Abstract
Cellular calmodulin binds to the SH2 domain of Src kinase, and upon Fas activation it recruits Src into the death-inducing signaling complex. This results in Src-ERK activation of cell survival pathway through which pancreatic cancer cells survive and proliferate. We had proposed that the inhibition of the interaction of calmodulin with Src-SH2 domain is an attractive strategy to inhibit the proliferation of pancreatic cancer. Thus we have performed screening of compound libraries by a combination of methods and identified some compounds (initial leads) that target the calmodulin-binding region on the SH2 domain and inhibit the proliferation of pancreatic cancer cells in in vitro assays. Most of these compounds also exhibited varying degrees of cytotoxicity when tested against immortalized breast epithelial cell line (MCF10A). These initial leads are likely candidates for development in targeted delivery of compounds to cancer cells without affecting normal cells.
Graphical abstract
Pancreatic cancer is one of the most lethal malignancies affecting Americans and is considered largely incurable. According to the NCI and the American Cancer Society, pancreatic cancer has the highest mortality rate of all major cancers. 93% of pancreatic cancer patients will die within five years of diagnosis – only 7% will survive more than five years. 74% of patients die within the first year of diagnosis. The average life expectancy after diagnosis with metastatic disease is just three to six months. This low survival rate is due to the difficulty in surgically removing the cancer at the time of diagnosis due its rapid spread in the body. The current treatment options include surgery, radiation therapy, and chemotherapy. But these therapies provide only marginal survival benefits for the treated patients1. The existing chemotherapy options are not satisfactory, e.g., use of the nucleoside analog gemcitabine to treat pancreatic cancer has not been very effective due to severe drug resistance2. Attempts at circumventing the drug resistance by combination chemotherapy that includes gemcitabine have not shown clinically meaningful survival benefits in numerous phase I and phase II trials2. Targeted therapy such as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors has offered new avenues for more effective treatment. Unfortunately, however, results of phase III studies with the EGFR tyrosine kinase inhibitor erlotinib were statistically significant but clinically of marginal benefit only1. Similarly, vatalanib and sorafenib that target VEGFR and Raf protein kinases respectively, as well as lestaurtinib that is a multitarget kinase inhibitor, failed to show promising benefits in phase I and phase II trials on human pancreatic cancer3,4. Src tyrosine kinase inhibitors saracatinib5 and dasatinib6 were not promising in clinical trials. Thus there is a critical need to identify new therapeutic targets for treating this lethal cancer.
It was thought until recently that the activation of the Fas death receptor-initiated apoptotic pathway might be a promising approach for treating pancreatic cancer7–9. The Fas death receptors, members of the tumor necrosis factor receptor family, are multimerized upon activation by the Fas ligands or pharmacological reagents, and form the death-inducing signaling complex (DISC) that consist of Fas, the Fas-associated death domain (FADD), caspase-8, and caspase-10. The caspase proteins undergo cleavage and subsequent activation within the DISC to activate additional caspases that are responsible for apoptotic cell death10. But, most intriguingly, in addition to its role as a death-inducing receptor, Fas activation has also has been shown to enhance tumorigenesis of various forms of cancers11, 12. In fact, some pancreatic cancer cells are resistant to Fas-mediated cell death, in spite of the expression of Fas death receptor13–15. The mechanisms for Fas-activated cancer cell survival/proliferative signals involve Fas-activated nuclear factor κB (NFκB) and extracellular signal regulated kinase (ERK) signaling pathways that promote cell survival and proliferation16–18. Most recently, a survival pathway by which pancreatic cancer cells escape apoptosis and proliferate has been described10. This involves the recruitment of Src to the DISC by cellular calmodulin (CaM) upon Fas activation. The activated Src in DISC initiates the Src-ERK signaling pathway which results in pancreatic cancer cell survival and proliferation. Most importantly, the recruitment of Src to the DISC is facilitated by the direct interaction of CaM with Src by binding to the CaM-binding regions on the Src-SH2 domain10. It was also shown that the CaM antaogonist tamoxifen (TMX) inhibits the interaction between CaM, Src and Fas. These results lead to our hypothesis that the inhibition of CaM/SH2 interaction by low molecular weight compounds will also inhibit the proliferation of pancreatic cancer. In the following, we describe results of our studies directed at identifying initial leads (low molecular weight compounds) that specifically target the calmodulin-binding region on the Src-SH2 domain and exhibit anti-proliferation activity against pancreatic cancer cells.
