Abstract
There is little published literature on the efficacy of strategies to reduce exposure to residential well water arsenic. The objectives of our study were to: 1) determine if water arsenic remained a significant exposure source in households using bottled water or point-of-use treatment systems; and 2) evaluate the major sources and routes of any remaining arsenic exposure. We conducted a cross-sectional study of 167 households in Maine using one of these two strategies to prevent exposure to arsenic. Most households included one adult and at least one child. Untreated well water arsenic concentrations ranged from <10 μg/L to 640 μg/L. Urine samples, water samples, daily diet and bathing diaries, and household dietary and water use habit surveys were collected. Generalized estimating equations were used to model the relationship between urinary arsenic and untreated well water arsenic concentration, while accounting for documented consumption of untreated water and dietary sources. If mitigation strategies were fully effective, there should be no relationship between urinary arsenic and well water arsenic. To the contrary, we found that untreated arsenic water concentration remained a significant (p ≤ 0.001) predictor of urinary arsenic levels. When untreated water arsenic concentrations were <40 μg/L, untreated water arsenic was no longer a significant predictor of urinary arsenic. Time spent bathing (alone or in combination with water arsenic concentration) was not associated with urinary arsenic. A predictive analysis of the average study participant suggested that when untreated water arsenic ranged from 100 to 500 μg/L, elimination of any untreated water use would result in an 8%–32% reduction in urinary arsenic for young children, and a 14%–59% reduction for adults. These results demonstrate the importance of complying with a point-of-use or bottled water exposure reduction strategy. However, there remained unexplained, water-related routes of exposure.
Keywords: Arsenic, Well water, Bottled water, Point-of-use, Bathing, Children
GRAPHICAL ABSTRACT
1. Introduction
Arsenic exposure is considered a worldwide public health problem (WHO, 2012). It is estimated that >200 million people worldwide could be exposed to elevated levels of naturally occurring arsenic in drinking water (Naujokas et al., 2013). Groundwater with elevated arsenic is prevalent in several regions of the United States, including the West, Midwest, parts of Texas and the Northeast (Ryker, 2001; Ayotte et al., 2003; Peters, 2008). In Maine, a state where over half the population relies on private wells for drinking water, 12% of wells have arsenic above the federal maximum contaminant level of 10 μg/L set for public water supplies (Loiselle et al., 2001). In more than 50 Maine towns, measured arsenic levels in private well water exceed 100 μg/L; the highest reported level is above 3000 μg/L (Maine Tracking Network, 2014).
Private well owners with elevated arsenic in their drinking water have several strategies available for reducing exposure. Strategies include switching to bottled water for beverage preparation and cooking, installing treatment systems that focus on a single area of water use, such as a kitchen sink (commonly referred to as point-of-use or POU), and treatment systems that treat all the water entering the home (commonly referred to as point-of-entry or POE). In a survey of central Maine residents with well water arsenic levels above 10 μg/L, more than 65% of respondents indicated they were using either bottled water or a POU treatment system to reduce exposure (Flanagan et al., 2015a). These two intervention strategies remained the most common even for households with water arsenic above 100 μg/L (Flanagan et al., 2015a).
There is little published literature, especially regarding children, on the efficacy of household exposure reduction strategies for well water with elevated arsenic levels. Josyula et al. (2006) reported only a modest reduction (21%) in urinary arsenic levels following bottled water intervention in Arizona homes with arsenic levels averaging 20 μg/L. In a small pilot study, Spayd et al. (2015) reported more substantial reductions (>60%) in urinary arsenic levels in New Jersey well owners using either POU or POE treatment systems for water arsenic levels averaging around 40 μg/L. Effective exposure reduction depends on the ability of the treatment system to reduce water arsenic to levels where the contribution to exposure is minor relative to dietary sources (Gilbert-Diamond et al., 2011; Kurzius-Spencer et al., 2013). Once arsenic levels in the primary drinking water source are reduced to <10 μg/L, diet is likely to be the major source of exposure to arsenic (Kurzius-Spencer et al., 2013, 2014).
For bottled water and POU treatment strategies, effective arsenic exposure reduction will also depend on behavioral factors such as willingness to use only treated water or bottled water for beverage and food preparation, as well as for drinking. Occasional use of untreated water for beverage or food preparation after switching to bottled water or installing a POU treatment system could lead to significant exposure, especially if water arsenic levels are high. Exposure may also result from bathing-related contact with untreated water (Spayd et al., 2015).
