ± 11.6 | 85.4 ± 11.6 | – |
| Body Weight (%change) | – | 0.69 ± 0.24 | – | – | 0.74 ± 0.29 | – |
= significantly increased from baseline (P < 0.05);
= (P < 0.01)
In Experiment 2, subjects’ tympanic temperature, HR, and thermal comfort responded similarly to subjects in Experiment 1. After 30 minutes of heat, temperature increased from 36.7 ± 0.2 to 38.6 ± 0.51° C (1.9° C increase from control), HR from 67.0 ± 7.4 to 126.1 ± 16.0 beats/minute. Subjects rated thermal comfort sensation as hot to very hot (10.7 ±1.3). All measures returned to baseline level 15 minutes after exiting the heat chamber.
Experiment 1: Cortical Map Excitability
The mapping and recruitment procedures commenced approximately 25 minutes after subjects left the chamber accounting for cooling time and replacement of recording electrodes. Representative examples of motor evoked potentials collected from a single male subject during the TMS mapping and recruitment procedures are shown in Figure 2A and 2B. Baseline MEP amplitude of the motor map for Control sessions previously showed high reliability (ICC = 0.80)(Littmann et al., 2013) Mean peak-to-peak MEP amplitude of the motor map decreased slightly after people sat quietly in the chamber set at an ambient temperature (Control condition) from 139.1 ± 120.0% MVC to 115.9 ± 91.6% MVC for the pre and post measurements, respectively. Conversely, after sitting in the chamber that was heated, the MEP increased from 111.6 ± 118.9% MVC to 142.26 ± 146.8% MVC. There was a significant Group (Heat vs. Control) × Time (pre vs. post) interaction of MEP amplitude (F(1,10)=10.23, P = 0.01). The MEP amplitude ratio was significantly increased after heat stress as compared to the no heat stress condition (P < 0.05). The mean MEP amplitude ratio was 0.96 ± 0.45 in the Control condition and 1.48 ± 1.1 for the Heat condition, indicating a mean 48% increase in MEP amplitude as a result of heat stress compared to a 4% decrease from the no heat stress condition (p < 0.05) (Figure 3A).
Figure 2.
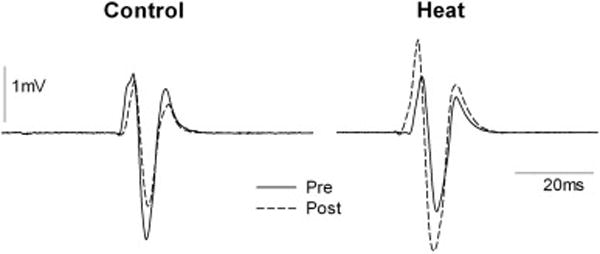
Representative examples of single motor evoked potentials from transcranial magnetic stimulation (TMS) collected from a single male subject during the control condition and the heat condition. MEPs were recorded at the motor hotspot at a fixed intensity of 120% RMT.
Figure 3.
