Summary
A fundamental feature of memory in humans is the ability to simultaneously work with multiple types of information using independent memory systems. Working memory is conceptualized as two independent memory systems under executive control [1, 2]. Although there is a long history of using the term working memory to describe short-term memory in animals, it is not known if multiple, independent memory systems exist in nonhumans. Here, we used two established short-term memory approaches to test the hypothesis that spatial and olfactory memory operate as independent working memory resources in the rat. In the olfactory memory task, rats chose a novel odor from a gradually incrementing set of old odors [3]. In the spatial memory task, rats searched for a depleting food source at multiple locations [4]. We presented rats with information to hold in memory in one domain (e.g., olfactory) while adding a memory load in the other domain (e.g., spatial). Control conditions equated the retention interval delay without adding a second memory load. In a further experiment, we used proactive interference [5–7] in the spatial domain to compromise spatial memory, and evaluated the impact of adding an olfactory memory load. Olfactory and spatial memory are resistant to interference from the addition of a memory load in the other domain. Our data suggest that olfactory and spatial memory draw on independent working memory systems in the rat.
Results and Discussion
The essential feature of working memory in humans involves the ability to work with information in one domain while maintaining a memory load in another domain, without performance suffering from between-domain interference. Lack of between domain interference in these so-called dual-task paradigms provides evidence for the existence of independent working memory subsystems, which is a fundamental attribute of human cognition. In an everyday example, one often suffers from overloaded memory in a single domain (e.g., too many digits are hard to remember). Yet, we are able to remember plentiful amounts of information if they come from two domains (e.g., watching a video with images and audio). The theoretical explanation for this remarkable ability in humans is the existence of dedicated working memory systems for two domains. In many experiments, tasks that place information in the visuospatial sketchpad (for manipulating visual images) do not interfere with tasks that tap the phonological loop (for storing speech-based information) [1, 2]. Although there is a long history of using the term working memory in animal research [8, 9], working memory has been used in the animal literature in a way that is quite different from the human conceptualization of working memory. In the animal literature, working memory refers to memory for information that changes in status during the completion of a test [10]; thus, working memory in the animal literature is not differentiated from basic short- or long-term memory.
We exploited the well-established proficiency of rats with olfactory and spatial information to test the hypothesis that rats have independent working memory systems for olfactory and spatial information. If rats rely on a single memory resource, then adding information in one domain (e.g., olfactory) to information in the other domain (e.g., spatial) would be expected to produce impaired performance. However, if rats have multiple, independent working memory resources, then the performance of rats would be expected to be resistant to interference when information is added to both domains. To provide a memory load in the olfactory domain, the rats received initial training in the odor task (see Supplemental Experimental Procedures for a description of preliminary training). After the first odor was presented in daily sessions, the rats were presented with pairs of odors; one odor in the pair was novel (not yet presented on that day), whereas the other odor had already been presented earlier in the day. Selection of the novel odor was rewarded with a small piece of food (i.e., selection of an old odor was considered an error). Thus, solving this task requires memory of recently presented odors. To provide a memory load in the spatial domain, we also trained the same rats (on other days) in a spatial task using the eight-arm radial maze (see Supplemental Experimental Procedures). Each arm was baited with a small piece of food once per day. When the rat visited an arm, it consumed the food; thus, a revisit to a food-depleted location is considered an error. In the study (encoding) phase, the rat choose from four open doors (randomly selected), thereby depleting these arms of food (i.e., closed doors prevented it from entering the remaining four arms). At the end of a retention interval, all eight doors opened and the rat searched for the last four baited locations (test phase; memory assessment). Thus, solving this task requires memory of spatial locations.