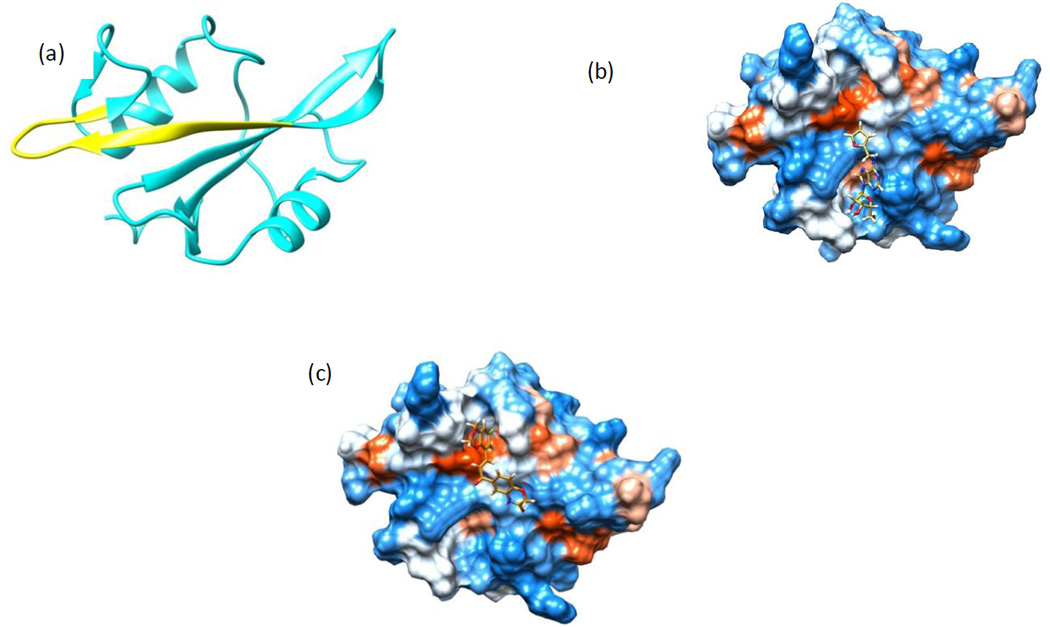
Several virtual compound libraries were assembled from the ZINC library which covers over 15 million commercially available compounds that have been screened and comply with the Lipinski’s19 rule of 5. The first is a focused library consisting of 5000 commercial analogs of known inhibitors of other protein SH2 domains. The second is a diverse library consisting of approximately 600,000 unique representative drug-like compounds. For the diverse library, we first selected the most diverse set of 100,000 structurally representative compounds from the ZINC library using the clustering and diversity analysis protocols of Pipeline Pilot20; then for each of the 100,000 compounds, 5 compounds that are structurally most similar were selected. Such an assembled library covers a large portion of chemical space, contains diverse structures and initial structure-activity relationships (SAR) information. Similarly, we also assembled another diverse library (374,000 compounds) from the Enamine collection (~1.7 M compounds) for screening. Based on the crystal structure of the c-Src-SH2/ligand complex (PDB ID: 1O47), structure-based virtual screenings (SBVS) were performed to identify compounds that can potentially block the SH2-CaM interaction. The total of approximate 605,000 compounds (from the focused and the diverse ZINC libraries together) were screened using a three-step docking protocol implemented in Glide21 with increased docking and scoring precision (HTVS, SP, and XP) at each step. Approximately 2000 top-scored compounds were obtained as output for further visual examinations. Since the calmodulin-binding region is conserved in many cellular kinases, we focused in particular on compounds that bind in non-conserved regions of the SH2 domain, while also potentially blocking the SH2/calmodulin interaction. After carefully examining the docked protein-ligand complex models, 107 compounds were identified that scored favorably and showed sufficient complementarity in shape. We further screened and identified an additional 199 compounds from the Enamine library. Figure 1a shows the SH2 domain with the calmodulin-binding region highlighted. Figures 1b and 1c show typical results of SBVS with two separate lead compounds, each docked into binding pockets encompassing the calmodulin-binding region on SH2. The total of 306 compounds were purchased in 5 to 10 mg quantities from three different vendors, Enamine, LLC (Monmouth Junction, NJ), MolPort (Riga, Latvia), and ChemBridge, Corp. (San Diego, CA) for screening in cell-based assays.
Figure 1.
(a) Model of Src-SH2 domain (PDB#1O47) showing the calmodulin-binding region highlighted in yellow (sequence KHYKIRKLDSGGF). (b) SBVS models showing the docked compound #2, and (c) compound #4 on the surface of the Src-SH2 domain. Models generated using UCSF-Chimera35.
Among the 306 compounds that were purchased, 6 compounds exhibited poor solubility in DMSO and were excluded. The remaining 300 compounds were dissolved in DMSO and then initially screened for activity (cell viability assay (MTS assay, Promega, Cat # G358A) at 10 µM concentration, 72 hours) against MiaPaCa-2 pancreatic cells, grown in Dulbeccos’ Modified Eagle’s Media (DMEM; Invitrogen, Carlsbad, CA)). 14 compounds with anti-proliferation activity were identified.
For a detailed screening of these 14 compounds, one wild type K-ras pancreatic cell line, BxPC-3, two mutant K-ras pancreatic cell lines, MiaPaCa-2 (mut 12 cys) and AsPC-1 (mut 12 Asp), and one immortalized breast epithelial cell line, MCF10A, were treated with these different compounds, tamoxifen, or a vehicle control of DMSO. 5,000 cells were seeded in each well of a 96-well plate. Eighteen hours later, the medium was removed and replaced with medium containing each of the compounds at 10 µM or DMSO. After treating for 48 and/or 72 hours, the medium was removed, and fresh medium containing MTS reagent (CellTiter 96® AQueous One Solution Cell Proliferation Assay; Promega) was added. The cells were placed at 37°C/5%CO2 to allow for color development. The results were read by taking spectrophotometric readings at 490 nm in a BioTek Eon plate reader. Results were plotted as a fold change relative to the vehicle control. The assay was done in triplicate. From these data, two of the above compounds were selected (compounds #1 and #2 in Table-1), and cells were plated as for the first assay. Eighteen hours later, the medium was removed and replaced with medium containing the compounds 1 and 2 for dose-dependent studies at concentrations of 10nM, 100nM, 1µM, and 10µM, 10µM tamoxifen or DMSO, as above. After treating for 48 or 72 hours, the MTS assay was performed, and results were plotted as above. The assays were done in triplicate.
