Abstract
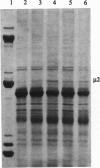
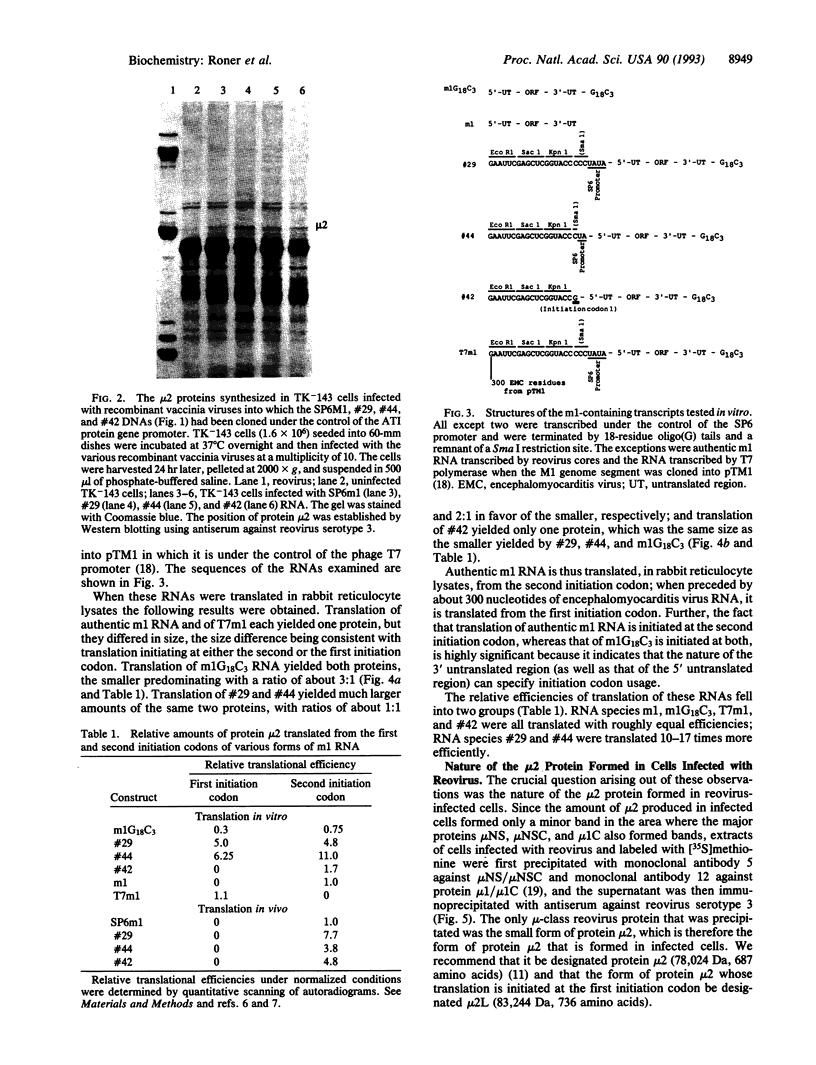
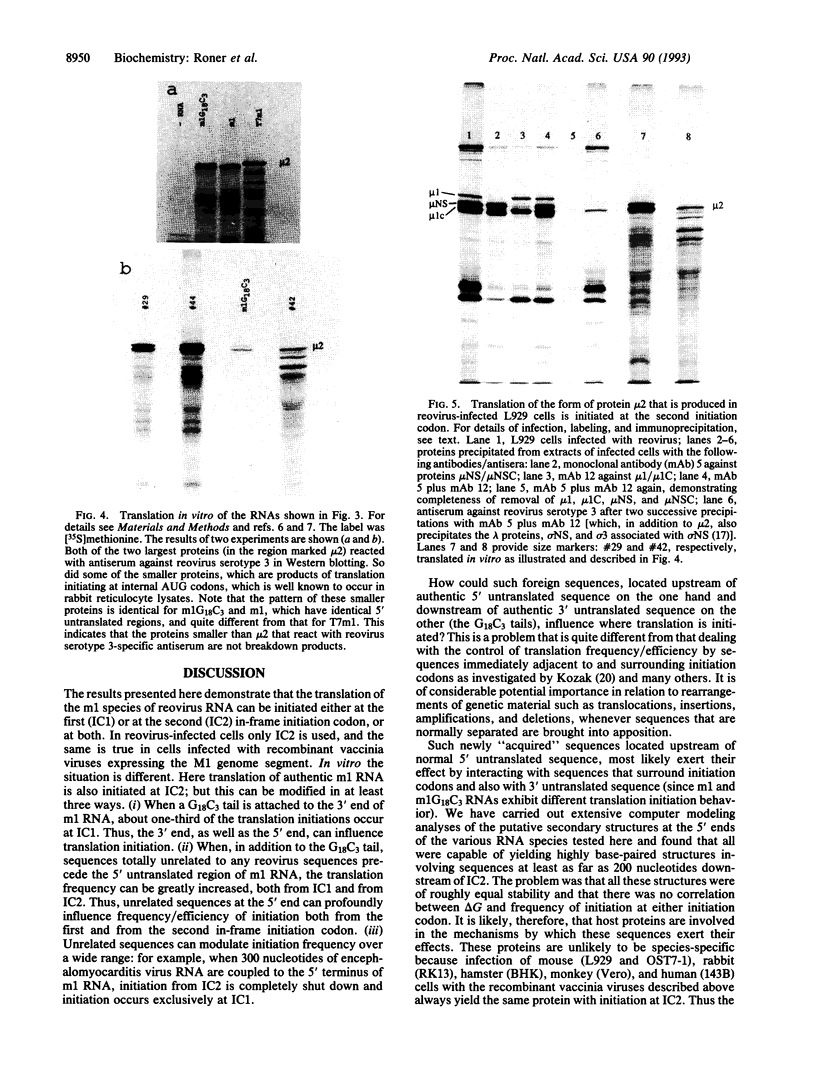
The m1 species of reovirus RNA, which encodes the minor protein component mu 2, possesses two initiation codons, one "strong" according to Kozak rules and preceded by 13 residues (IC1), the other "weak" and located 49 codons downstream of the first (IC2). In reovirus-infected cells only IC2 is used, but initiation from IC1 can be activated, and efficiency of initiation from either initiation codon modulated over a wide range, by coupling unrelated sequences to either or both ends of m1 RNA. For example, when the M1 genome segment is cloned into the thymidine kinase gene of vaccinia virus in such a way that various "irrelevant" stretches of nucleotides comprising restriction endonuclease cleavage sites or promoter remnants are coupled to the 5' end of m1 RNA, translation of the resultant transcripts is also initiated at IC2, with frequencies controlled by the nature of the attached sequences. However, in rabbit reticulocyte lysates these same transcripts are translated from IC1 as well as from IC2, and transcripts in which m1 RNA is preceded by long sequences of encephalomyocarditis virus RNA (from the T7 polymerase-controlled pTM1 vector) are translated exclusively from IC1. By contrast, m1 RNA itself is translated only from IC2. It appears that the most important factor that controls the extent to which translation is initiated from IC1 and IC2 is their "availability," which is likely to be a function of the extent to which the regions on either side of them interact with each other (and also, to a lesser extent, with the 3' untranslated region) either directly or via interaction with host cell proteins. The effects described here are of considerable potential significance when genetic material is rearranged as a result of translocations, insertions, deletions, and amplifications--that is, when sequences that are normally separated are brought into apposition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antczak J. B., Patel D. D., Ray C. A., Ink B. S., Pickup D. J. Site-specific RNA cleavage generates the 3' end of a poxvirus late mRNA. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12033–12037. doi: 10.1073/pnas.89.24.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjea A. C., Brechling K. A., Ray C. A., Erikson H., Pickup D. J., Joklik W. K. High-level synthesis of biologically active reovirus protein sigma 1 in a mammalian expression vector system. Virology. 1988 Dec;167(2):601–612. [PubMed] [Google Scholar]