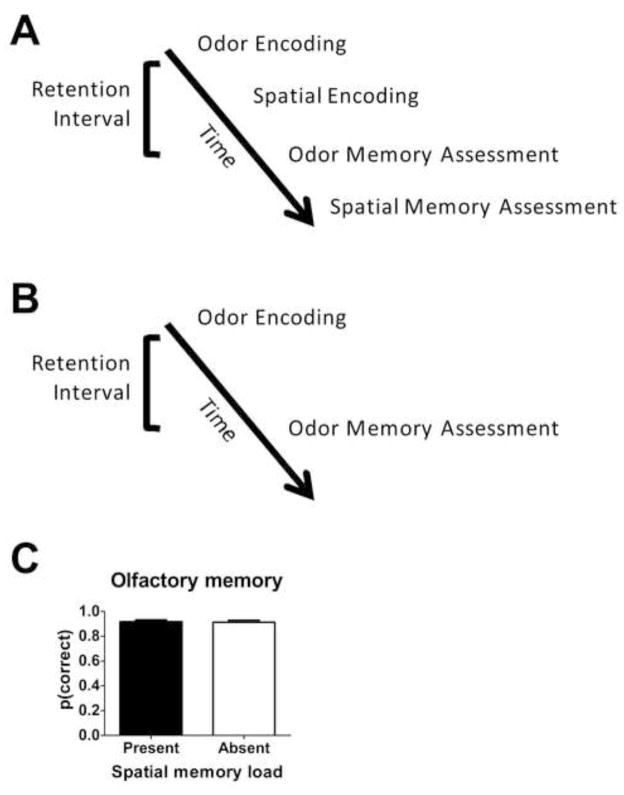
To arrange conditions in which a memory load was imposed in both olfactory and spatial memory, we interleaved odor and spatial tasks. In Experiment 1 (Figure 1), we began with encoding of olfactory information to evaluate the impact of adding a spatial memory load. When a spatial memory load is present, the sequence of events (Figure 1A) is: olfactory encoding, spatial encoding, olfactory memory assessment, spatial memory assessment. On other days (randomly selected) we used only the olfactory memory task (shown in Figure 1B) without the addition of a spatial memory load. When a spatial memory load is absent, the sequence of events is: olfactory encoding, olfactory memory assessment. Importantly, the delay between olfactory encoding and memory assessment was equated by extending the delay in this condition to match the time taken on other days to complete the spatial encoding. To evaluate the impact of adding a spatial memory load to a pre-existing olfactory memory load, we compared performance on the olfactory memory assessment in the presence and absence of the spatial memory load (Figure 1C). When a spatial memory load was present, olfactory memory was high, similar to the high performance observed when the spatial memory load was absent (t(10)=0.73, p=0.48). Moreover, olfactory memory was above chance when spatial memory was present (t(10)=27.4, p<0.001) and absent (t(10)=26.4, p<0.001). Resistance to interference is consistent with the hypothesis that adding a spatial memory load does not impair olfactory memory, as expected if rats process information with multiple, independent memory systems.
Figure 1. Olfactory memory is resistant to interference from the addition of a spatial memory load.
(A–B) Schematic of timeline illustrating experimental design. Olfactory memory is assessed in the presence (A) or absence (B) of an added spatial memory load. (C) Adding a spatial memory load does not impair olfactory memory, as expected with multiple, independent memory systems. See also Figure S1 and Table S2.
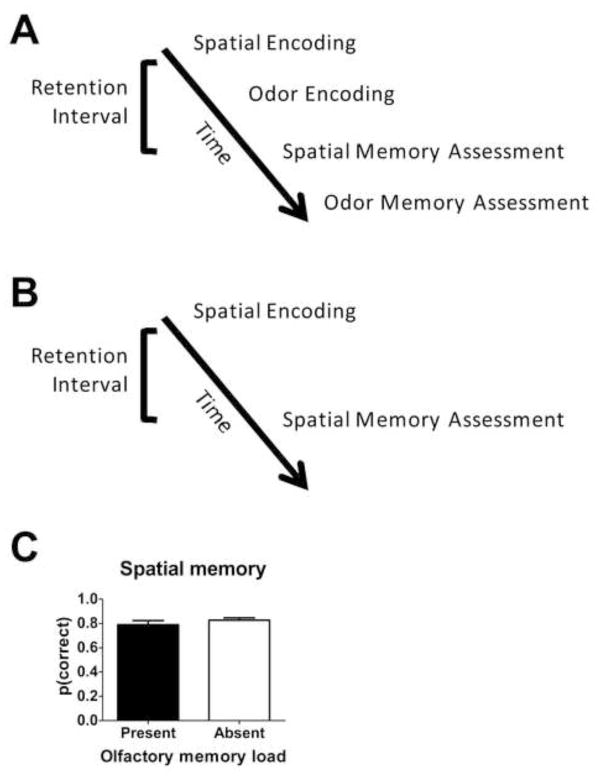
In Experiment 2 (Figure 2), we began with encoding of spatial information to evaluate the impact of adding an olfactory memory load. When an olfactory memory load is present, the sequence of events (Figure 2A) is: spatial encoding, olfactory encoding, spatial memory assessment, olfactory memory assessment. On other days (randomly selected), we used only the spatial memory task (shown in Figure 2B) without the addition of an olfactory memory load. When an olfactory memory load is absent, the sequence of events is: spatial encoding, spatial memory assessment. Importantly, the delay between spatial encoding and memory assessment was equated by extending the delay in this condition to match the time taken on other days to complete the olfactory encoding. To evaluate the impact of adding an olfactory memory load to a pre-existing spatial memory load, we compared performance on the spatial memory assessment in the presence and absence of the olfactory memory load (Figure 2C). When a olfactory memory load was present, spatial memory was high, similar to the high performance observed when the olfactory memory load was absent (t(10)=−1.77, p=0.11). Moreover, spatial memory was above chance when olfactory memory was present (t(10)=11.3 p<0.001) and absent (t(10)=22.3, p<0.001). Resistance to interference is consistent with the hypothesis that adding a between-domain memory load does not impair spatial memory, as expected if rats process information with multiple, independent memory systems.