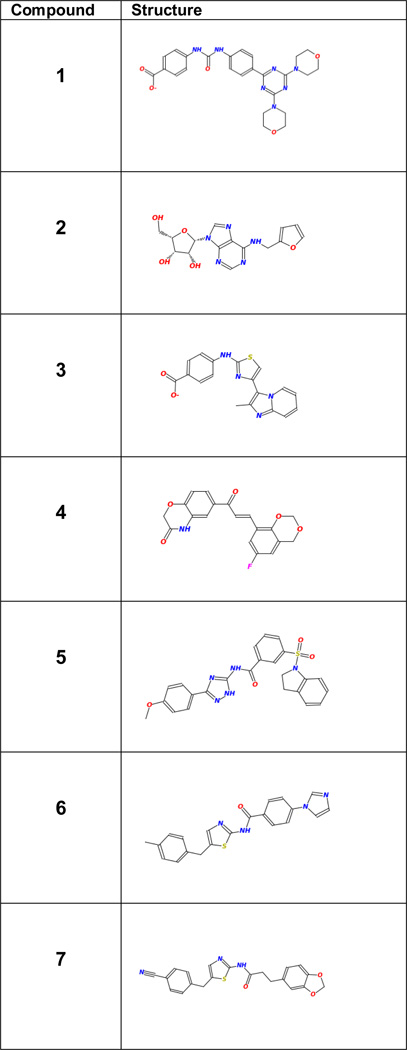
Table-1.
Initial leads identified in this study
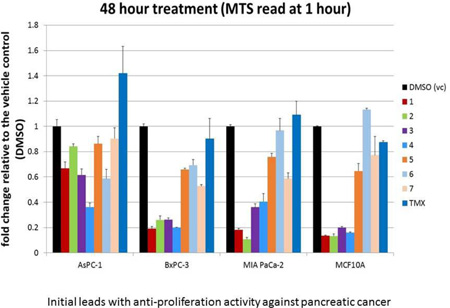
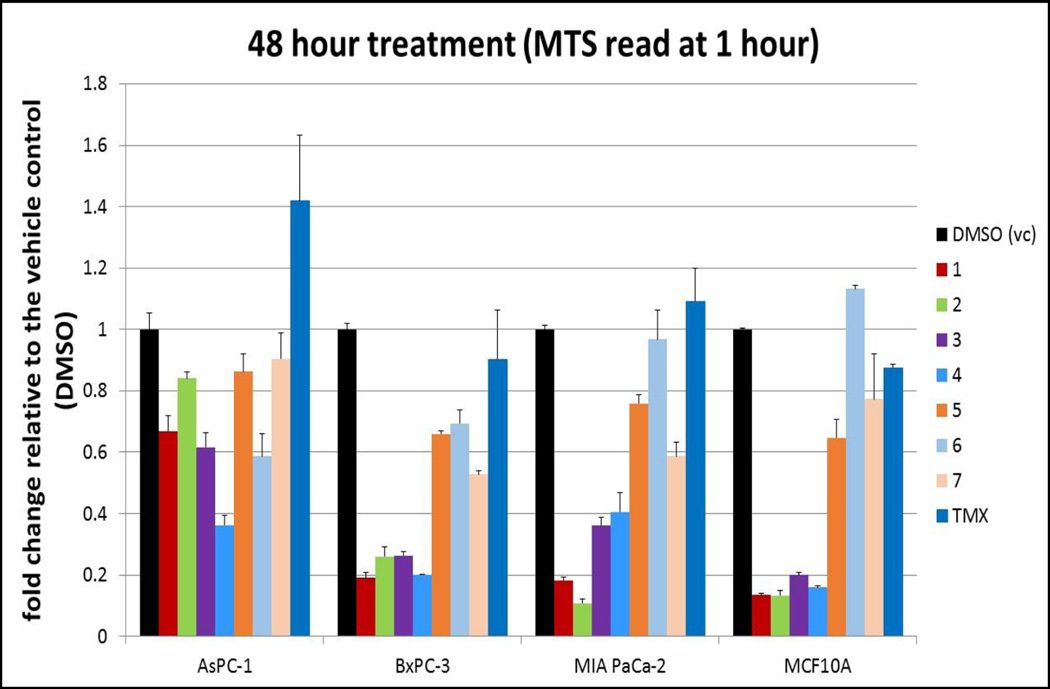
From these assays, a final set of 7 compounds were identified as promising candidates for further investigations. An eighth compound that showed activity against the three pancreatic cancer cell lines with very little toxicity toward MCF10A proved to be insoluble in water/DMSO mixed solvents for binding studies with purified SH2, and was deferred to future investigations to develop soluble analogs. The remaining 7 compounds that were considered as potential candidates for further investigations are listed in Table-1. The results of cell-based assays on these compounds are shown in Figures 2 and 3.
Figure 2.
Cell-based Functional Assays on seven compounds and tamoxifen at 10µM on three pancreatic cancer cell lines (MIAPaCa-2, AsPC-1, BxPC-3) and normal mammary cells (MCF10A). DMSO was used as the control.
Figure 3.
Dose-dependence studies with two compounds (#1 at the left, and #2 at the right) on three pancreatic cancer cell lines (MIAPaCa-2, AsPC-1, BxPC-3) and MCF10A cells. The calmodulin antagonist tamoxifen (TMX) at 10 µM concentration was used as the control.