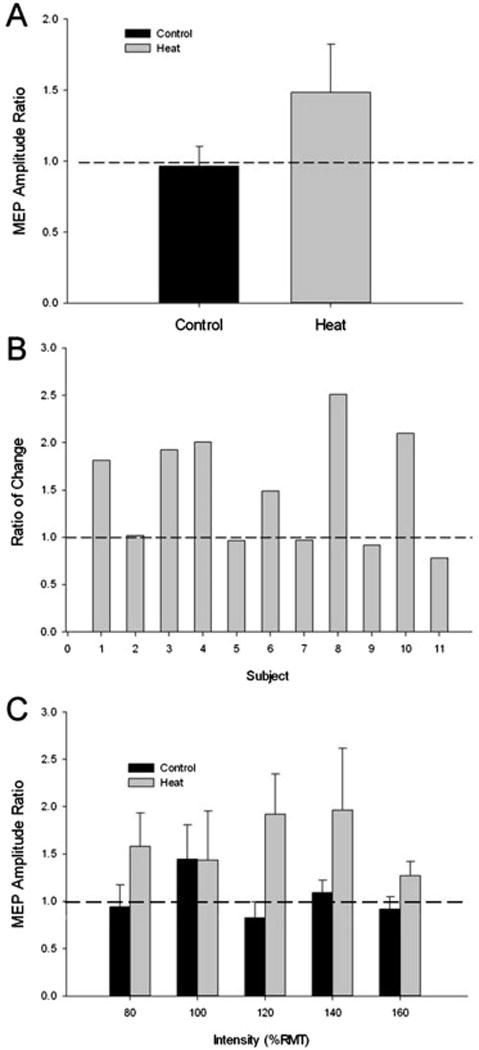
The MEP responses to heat stress. (A) The Ratio (post/pre) of the MEP amplitude measured after 30 minutes of no heat stress (Control) or after 30 minutes of heat stress (Heat) (p < 0.05). (B) The Ratio of the change in MEP amplitude ((Heat (post/pre)/Control (post/pre)) for each subject in the study. Six of the eleven subjects (5 male; subjects 1, 3, 4, 8, 10) showed 49% or greater increase in MEP amplitude ratio after the heat condition compared to the control condition. (C) The MEP amplitude ratio for each intensity assessed during the TMS protocol. There was an overall significant main effect for heat stress in the Heat condition, (P = 0.05).
We performed a comparison of the magnitude of change displayed by each subject between conditions. Figure 3B displays a comparison of the magnitude of change in the Heat condition relative to the Control condition (Ratio of Change) for each subject in the study. Six subjects (5 male) showed greater than 49% increase in MEP amplitude with heat stress compared to no heat stress. To assess for differential responses to heat stress between sexes, we tested for Group (male vs. female) by Condition (Heat vs. Control) effects. Male MEP amplitude ratios were 2.02 ± 1.3 and 1.07 ± 0.48 for the Heat and Control conditions, respectively, while female MEP amplitude ratios were 0.84 ± 0.43 and 0.84 ± 0.35 for Control and Heat, respectively. There was a significant Group × Condition interaction (F(1,9) = 5.89, P = 0.038), where males showed significantly greater increase in MEP amplitude ratio in the Heat condition (P = 0.02) but not in the control condition (P = 0.61).
For the MEP recruitment procedure, there was no significant Condition × Intensity interaction (F(4,40) = 1.45, P = 0.47) or significant main effects of Intensity (F(4,40) = 0.617, P = 0.67) or Condition (F (1,10) = 9.63, P = 0.056) though MEP amplitude ratio showed a trend to increase after heat stress. MEP amplitude ratio was 1.63 ± 0.86 for the Heat group and 1.04 ± 0.56 for the Control group. Separate analysis of males and females revealed a significant main effect of Condition in male subjects, (F(1,5) = 7.66, P = 0.039) wherein MEP amplitude ratio was significantly greater after heat stress. No significant interaction or main effect was present in female subjects.
Pre and post-heat stress coordinates of the center of gravity (CoG) of the motor map were calculated to determine whether heat stress exerted a directional influence on the distribution of excitability in M1. Mean CoG shift was 2.46 ± 1.2 mm and 2.68 ± 1.2 mm for the Control and Heat conditions, respectively. In the Control condition, reliability of the CoG was high for the X-coordinate (ICC = 0.83) and low to moderate for the Y-coordinate (ICC = 0.32). In the Heat condition, X-coordinate reliability was high (ICC = 0.92) and moderate for the Y-coordinate (ICC = 0.62). We have previously demonstrated high reliability of this measurement in the current protocol with mean CoG shift of 2.79 ± 1.3 mm in control subjects (Littmann et al., 2013). There was no significant shift in CoG position following heat stress.
Motor Skill Acquisition Task
All subjects showed rapid learning of the tracking task. Absolute error decreased by 50.1% and 55.4% from Trial 1 to Trial 10 for the Control and Heat groups, respectively, while variable error decreased by 33.6% and 40.8% for control and heat groups, respectively (Figure 4). No significant Group × Block interaction was found for either absolute (F (9,199) = 0.54, P < 0.85) or variable error (F (9,199) = 1.21, P < 0.29). There was a significant main effect for Group indicating that the absolute error (F (9,199) = 45.2, P < 0.001) and variable error (F (9,199) = 39.14, P < 0.001) were less for the heat condition. Follow-up tests also showed absolute and variable error during Trials 2–10 were significantly less than Trial 1 (P < 0.001) but did not decrease after Trial 7 for absolute error and after Trial 5 for variable error. An analysis of covariance (ANCOVA) using baseline error measures as the covariate supported that groups were not different at the start of the skill acquisition trial.