Figure 2. Spatial memory is resistant to interference from the addition of an olfactory memory load.
(A–B) Schematic of timeline illustrating experimental design. Spatial memory is assessed in the presence (A) or absence (B) of an added olfactory memory load. (C) Adding an olfactory memory load does not impair spatial memory, as expected with multiple, independent memory systems. See also Tables S1 and S2.
Olfactory and spatial memory performance was excellent in Experiments 1 and 2. Correcting for different levels of chance (chance is 0.50 and 0.41 for olfactory and spatial memory assessments, respectively), olfactory and spatial memory performance was 0.42 ± 0.02 and 0.40 ± 0.03 above chance, respectively. Experiments 1 and 2 suggest that adding an extra memory load does not impair performance. According to the independent-memory-system hypothesis, resistance to interference is expected to occur not only when performance is high (as in Experiments 1 and 2), but also when performance is compromised (i.e., when memory performance is at a relatively low level). Alternatively, perhaps the observed resistance to interference is limited to conditions in which performance is quite high. To test these alternative hypotheses, we characterized the impact of adding a working memory load when one of the domains was compromised. To compromise spatial memory, we used proactive interference [5–7] in the spatial domain and evaluated the impact of adding an olfactory memory load. If resistance to interference from the addition of a memory load is restricted to conditions in which performance is high, susceptibility to interference may be observed when performance is compromised. Alternatively, resistance to interference, despite compromised performance, would validate our conclusion that rats use multiple, independent memory systems.
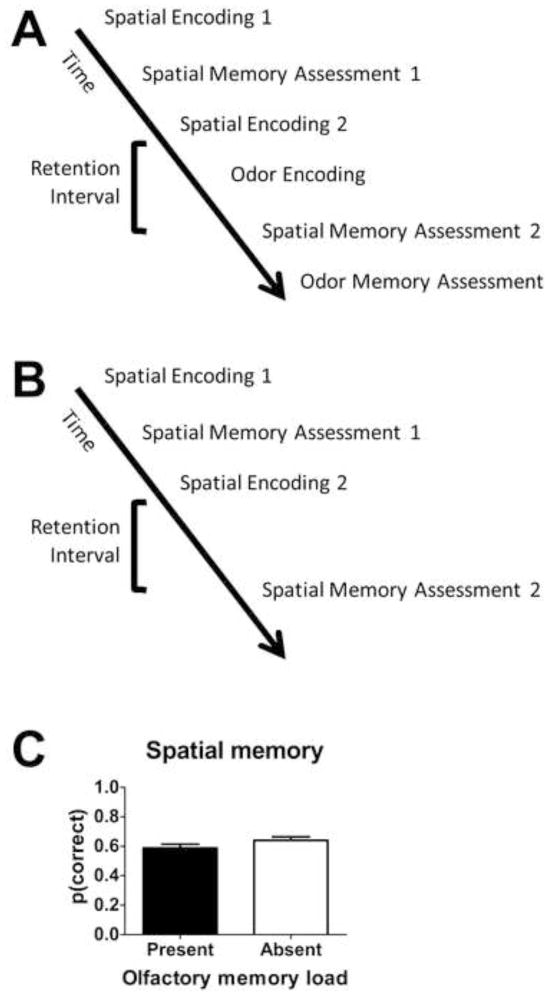
To generate proactive interference, we conducted two successive spatial memory trials (i.e., spatial encoding trial 1 and spatial memory assessment trial 1, followed by a new trial: spatial encoding trial 2 and spatial memory assessment trial 2). Performance on the second trial is expected to decline relative to performance on the first trial [5–7]. Critically, poor performance on the second trial occurs because the animal remembers information from the first trial [6]. Thus, in Experiment 3 (Figure 3), we evaluated the impact of adding an olfactory memory load after the development of proactive interference. When an olfactory memory load is present, the sequence of events (Figure 3A) is: spatial encoding trial 1, spatial memory assessment trial 1, spatial encoding trial 2, olfactory encoding, spatial memory assessment trial 2, olfactory memory assessment. On other days (randomly selected), we used only the spatial memory task (shown in Figure 3B) without the addition of an olfactory memory load. When an olfactory memory load is absent, the sequence of events is: spatial encoding trial 1, spatial memory assessment trial 1, spatial memory encoding trial 2, and spatial memory assessment trial 2. Importantly, the delay between spatial encoding and memory assessment on trial 2 was equated by extending the delay in this condition to match the time taken on other days to complete the olfactory encoding. To evaluate the impact of adding an olfactory memory load to a pre-existing and compromised spatial memory load, we compared performance on the spatial memory assessment in the presence and absence of the olfactory memory load (Figure 3C). When an olfactory memory load was present, spatial memory was modest, similar to the modest performance observed when the olfactory memory load was absent (t(10)=−1.72, p=0.12). Critically, performance was reduced on the second spatial memory trial by 0.18 ± 0.03, relative to first trial performance, thereby documenting the development of proactive interference (t(10)=−6.89, p<0.001). Despite the reduced level of performance on the second spatial memory trial, spatial memory was still above chance when olfactory memory was either present (t(10)=7.00 p<0.001) or absent (t(10)=9.40, p<0.001), documenting that the rats were successfully attending to the spatial domain despite their impaired performance. Resistance to interference is consistent with the hypothesis that adding an olfactory memory load does not impair spatial memory even when performance is compromised, as expected if rats process information with multiple, independent memory systems.