- Cashdollar L. W., Chmelo R., Esparza J., Hudson G. R., Joklik W. K. Molecular cloning of the complete genome of reovirus serotype 3. Virology. 1984 Feb;133(1):191–196. doi: 10.1016/0042-6822(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Cashdollar L. W., Esparza J., Hudson G. R., Chmelo R., Lee P. W., Joklik W. K. Cloning the double-stranded RNA genes of reovirus: sequence of the cloned S2 gene. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7644–7648. doi: 10.1073/pnas.79.24.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R. K., Jr, Joklik W. K. The relative translation efficiencies of reovirus messenger RNAs. Virology. 1985 Dec;147(2):336–348. doi: 10.1016/0042-6822(85)90136-9. [DOI] [PubMed] [Google Scholar]
- González S. A., Burrone O. R. Rotavirus NS26 is modified by addition of single O-linked residues of N-acetylglucosamine. Virology. 1991 May;182(1):8–16. doi: 10.1016/0042-6822(91)90642-o. [DOI] [PubMed] [Google Scholar]
- Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol. 1992;27(4-5):385–402. doi: 10.3109/10409239209082567. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z. X., Joklik W. K. Isolation and enzymatic characterization of protein lambda 2, the reovirus guanylyltransferase. Virology. 1991 Nov;185(1):377–386. doi: 10.1016/0042-6822(91)90785-a. [DOI] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J., Joklik W. K. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology. 1986 Mar;149(2):174–189. doi: 10.1016/0042-6822(86)90119-4. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Ray C. A., Drucker R. P., Pickup D. J. A poxvirus-derived vector that directs high levels of expression of cloned genes in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9431–9435. doi: 10.1073/pnas.85.24.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roner M. R., Gaillard R. K., Jr, Joklik W. K. Control of reovirus messenger RNA translation efficiency by the regions upstream of initiation codons. Virology. 1989 Feb;168(2):292–301. doi: 10.1016/0042-6822(89)90269-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Starnes M. C., Joklik W. K. Reovirus protein lambda 3 is a poly(C)-dependent poly(G) polymerase. Virology. 1993 Mar;193(1):356–366. doi: 10.1006/viro.1993.1132. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Bartlett J. A., Joklik W. K. The sequences of reovirus serotype 3 genome segments M1 and M3 encoding the minor protein mu 2 and the major nonstructural protein mu NS, respectively. Virology. 1989 Apr;169(2):293–304. doi: 10.1016/0042-6822(89)90154-2. [DOI] [PubMed] [Google Scholar]