Figure 4.
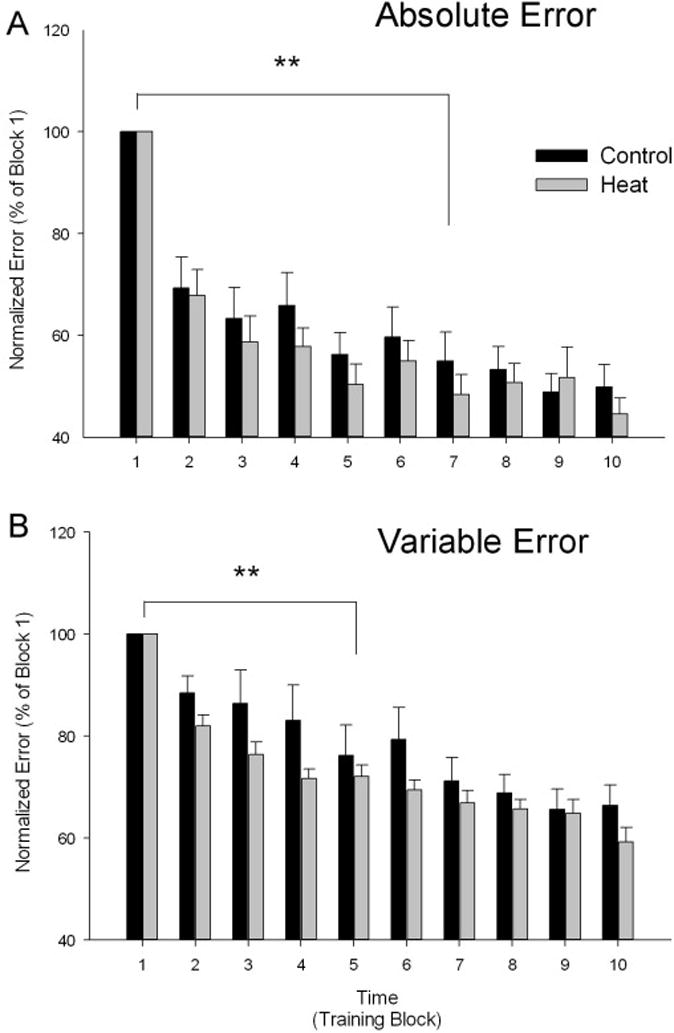
(A) Absolute error, normalized as the percent of block 1 error, depicted for the Control (black bars) and Heat (gray bars) groups showing an overall reduction in error during the Heat condition (p < 0.05). (B) Variable error, normalized as the percent of block 1 error, depicted by for the Control (black bars) and Heat (gray bars) groups showing an overall reduction in error during the Heat condition (p < 0.05). Error bars are standard errors.
**indicates the first Block error that was significantly reduced from baseline (P <0.01) with all subsequent Block error groups less than baseline (p <0.01).
We hypothesized that those subjects showing the greatest sympathetic response from the stress (HR, tympanic temperature, or thermal sensation) may show a greater change in MEP amplitude of the motor map, MEP intensity curve, or magnitude of motor skill acquisition. However, no significant correlations were present between the magnitude of the sympathetic responses to stress and the magnitude of change in skill acquisition or MEP amplitude measurements. Importantly, males did not show a greater stress response than females as determined by HR.
Discussion
Maintaining central nervous system health and performance across the lifespan is an important public health care goal. Low exercise compliance or limited participation in many individuals after injury suggests the need for interventions that supplement physical activity to achieve a desired rehabilitation outcome. Whole body heat stress may serve as an adjunct that will assist those who are not able to exercise at a level to trigger systemic stressors to adapt CNS responsiveness. However, additional studies are necessary to understand the physiological responses of passive heat stress.