Figure 3. Spatial memory, when compromised by the development of proactive interference, is resistant to interference from the addition of an olfactory memory load.
(A–B) Schematic of timeline illustrating experimental design. Spatial memory is assessed in the presence (A) or absence (B) of an added olfactory memory load after the development of proactive interference. (C) Adding an olfactory memory load does not impair spatial memory even when performance is compromised, as expected with multiple, independent memory systems. See also Tables S1 and S2.
The resistance to between-domain interference in olfactory and spatial memory (Figures 1C, 2C, and 3C) is noteworthy because within-domain memory appears to have a limited capacity. Limited capacity is documented by a decline in accuracy as within-domain memory load increases. To provide a memory load in the olfactory domain, without a concurrent spatial task, we assessed olfactory memory using 101 odors (see supplemental information and Figure S1); the olfactory memory load increased as the animal progressed through the session because an increasing number of odors needed to be remembered as the session progressed. Accuracy declined as a function of olfactory memory load (F(8,80)=2.20, p<0.05; Figure S1). The decline in accuracy as a function of memory load, within a single domain, documents that olfactory memory is capacity limited. Similarly, spatial working memory declines as a function of the number of arms used in radial maze experiments [11] (see supplemental information and Table S1). The increase in spatial errors as a function of spatial memory load, within a single domain, documents that spatial memory is capacity limited. Although the declines in accuracy are relatively small, they establish the principle that capacity is limited in olfactory and spatial domains. From a comparative perspective, the quantities of olfactory and spatial information that can be maintained in working memory systems in rats are higher than estimates of human working memory [12].
Our findings suggest that independence of working memory systems is evolutionarily quite old. Moreover, our findings support the view that rats may be used to model fundamental aspects of human cognition. Working memory is impaired in aging and Alzheimer’s disease [13–16]. The ability to translate successfully from animals to humans will be improved by development of approaches that include modeling of the specific memory impairments observed in clinical populations (i.e., working memory as validated here, and other aspects of memory, such as episodic memory, source memory, retrieval proactive, and prospective memory e.g., [17–28]). This approach will advance translational research that may ultimately foster the development of therapeutic approaches to disorders of human memory.
Supplementary Material
Acknowledgments
All procedures were approved by the Institutional Animal Care and Use Committee at Indiana University Bloomington and followed national guidelines. This work was supported by National Institute of Mental Health R01MH098985 and National Institute on Aging R21AG044530 to JDC, National Science Foundation grants DBI-1440865 and DBI-1460949 REU Site in Animal Behavior, and Indiana University Project STEM.
Footnotes
Supplemental Information includes Figure S1, Table S1, Table S2 and Supplemental Experimental Procedures.