Next, we extended the efficacy testing to human 3D-Microtumor assay with one of the lead compounds (#4). The results are shown in Figure 4. Anti-tumor activity profile was evaluated with new 3D tumor screening system developed using HuBiogel culture technology22–26. In brief, viable Microtumors are produced by mixing 10K MiaPaCa-2 cells in ice-cold HuBiogel solution (3 mg/ml). HuBiogel/cell mixture was dispensed into beads (10uL) and allowed to polymerize for 5 min at 37C using custom tools. Tumor beads (2mm) were transferred to assay plates and cultured for 1–14 days in complete medium (DMEM, 10% FBS) for drug testing and functional analysis. In treatment groups, increasing concentration of compound or drug (1–50 µM) was added every other day (final: 5% FBS, 0.5% DMSO). The control set included media or DMSO vehicle alone. On day 1, 7 and 14, Microtumors were stained with 1.0µM Calcein-AM (Life Technologies) for live cell imaging/viability analysis. Proliferation activity or index was determined using CellTiter Glo assay kit (Promega). Anti-tumor data analysis was performed with 6–8 tumor beads per assay group.
Figure 4.
Evaluation of compound #4 using a 3D microtumor assay system with MiaPaCa-2 pancreatic cancer cells. The calmodulin antagonist tamoxifen (TMX) was used as a drug control. The live cells or colonies are bright green (bottom images). The top bar graph shows the corresponding proliferation Index (relative luminescence units with DMSO as the control at 100%). The total concentration (amount) of test compounds at Day 8 ranged 1µM each (4.26 µg for #4 and 4.46 µg for TMX) to 50µM each (213.2 µg for #4 and 222.9 µg for TMX).
For screening experiments on the compounds by NMR, we have undertaken the cloning, expression and purification of the SH2 and calmodulin proteins. PET28-SUMO-hCaM was a gift from Dr. Jin-Biao Ma for the production of human calmodulin. MGC:59921 was obtained from Open Biosystems (Huntsville, AL) and used as a template. The coding sequence for the SH2 domain (143–249) of CSRC was amplified and inserted into pET28-SUMO to obtain pET28-SUMO-SH2. Both pET28-SUMO-hCaM and pET28-SUMO-SH2 were transformed into E. coli Rosetta2 pLYSE for recombinant protein production. Auto-inducing media ZYM5052 and 15N5052 were used to express unlabeled and 15N-labeled proteins, respectively. These SUMO-fused proteins were extracted with a French press and bound to a Ni-column with binding buffer (25mM Tris, 100mM NaCl, 25mM Imidazole, pH7.2). After multiple thorough washes, proteins were eluted with elution buffer (25mM Tris, 100mM NaCl, 500mM Imidazole, pH7.2) and SUMO tags were cleaved with SUMO protease under dialysis conditions (25mM Tris, 150mM NaCl, pH7.2, 4 °C, overnight). SUMO tags and SUMO protease were absorbed with another Ni-column, and high purity SH2 or CaM was dialyzed and buffer exchanged with the desired buffer. Protein contents were measured by NanoDrop ND-1000 (Thermo Scientific, Wilmington, DE) with extinction coefficients of 1.12 (mg/ml)−1 cm−1 for SH2 and 0.15 (mg/ml) −1 cm−1 for calmodulin.