The primary aim of this study was to determine whether whole-body heat stress induced acute enhancement of CNS excitability and skill acquisition. The main findings were: (1) a single 30-minute bout of heat stress increased resting excitability of the FDI motor cortex. Males showed significantly greater M1 excitability after heat stress than females, (2) MEP stimulus-response amplitudes were significantly increased after heat stress, but did not differ according to stimulus intensity between groups. Males showed significantly greater MEP enhancement across stimulus intensities than female subjects and, (3) motor skill acquisition was improved in subjects who experienced the heat stress condition.
Cardiovascular, Heart Rate and Subjective Response to Heat Stress
The increase in HR with heat stress in the present study was consistent with previous findings from our laboratory using the same heat stress protocol (Iguchi et al., 2011). The increase in HR is a compensatory response to avoid a large drop in mean arterial blood pressure, so that the cardiac output can be relatively stable even with reduced stroke volume (Kiss et al., 1994). HR for subjects in both experiments increased to roughly 65% of age-predicted maximum HR (220 – age), which is a HR response consistent with moderate exercise (Gibbons et al., 1997). Tympanic temperature rose rapidly by approximately 2.0° C. This change in temperature is roughly equivalent to a rectal temperature of 0.8° C (Iguchi et al., 2011). By the end of the heat stress, subjects felt “very hot” on average. All three cardiovascular indicators returned to baseline prior to subsequent testing in evoked potentials or motor learning. Importantly, the cardiovascular and skin changes induced in the present study are similar in magnitude to those previously demonstrated to increase serum concentrations of prolactin, catecholamine hormones, skin temperature, and heat shock proteins (Iguchi et al., 2011).
Motor Cortical Excitability after Heat Stress
The mean MEP amplitude of the 15-point cortical map increased approximately 48% in response to 30 minutes of heat exposure. This contrasts with a 4% decrease in MEP amplitude measured during the control condition. The minimal change in mean MEP amplitude observed in the control condition supports that excitability after heat stress was not significantly influenced by the TMS procedures themselves; in fact, the time sitting in the chamber at an ambient room temperature induced a reduction in cortical excitability. MEPs after heat stress showed a global pattern of increased excitability without an obvious directional effect on the location of the motor map CoG. The mean shift in CoG map after heat stress was within the range of natural variability (~2.7 mm) previously established for the mapping protocol (Littmann et al., 2013). The absence of a shift in CoG after heat stress suggests there was no redistribution of the center of cortical excitability in response to the intervention that might be observed if excitation and inhibition modulated differently among adjacent M1 regions.
Somewhat unexpectedly, males showed significantly greater increase in MEP amplitude despite no differences in mean increase of tympanic temperature, heart rate, or perceived level of thermal sensation between sexes. Five of six male subjects showed MEP enhancement of 49–150% after heat stress compared to the control condition. Only 1 of 5 female subjects showed similar MEP enhancement. Several factors may contribute to this result. Kuo and colleagues (2006) reported gender differences in response to transcranial direct current stimulation (tDCS) wherein excitability-diminishing neuroplasticity was enhanced in female subjects (Kuo et al., 2006). The authors hypothesized that sex hormone may contribute to the observed differences between male and female subjects.
Additionally, cerebral blood flow decreases during passive heat stress (Wilson et al., 2006, Nelson et al., 2011); though blood flow differences have not been detected between sexes (Nelson et al., 2011) or were established for male subjects only (Wilson et al., 2006). Ross and colleagues recently evoked motor potentials from the vastus lateralis measured in parallel with cerebral blood flow velocity (CBFv) during progressive passive hyperthermia to 39° C (Ross et al., 2012). CBFv and cortical voluntary drive both decreased with core temperatures elevated 0.5–2.0°C; however, corticospinal excitability measured by the MEP response to TMS was unaltered. This study differed from the present investigation in that core temperature was elevated over a period of 160 minutes (40 minutes per 0.5° C of temperature increase). Measures were obtained in the hyperthermic state, such that brain temperature likely differed between the studies. In our study, all subjects returned to their baseline condition as determined by HR, thermal tolerance, and temperature; however, we have previously shown elevation of prolactin, catecholamines, and heat shock proteins at 30 minutes post heat stress.