Author Contributions
Conceptualization, A.B. and J.D.C.; Formal Analysis, A.B. and J.D.C.; Investigation, A.B., S.K., J.A.C., J.-E. W., N.R.-R., S.D., D.A., A.D., S.C., H.E.C., and M.J.P.; Writing, A.B. and J.D.C.; Visualization, A.B. and J.D.C.; Supervision, A.B., A.R.D., M.J.P., A.E.S., and J.D.C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baddeley A, Hitch G. Working memory. In: Bower GH, editor. The psychology of learning and motivation. Vol. 8. New York: Academic Press; 1974. pp. 47–90. [Google Scholar]
- 2.Baddeley A. Working Memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 3.April LB, Bruce K, Galizio M. The magic number 70 (plus or minus 20): Variables determining performance in the Rodent Odor Span Task. Learn Motiv. 2013;44:143–158. doi: 10.1016/j.lmot.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J Exp Psychol Anim Behav Process. 1976;2:97–116. [Google Scholar]
- 5.Wright AA, Santiago HC, Sands SF, Kendrick DF, Cook RG. Memory processing of serial lists by pigeons, monkeys, and people. Science. 1985;229:287–289. doi: 10.1126/science.9304205. [DOI] [PubMed] [Google Scholar]
- 6.Wright AA. An experimental analysis of memory processing. J Exp Anal Behav. 2007;88:405–433. doi: 10.1901/jeab.2007.88-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts WA, Dale RHI. Remembrance of places lasts: Proactive inhibition and patterns of choice in rat spatial memory. Learn Motiv. 1981;12:261–281. [Google Scholar]
- 8.Olton DS. Spatial memory. Sci Am. 1977:82–98. doi: 10.1038/scientificamerican0677-82. [DOI] [PubMed] [Google Scholar]
- 9.Honig WK. Studies of working memory in the pigeon. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 211–248. [Google Scholar]
- 10.Roberts WA. Principles of Animal Cognition. Boston: McGraw-Hill; 1998. [Google Scholar]
- 11.Meck WH, Williams CL. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 12.Cowan N. The Magical Mystery Four: How Is Working Memory Capacity Limited, and Why? Curr Directions Psych Sci. 2010;19:51–57. doi: 10.1177/0963721409359277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddeley A, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 14.Belleville S, Peretz I, Malenfant D. Examination of the working memory components in normal aging and in dementia of the Alzheimer type. Neuropsychologia. 1996;34:195–207. doi: 10.1016/0028-3932(95)00097-6. [DOI] [PubMed] [Google Scholar]
- 15.Calderon J, Perry R, Erzinclioglu S, Berrios G, Dening T, Hodges J. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley M, Simpson S, Dagenbach D, Lyday R, Burdette J, Laurienti P. Changes in Brain Network Efficiency and Working Memory Performance in Aging. PLoS ONE. 2014;10:e0123950–e0123950. doi: 10.1371/journal.pone.0123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learn Motiv. 2005;36:177–189. [Google Scholar]
- 18.Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proc Natl Acad Sci USA. 2009;106:9525–9529. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou W, Hohmann AG, Crystal JD. Rats answer an unexpected question after incidental encoding. Curr Biol. 2012;22:1149–1153. doi: 10.1016/j.cub.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crystal JD, Smith AE. Binding of episodic memories in the rat. Curr Biol. 2014;24:2957–2961. doi: 10.1016/j.cub.2014.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crystal JD, Alford WT, Zhou W, Hohmann AG. Source memory in the rat. Curr Biol. 2013;23:387–391. doi: 10.1016/j.cub.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crystal JD, Alford WT. Validation of a rodent model of source memory. Biol Letters. 2014;10:20140064. doi: 10.1098/rsbl.2014.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crystal JD. Animal models of source memory. J Exp Anal Behav. doi: 10.1002/jeab.173. in press. [DOI] [PubMed] [Google Scholar]
- 25.Crystal JD, Ketzenberger JA, Alford WT. Practicing memory retrieval improves long-term retention in rats. Curr Biol. 2013;23:R708–709. doi: 10.1016/j.cub.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson AG, Crystal JD. Prospective memory in the rat. Anim Cogn. 2012;15:349–358. doi: 10.1007/s10071-011-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson AG, Pizzo MJ, Crystal JD. Event-based prospective memory in the rat. Curr Biol. 2013;23:1089–1093. doi: 10.1016/j.cub.2013.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crystal JD. Prospective memory. Curr Biol. 2013;23:R750–751. doi: 10.1016/j.cub.2013.07.081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.