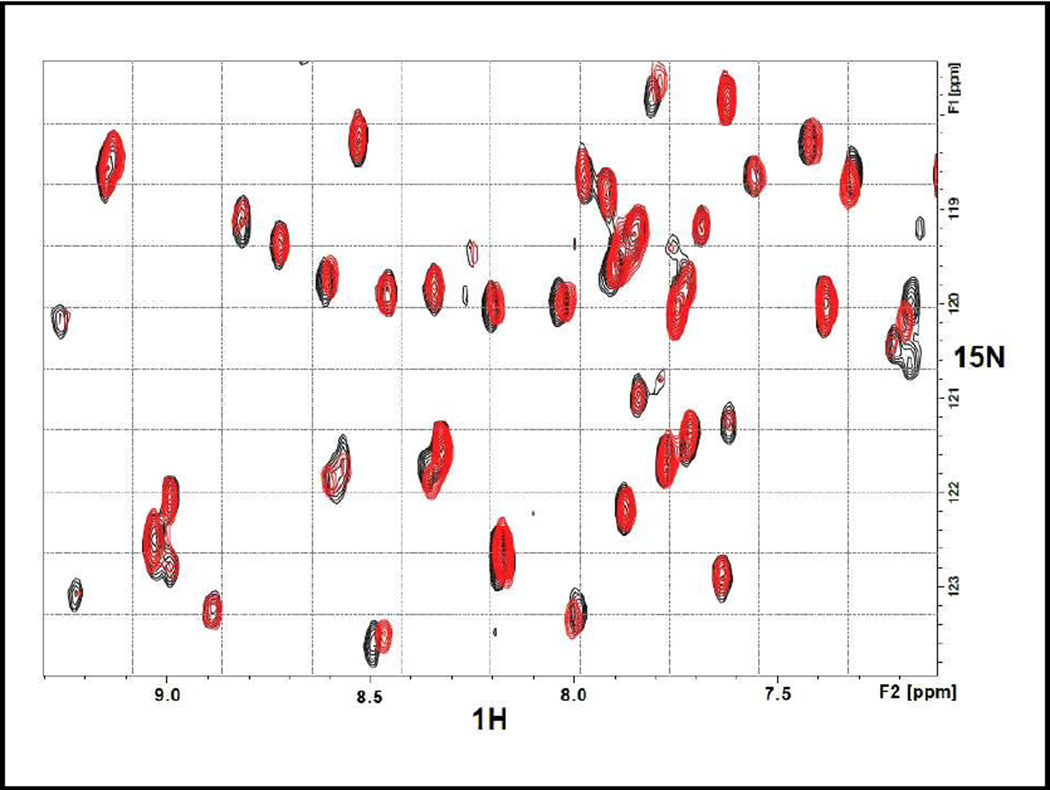
The 7 compounds were soluble in DMSO which was used to prepare stock solutions (for NMR studies, DMSO-d6 was used). However, in water/DMSO mixtures, the compounds exhibited varying degrees of solubility and often posed problems when recording NMR spectra. Thus the NMR measurements were limited to compounds that were reasonably soluble in water/DMSO mixed solvent. For the 15N-SH2 sample containing compound #3 in 10mM Bis-Tris pH 6.3, the 15N-HSQC experiments were run on a Bruker AVANCE III-HD 850 MHz spectrometer, equipped with a TCI Cryoprobe. The pulse program hsqcetfpf3gpsi, using 8 scans and 128 indirect points, was used to generate both the control HSQC spectrum (15N-SH2 alone) as well as the complex’s HSQC (15N SH2 + #3) spectrum. The spectra were processed using a Gaussian function in the acquisition dimension and a 90 degree sinebell squared function in the indirect dimension, using forward linear prediction. The experiment was carried out with a final concentration ratio of 1: 4 (protein to ligand). Figure 5 shows the 15N-HSQC-foot printing showing the binding of compound #3 to the 15N-labeled SH2. Both the control sample (15N-SH2) and the sample with the compound (15N-SH2 + #3) contained identical trace amounts of DMSO. The observed differential shifts in the protein signals are consistent with #3 binding in the region encompassing the calmodulin-binding sequence.
Figure 5.
15N-HSQC Foot-printing assay of the binding of compound #3 to the SH2 domain. The red-colored peaks are for the isolated SH2 domain while the black-colored peaks are for the SH2 domain in the presence of compound #3. Differential shifts indicate specific binding of the compound to the SH2 domain.
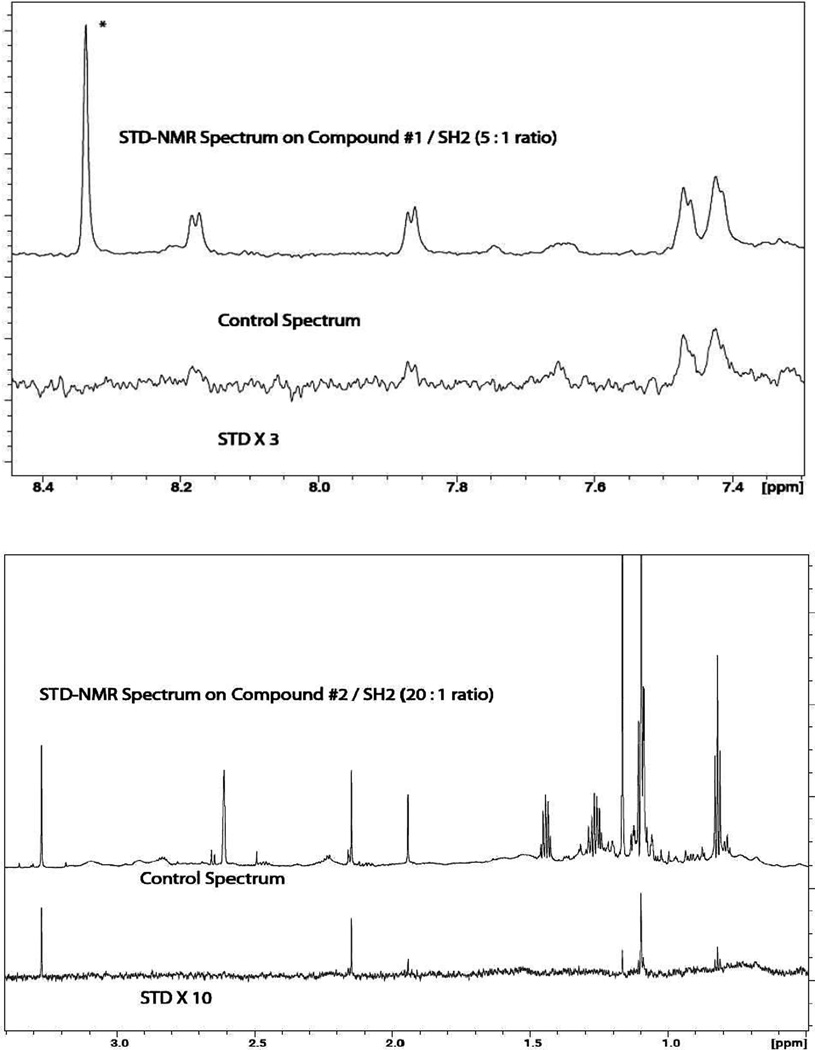
For compounds #1 and #2, their binding to SH2 was tested using Saturation Transfer Difference NMR (STD-NMR27) spectroscopy on a Bruker AVANCE III-HD 850 MHz spectrometer equipped with a TCI Cryoprobe. The pulse program of stddiffesgp.3 was used. Saturation and delay times were set at 5.0 seconds. 16 scans, using 720 averages, were used to obtain the spectrum. A 50 ms Gaussian pulse was used for saturation. The experiment was carried with a final ligand/protein ratios of 5:1 and 20:1 for compounds #1 and #2, respectively, in 100% D2O (with a trace amount of DMSO-d6 from the stock solution). The ligand concentrations were 25µM for #1 and 100µM for #2. The STD-NMR data are shown in Figure 6, clearly establishing the reversible binding of the compounds to the protein.