A novel component of the current study was that all post-heat measures of cortical excitability and motor performance were collected at 30 minutes after the heat stress terminated and tympanic temperature had returned to or near baseline. It has been shown in our laboratory and others that young females are more fatigue resistant than young males in certain muscular fatigue tasks (Hunter et al., 2004, Martin et al., 2006, Iguchi et al., 2011). Though subjects did not perform muscular exertion in this study, heat stress is implicated in the development of central fatigue (Todd et al., 2005, Periard et al., 2014) wherein loss of performance develops secondary to reduced neural output, independent of the force generating capacity of a muscle [for review see (Gandevia, 2001)]. The onset of central fatigue is likely influenced by the interaction of serotonin, dopamine, and norepinephrine. We demonstrated that prolactin, an indirect marker of serotonin availability, increases nearly three-fold in the present heat stress protocol (Iguchi et al., 2011). Thus, it is conceivable that heat stress modulates neurotransmitters in distinct patterns based on sex.
Todd et al. reported central fatigue measured by twitch interpolation of the elbow flexors during hyperthermia to 38.5° C (Todd et al., 2005). In the present study, cortical excitability measures were taken at rest rather than during voluntary activation thus it is not possible to determine whether excitability changes resulted from decreased cortical drive upstream from the motor cortex or at the spinal level. The neurochemical responses of this protocol suggest that serotonin would still be elevated in all subjects during testing. However in the present study it is not possible to determine whether modulations in the MEP are due to purely cortical or a combination of cortical and spinal circuitry changes.
Heat Stress and Motor Skill Acquisition
Movement or muscle-specific learning interventions are associated with an increase in global excitability or localized expansion of the cortical representation of the muscles of interest (Pascual-Leone et al., 1995, Classen et al., 1998). Facilitation of MEP amplitude observed during task acquisition and practice is considered to be a key component of early motor skill acquisition (Sanes et al., 2000, Muellbacher et al., 2001). The tracking task used in the present study induced significant global potentiation of MEP amplitude in the FDI representation during resting motor recruitment using TMS (unpublished data). For this reason we hypothesized that increases in motor cortex excitability induced by heat stress might translate into greater motor skill acquisition. Because we observed significantly greater cortical excitability in males during Experiment 1, we carried out Experiment 2 using only male subjects, working under the assumption that greater cortical excitability should enhance motor learning. In experiment 2, both Heat and Control group subjects (males) showed motor skill learning with each trial of the visual motor task. This response was expected. Most interesting, however, was the consistent reduction in error in the group of subjects who experienced the heat condition. While there was no significant interaction (indicating that the control group and heat group responded similarly), there was a significant group main effect. When tracking error was analyzed using the non-normalized absolute error or the normalized absolute error, the heat group showed consistently showed higher rates of error reduction when compared to the control group. Interestingly, we recently observed a similar trend in our laboratory while measuring a tracking task performance task of the lower extremities following a similar heat stress condition (unpublished data). The question arises whether the two groups had different pre-existing capacities in the rate or degree of motor skill acquisition. Similarity between the groups in BMI, body fat percentage and activity level (Table 1) suggest that these factors did not account for differences in skill acquisition. Kleim and colleagues (2006) reported a reduction in training-dependent increases in the amplitude of MEP’s in healthy subjects with a common single nucleotide polymorphism in the BDNF gene (Val66Met) in response to precision motor task performance using the hand (Kleim et al., 2006). Reduced cortical excitability has also been observed in individuals with the Val66Met polymorphism with transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) (Cheeran et al., 2008, Antal et al., 2010). A limitation of the present study was that any potential group differences in BDNF genotype that may influence cortical excitability was not determined.
Coupled to the enhanced skill acquisition, the potentiation of MEPs seen in males in Experiment 1 may have important ramifications for long term retention and learning. Positive correlations between motor learning success and the magnitude of MEP facilitation have been reported in the upper extremity (Muellbacher et al., 2001, Ziemann et al., 2001, Muellbacher et al., 2002, Smyth et al., 2010); with significant increases induced by ballistic contractions using concentric biceps brachii flexion (Ziemann et al., 2001) or isometric pinch force (Muellbacher et al., 2001). In the latter study, no relationship was found between excitability and motor performance of graded ramp contractions using visual feedback of the EMG signal, similar to the position feedback of the present study. At the same time, early enhancement of MEPs is implicated in motor skill consolidation, wherein newly acquired motor skills become resistant to contextual interference over time (Sanes et al., 2000, Muellbacher et al., 2001, Muellbacher et al., 2002). Facilitation of MEPs due to systemic stress during the initial stages of learning may lead to increased learning retention over time. In fact, mild systemic stress in mice facilitates spatial learning retention over 1 week and up-regulates BDNF gene expression in the hippocampus (Adlard et al., 2011). Future investigations of the influence of heat stress on learning should include within subject comparison of M1 excitability and learning success to direct comparison of heat-induced excitability changes and the magnitude of task learning. Moreover, longitudinal studies of heat stress and learning will provide valuable information on the nature of systemic stress and learning after the motor consolidation period.
Conclusions
Whole body heat stress may be an effective intervention to enhance movement control and learning. Establishing the acute effects of passive heat stress on the CNS excitability and motor skill acquisition is an important first step to developing an effective intervention to enhance long-term CNS health and performance. Whole-body heat stress acutely increased motor cortex excitability globally across the cortical representation of a hand intrinsic muscle. Future studies should examine the effect of chronic whole body heat stress training in healthy individuals and those with CNS impairments to determine whether this novel intervention is a plausible strategy to enhance CNS plasticity and movement control. In addition, the modulation of heat stress response between males and females and the influence of heat stress on neurotrophic factors warrant further examination.
Highlights.
Heat stress may be a useful way to prime the central nervous system to enhance movement skill acquisition.
Heat stress increased motor cortical excitability.
Heat stress enhanced learning during a motor skill acquisition task.
Acknowledgments
This study was supported in part by awards to RKS from the National Institute of Health (R01HD084645, R01HD082109).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
References
- Adlard PA, Engesser-Cesar C, Cotman CW. Mild stress facilitates learning and exercise improves retention in aged mice. Exp Gerontol. 2011;46:53–9. doi: 10.1016/j.exger.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–7. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bakken RC, Carey JR, Di Fabio RP, Erlandson TJ, Hake JL, Intihar TW. Effect of aerobic exercise on tracking performance in elderly people: a pilot study. Phys Ther. 2001;81:1870–9. [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9:132–6. [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo J, Lavender AP, Ridding MC, Semmler JG. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol. 2009;587:5831–42. doi: 10.1113/jphysiol.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–23. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci. 2006;24:1265–76. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108:3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Gray H. The Arteries Anatomy, descriptive and surgical. New York: Bounty Books; 1977. [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Hollies NRS, Goldman RF. Clothing Comfort: Interaction of Theram, Ventilation, Construction, and Assessment Factors. Science; Ann Arbor, MI: 1977. 1977 ed. [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol. 2004;96:2125–32. doi: 10.1152/japplphysiol.01342.2003. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Littmann AE, Chang S-H, Wester L, Knipper J, Shields RK. Heat stress and cardiovascular, hormonal, and heat shock proteins in humans. J Athl Train. 2012;47:184–90. doi: 10.4085/1062-6050-47.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi M, Shields RK. Prior heat stress effects fatigue recovery of the elbow flexor muscles. Muscle Nerve. 2011;44:115–25. doi: 10.1002/mus.22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kiss D, Popp W, Wagner C, Zwick H, Sertl K. Effects of the sauna on diffusing capacity, pulmonary function and cardiac output in healthy subjects. Respiration. 1994;61:86–8. doi: 10.1159/000196312. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–8. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kruk J. Physical activity in the prevention of the most frequent chronic diseases: an analysis of the recent evidence. Asian Pac J Cancer Prev. 2007;8:325–38. [PubMed] [Google Scholar]