Figure 6.
STD-NMR assay showing the reversible binding of compounds #1 (top) and #2 (bottom) to the SH2 domain, pH 6.3. The ligand/protein ratios were 5:1 and 20:1, respectively, for compounds #1 and #2. Selected regions of the STD-NMR spectra are shown at the bottom in each figure. The STD peaks reflect the binding epitope of the compound. The top spectrum is the control 1D-NMR. The peak at low-field with an asterick (*) in the top spectrum is from an impurity. The broad peak at 7.65 ppm is from residual slowly exchanging NH proton in SH2.
The inhibition of the CaM/SH2 interaction by the compounds was examined by isothermal titration calorimetry (ITC). In preparation for the ITC measurements, both SH2 and CaM were dialyzed with 8kD MWCO tubing in 10mM Bis-Tris pH6.5, 50mM NaCl, 1mM CaCl2, 0.1mM Tris (2-carboxyethyl) phosphine (TCEP), overnight at cold room. The concentrations of CaM and SH2 were adjusted to 0.2mM and 0.02mM respectively. Compounds dissolved in DMSO were premixed with SH2 before loading into the cell of VP-ITC MicroCalorimeter (MicroCal, Northampton, MA) for isothermal titration calorimetry (ITC). Additional DMSO was also added to CaM to keep the DMSO concentration identical to that in SH2 solution. Each titration point, 20µl of CaM was released into the cell for the kinetics measurements at 27°C. We extended the space between each titration point to 300 seconds and set all other parameters system’s default. The results were analyzed with Origin 7.0 SR4 (OriginLab, Northampton, MA). Typical results of the inhibition of SH2/CaM interaction by a couple of the promising compounds (i.e., #1 and #2) by ITC are shown in Figure 7. According to the data, calmodulin forms a 1:1 complex with SH2. In the presence of 10-fold excess of compound #1, the apparent association constant Ka drops from 1.63 × 107 M−1 to 2.49 × 106 M−1. In the presence of 25-fold excess of compound #2, the apparent Ka drops from 1.95 × 107 M−1 to 2.03 × 106 M−1. These data clearly confirm the inhibitory effects of compounds #1 and #2 on the SH2/CaM interaction. We have also confirmed by ITC (the black data points in Fig.7) that the compounds do not directly bind to CaM. Thus, the compounds specifically bind to the SH2 domain only, and inhibit the interaction of CaM with SH2. We had also confirmed that compounds #3 and #4 do not bind to free calmodulin. In initial ITC runs without any optimization, compound # 4 showed a slight inhibition of the CaM/SH2 interaction (by a factor of ~ 2.5 at 20 fold excess), but we had some difficulty in further improving this inhibition assay by optimizing the conditions for co-solubilizing the proteins and the compound (in DMSO) without precipitation or solubility issues, and in estimating accurate protein concentrations in the ITC cell.
Figure 7.
Isothermal Titration Calorimetry (ITC) data on CaM-Ca++/SH2 interaction without (red) and with (blue) 10-fold excess of compound #1 over SH2 (a), and 25-fold excess of compound #2 over SH2 (b). The “apparent association constant Ka” decreases in the presence of compounds, clearly showing inhibition of interaction by the compounds. The stoichiometry of SH2/CaM-Ca++ complex is 1:1. The compounds do not bind to calmodulin (black dots).
In this study, we have identified a new set of inhibitors by focusing on those that target the calmodulin-binding domain on the Src-SH2 domain (Fig. 1a) with the goal of inhibiting its interaction with calmodulin. They exhibit anti-proliferation activity against pancreatic cancer in cell-based assays (Figs. 2–4). The four most promising compounds (#1 to #4) that we tested do not bind to free calmodulin, thereby enabling CaM to be free to fulfill all its essential cellular functions. This strategy avoids any potential toxicity associated with the inhibition of calmodulin function. The initial leads identified in this study add to a growing list of inhibitors that target the SH2 domain and modulate its diverse biological activities. Previously, an inhibitor AP22161 that specifically targets the cysteine residue within the phosphotyrosine (pTyr) binding pocket of Src-SH2 and inhibits osteoclast-mediated resorption has been reported28 for treating osteoporosis. Several other non-peptide inhibitors that mimic pTyr as well as some natural and semi-synthesis inhibitors of Src-SH2 have also been reported29–32. Many of these compounds appear to bind in the same general vicinity on the Src-SH2 surface as our inhibitors.