- Kukkonen-Harjula K, Kauppinen K. How the sauna affects the endocrine system. Ann Clin Res. 1988;20:262–6. [PubMed] [Google Scholar]
- Kuo MF, Paulus W, Nitsche MA. Sex differences in cortical neuroplasticity in humans. Neuroreport. 2006;17:1703–7. doi: 10.1097/01.wnr.0000239955.68319.c2. [DOI] [PubMed] [Google Scholar]
- Laatikainen T, Salminen K, Kohvakka A, Pettersson J. Response of plasma endorphins, prolactin and catecholamines in women to intense heat in a sauna. Eur J Appl Physiol Occup Physiol. 1988;57:98–102. doi: 10.1007/BF00691246. [DOI] [PubMed] [Google Scholar]
- Littmann AE, McHenry CL, Shields RK. Variability of motor cortical excitability using a novel mapping procedure. J Neurosci Methods. 2013;214:137–43. doi: 10.1016/j.jneumeth.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I, Pariente J, Rascol O, Celsis P, Chollet F. Selective serotonin reuptake inhibitor paroxetine modulates motor behavior through practice. A double-blind, placebo-controlled, multi-dose study in healthy subjects. Neuropsychologia. 2002;40:1815–21. doi: 10.1016/s0028-3932(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Madhavan S, Shields RK. Neuromuscular responses in individuals with anterior cruciate ligament repair. Clin Neurophysiol. 2011;122:997–1004. doi: 10.1016/j.clinph.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol. 2006;95:3512–8. doi: 10.1152/jn.01230.2005. [DOI] [PubMed] [Google Scholar]
- Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29:213–8. doi: 10.1177/03635465010290021601. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–8. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–4. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Haykowsky MJ, Stickland MK, Altamirano-Diaz LA, Willie CK, Smith KJ, et al. Reductions in cerebral blood flow during passive heat stress in humans: partitioning the mechanisms. J Physiol. 2011;589:4053–64. doi: 10.1113/jphysiol.2011.212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–45. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Periard JD, Christian RJ, Knez WL, Racinais S. Voluntary muscle and motor cortical activation during progressive exercise and passively induced hyperthermia. Exp Physiol. 2014;99:136–48. doi: 10.1113/expphysiol.2013.074583. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Cohen LG, Gerloff C. Improved motor skill acquisition after selective stimulation of central norepinephrine. Neurology. 2004;62:2124–6. doi: 10.1212/01.wnl.0000128041.92710.17. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Hoppe J, Hiemke C, Bartels M, Cohen LG, Gerloff C. Enhancement of human cortico-motoneuronal excitability by the selective norepinephrine reuptake inhibitor reboxetine. Neurosci Lett. 2002;330:231–4. doi: 10.1016/s0304-3940(02)00803-0. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Williamon A, Rothwell JC. Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci. 2007;27:5200–6. doi: 10.1523/JNEUROSCI.0836-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross EZ, Cotter JD, Wilson L, Fan JL, Lucas SJ, Ainslie PN. Cerebrovascular and corticomotor function during progressive passive hyperthermia in humans. J Appl Physiol. 2012;112:748–58. doi: 10.1152/japplphysiol.00988.2011. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–7. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Schweitzer NB, Alessio HM, Berry SD, Roeske K, Hagerman AE. Exercise-induced changes in cardiac gene expression and its relation to spatial maze performance. Neurochem Int. 2006;48:9–16. doi: 10.1016/j.neuint.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Shields RK, Madhavan S, Gregg E, Leitch J, Petersen B, Salata S, et al. Neuromuscular control of the knee during a resisted single-limb squat exercise. Am J Sports Med. 2005;33:1520–6. doi: 10.1177/0363546504274150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C, Summers JJ, Garry MI. Differences in motor learning success are associated with differences in M1 excitability. Hum Mov Sci. 2010;29:618–30. doi: 10.1016/j.humov.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Stewart KJ. Physical activity and aging. Ann N Y Acad Sci. 2005;1055:193–206. doi: 10.1196/annals.1323.029. [DOI] [PubMed] [Google Scholar]
- Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, et al. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation. 1995;91:2582–90. doi: 10.1161/01.cir.91.10.2582. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–31. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–56. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–34. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–95. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, et al. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–46. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1443–8. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–81. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]