The data in Figures 2–4 confirm the varying degrees of anti-proliferation activity of the compounds 1–7 in Table-1 against the three pancreatic cancer cells MiaPaCa-2, BxPC-3 and AsPC-1, with compounds 1 to 4 showing higher activity than the remaining three compounds 5 to 7. In general, effects were observed only at 10µM concentrations and above. Interestingly, as can be seen in Figure 3, the AsPC-1 cells seem to be somewhat resistant to compound #2 compared to MiaPaCa-2 and BxPC-3 cells. Further, as shown in Figures 2 and 3, the compounds also exhibit varying degrees of cytotoxicity against the normal mammary cells MCF10A, with compounds 1 to 4 exhibiting greater cytotoxicity than the compounds 5 to 7 (with compound 6 showing no cytotoxicity). All these results indicate, not surprisingly, that these initial leads in Table-1 are far from optimal, and are potential candidates for further development by structure-guided design and chemical synthesis. Since some of these initial leads exhibit varying degrees of cytotoxicity against the normal cells that we tested against (i.e., MCF10A), they and their derivatives are also excellent candidates for targeted delivery by antibody-conjugation33 or nanoparticles34 to deliver the inhibitors to pancreatic cancer cells with high specificity and with minimal effects on normal cells.
Acknowledgments
This work was supported by the grant 1R21 CA176267-01 from the NCI. We thank Dr. Edward Partridge, the Director of the UAB Comprehensive Cancer Center, and Dr. Michael Bertram, the Associate Director, for providing a Pancreatic Cancer Pilot Research Fund to purchase the compounds used in this study. The High-Field NMR Facility where the NMR-binding assays were performed was supported by the NCI CCSG grant 1P30 CA-13148 and by the NCRR/NIH High End Instrumentation grant 1S10 RR022994-01A1. Lalita Shevde acknowledges the support from a Career Development Award from the UAB Pancreatic Cancer SPORE grant (P50CA101955). We thank Prof. Kiril Popov for access to his laboratory. The encouragement of Prof. Jay McDonald of the UAB Department of Pathology in pursuing this research is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Philip PA. Targeted Therapies for Pancreatic Cancer, Gastrointest. Cancer Res. 2008;2:S16. [PMC free article] [PubMed] [Google Scholar]
- 2.Gounaris I, Zaki K, Corrie P. Options for the treatment of gemcitabine-resistant advanced pancreatic cancer. J. Pancreas. 2010;11:113. [PubMed] [Google Scholar]
- 3.El-Khoueiry AB, Ramanathan RK, Yang DY, Zhang W, Shibata S, Wright JJ, Gandara D, Lenz HJ. A randomized phase II of gemcitabine and sorafenib versus sorafenib alone in patients with metastatic cancer. Invest. New Drugs. 2011;30:1175. doi: 10.1007/s10637-011-9658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan E, Mulkerin D, Rothenberg M, Holen KD, Lockhart AC, Thomas J, Berlin JA. Phase I trial of CEP-701 + gemcitabine in patients with advanced adenocarcinoma of the pancreas. Invest. New Drugs. 2008;26:241. doi: 10.1007/s10637-008-9118-3. [DOI] [PubMed] [Google Scholar]
- 5.Renouf DJ, Moore MJ, Hedley D, Gill S, Jonker D, Chen E, Walde D, Goel R, Southwood B, Gauthier I, Walsh W, McIntosh L, Seymour L. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest. New Drugs. 2012;30:779. doi: 10.1007/s10637-010-9611-3. [DOI] [PubMed] [Google Scholar]
- 6.Chee CE, Krishnamurthi S, Nock CJ, Meropol NJ, Gibbons J, Fu P, Bokar J, Teston L, O'Brien T, Gudena V, Reese A, Bergman M, Saltzman J, Wright JJ, Dowlati A, Brell J. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist. 2013;18:1091. doi: 10.1634/theoncologist.2013-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MA, Zhang G, Mitchell C, Rahmani M, Hamed H, Hagan MP, Yacoub A, Curiel DT, Fisher B, Grant S, Dent P. Mitogen-activated protein kinase kinase 1/2 inhibitors and 17-allylamino-17-demethoxygeldanamycin synergize to kill human gastrointestinal tumor cells in vitro via suppression of c-FLIP- s levels and activation of CD95. Mol. Cancer Ther. 2008;7:2633. doi: 10.1158/1535-7163.MCT-08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MA, Mitchell C, Zhang G, Yacoub A, Allegood J, Häussinger D, Reinehr R, Larner A, Spiegel S, Fisher PB, Voelkel-Johnson C, Ogretmen B, Grant S, Dent P. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway. Cancer Res. 2010;70:6313. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, Houghton PJ, Voelkel-Johnson C, Grant S, Dent P. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and - independent mechanisms. Mol. Pharmacol. 2009;76:342. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan K, Jing G, Chen J, Liu H, Zhang K, Li Y, Wu H, McDonald JM, Chen Y. Calmodulin mediates Fas-induced FADD-independent survival signaling in pancreatic cancer cells via activation of Src-extracellular signal-regulated kinase (ERK) J. Biol. Chem. 2011;286:24776. doi: 10.1074/jbc.M110.202804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ametller E, García-Recio S, Costamagna D, Mayordomo C, Fernández-Nogueira P, Carbo N, Pastor-Arroyo EM, Gascón P, Almendro V. Tumor promoting effects of CD95 signaling in chemoresistant cells. Mol. Cancer. 2010;9:161. doi: 10.1186/1476-4598-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Park SM, Tumanov AV, Hau A, Sawada K, Feig C, Turner JR, Fu YX, Romero IL, Lengyel E, Peter ME. CD95 promotes tumour growth. Nature. 2010;465:492. doi: 10.1038/nature09075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christgen M, Schniewind B, Jueschke A, Ungefroren H, Kalthoff H. Gemcitabine-mediated apoptosis is associated with increased CD95 surface expression but is not inhibited by DN-FADD in Colo357 pancreatic cancer cells. Cancer Lett. 2005;227:193. doi: 10.1016/j.canlet.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Kornmann M, Ishiwata T, Maruyama H, Beger HG, Korc M. Coexpression of FAS and FAS-ligand in chronic pancreatitis: correlation with apoptosis. Pancreas. 2000;20:123. doi: 10.1097/00006676-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;58:1741. [PubMed] [Google Scholar]
- 16.Reinehr R, Sommerfeld A, Häussinger D. CD95 ligand is a proliferative and antiapoptotic signal in quiescent hepatic stellate cells. Gastroenterology. 2008;134:1494. doi: 10.1053/j.gastro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, Wallach D, Wiltrout RH, Zörnig M, Lynch DH. The CD95 Receptor: Apoptosis Revisited. Cell. 2007;129:447. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat. Cell Biol. 2003;5:118. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 19.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 2004;1:337. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Baurin N, Baker R, Richardson C, Chen I, Foloppe N, Potter A, Jordan A, Roughley S, Parratt M, Greaney P, Morley D, Hubbard RE. Drug-like annotation and duplicate analysis of a 23-supplier chemical database totalling 2.7 million compounds. J. Chem. Inf. Comput. Sci. 2004;44:643. doi: 10.1021/ci034260m. [DOI] [PubMed] [Google Scholar]
- 21.Glide, version 6.3. New York, NY: Schrödinger, LLC; 2014. [Google Scholar]
- 22.Piazza G, Keeton AB, Tinsley H, Gary B, Whitt J, Singh R, Reynolds B. Cyclooxygenase independent antineoplastic properties of NSAIDs. Pharma. Rev. 2010;3:1652. doi: 10.3390/ph3051652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong M, Jin S, Rane M, Singh R, Gupta R, Kakar S. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS ONE. 2012;7:e42265. doi: 10.1371/journal.pone.0042265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Felder R, Cano J, Shevin R, Justice B, Singh R. Replicating human tumor biology in vitro. 3D Culture Systems. Genetic & Engineering News. 2013;33(1) [Google Scholar]
- 25.Chandra D, Hansel J, Isayeva T, Reilly S, Siegal G, Singh R, Ponnazhagan S. AGP-2 promotes prostate cancer metastasis by regulating cellular adhesion. PLoS ONE. 2014;9:e89940. doi: 10.1371/journal.pone.0089940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willey C, Gilbert A, Shevin R, Langford C, Singh R, Anderson J, Gillespie Y. Profiling drug sensitivity and kinomic pathways in glioma Microtumors. JOVE. 2016 In press. [Google Scholar]
- 27.Mayer M, Meyer B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 1999;38:1784. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1784::AID-ANIE1784>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Violette SM, Shakespeare WC, Bartlett C, Guan W, Smith JA, Rickles RJ, Bohacek RS, Holt DA, Baron R, Sawyer TK. A Src SH2 selective binding compound inhibits osteoclast-mediated resorption. Chem. Biol. 2000;7:225. doi: 10.1016/s1074-5521(00)00090-9. [DOI] [PubMed] [Google Scholar]
- 29.Shakespeare W, Yang M, Bohacek R, Cerasoli F, Stebbins K, Sundaramoorthi R, Azimioara M, Vu C, Pradeepan S, Metcalf C, 3rd, Haraldson C, Merry T, Dalgarno D, Narula S, Hatada M, Lu X, van Schravendijk MR, Adams S, Violette S, Smith J, Guan W, Bartlett C, Herson J, Iuliucci J, Weigele M, Sawyer T. Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc. Nat’l. Acad. Sci. U S A. 2000;97:9373. doi: 10.1073/pnas.97.17.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandine E, Jean-Baptiste V, Vayssière B, Gofflo D, Bénard D, Sarubbi E, Deprez P, Baron R, Superti-Furga G, Lesuisse D. High-affinity Src-SH2 ligands which do not activate TyR527)-phosphorylated Src in an experimental in vivo system. Biochem. Biophys. Res. Commun. 2002;298:185. doi: 10.1016/s0006-291x(02)02424-5. [DOI] [PubMed] [Google Scholar]
- 31.Sperl B, Seifert MH, Berg T. Natural product inhibitors of protein-protein interactions mediated by Src-family SH2 domains. Bioorg. Med. Chem. Lett. 2009;19:3305. doi: 10.1016/j.bmcl.2009.04.083. [DOI] [PubMed] [Google Scholar]
- 32.Lu XL, Cao X, Liu XY, Jiao BH. Recent progress of Src SH2 and SH3 inhibitors as anticancer agents. Curr. Med. Chem. 2010;17:1117. doi: 10.2174/092986710790827861. [DOI] [PubMed] [Google Scholar]
- 33.Govindan SV, Cardillo TM, Rossi EA, Trisal P, McBride WJ, Sharkey RM, Goldenberg DM. Improving the therapeutic index in cancer therapy by using antibody-drug conjugates designed with a moderately cytotoxic drug. Mol. Pharm. 2015;12:1836. doi: 10.1021/mp5006195. [DOI] [PubMed] [Google Scholar]
- 34.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008;26:57. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]