We report results from a study of exposure to arsenic in households after implementing common mitigation exposure reduction strategies. We enrolled families residing in Maine that relied on private well water and used either bottled water or a POU treatment system to reduce their arsenic exposure from untreated well water. As there is a paucity of studies regarding arsenic exposure in young children, a focus of the study was to examine households with children younger than 6 years. The primary aim of this cross-sectional study was to determine whether arsenic in untreated well water was a significant exposure source in households employing either bottled water or POU exposure reduction strategies. A secondary aim was to evaluate sources and routes of remaining arsenic exposure, including lack of compliance with use of treated or bottled water, bathing habits, and diet.
2. Methods
2.1. Study design and population
Participant recruitment was aimed at constructing a convenience sample of families with young children (<6 years) and a wide range of private well water arsenic levels. Briefly, recruitment was done in one of two ways: by identifying households with elevated water arsenic levels (>10 μg/L) from state laboratory testing data or treatment company mailings to customers; or by identifying households with young children in areas likely to have elevated water arsenic levels through state birth records (see Supplemental Information for more detail). Participant recruitment and study sampling took place from 2001 to 2003.
All recruitment and study procedures were approved by the Institutional Review Boards of the Maine Center for Disease Control and Prevention (MECDC) and the U.S. Centers for Disease Control and Prevention (CDC). Families received study information along with recruitment letters, and those who chose to participate provided written informed consent. Participating households received reports summarizing their water and urine arsenic test results.
2.2. Survey and diary information
Each participating household completed a household survey, and each participating individual recorded a three-day diet and bathing diary. Home visits were conducted with each household the day after the three-day diet and bathing diary period ended. During the home visit, trained study personnel administered the household survey and reviewed and collected the three-day diet and bathing diaries. The household survey solicited information on the home’s well type; information on the treatment system, if any; descriptions of other water sources; and the presence of other potential sources of arsenic exposure, such as pressure-treated wood or pesticides. The survey also queried individuals regarding their prior habitual use of untreated and treated water for drinking and beverage preparation, cooking, and brushing teeth, as well as recent seafood or seaweed consumption and adult smoking behavior. The diet and bathing diary was used to record the types and volumes of foods and beverages consumed, and the volumes and sources (e.g., bottled water, filtered water, untreated tap water) of any water used to prepare food. For bathing, participants recorded the number of daily bathing and/or showering events, including the duration of each event. A three day period for the diet and bathing diary was selected to avoid respondent fatigue (Thompson and Byers, 1994), and to capture any dietary arsenic exposure sources that would contribute to urinary arsenic levels based on the biological half-life for inorganic arsenic of 2 to 4 days (NRC, 1999; Zheng et al., 2002). One adult participant in each household was asked to keep proxy diaries for all child participants in the household. Study personnel reviewed diaries on the day of the home visit, and attempted to follow up on any missing or incomplete information.
2.3. Water and urine samples
Trained study staff collected untreated and treated water samples from each household at the time of the home visit. Untreated water was collected from a bathtub faucet, after removing any screen or sediment filter from the faucet and letting the water run for 15 minutes(min). If the treated water source was filtered tap water, water was collected from the sink where the filter was installed (usually the kitchen). If the treated water source was bottled water, a sample was collected directly from the bottle in use at the time of the home visit. If the household reported use of any other water sources (e.g., alternative bottled water source, secondary well or water filter), a sample of these was also collected. All water samples were collected into acid-cleaned polyethylene bottles, placed immediately into a cooler with ice, and transported to MECDC’s Health and Environmental Laboratory (HETL). Standard quality control procedures were followed (see Supplemental Information for more detail). Samples were analyzed for total arsenic using inductively coupled plasma mass spectrometry (ICP-MS), following EPA Method 200.8 (EPA, 1994). The laboratory method limit of detection for total arsenic was 0.5 μg/L.
On the day of the home visit, participants were asked to collect first morning void urine samples into prescreened sample collection cups provided by study staff and store them in their home freezers. Young children participating in the study were all toilet-trained. During the home visit, study staff gathered and labeled all urine samples and transferred them immediately to either a cooler filled with ice or a portable freezer (−18 °C) for transport to HETL. Once samples were received at HETL, they were thawed, aliquoted for subsequent analysis, and stored in a −70 °C freezer. Samples were shipped overnight on dry ice to the CDC for total arsenic measurement, arsenic speciation, and creatinine analysis. Total arsenic was measured by inductively coupled plasma dynamic reaction cell mass spectrometry (ICP-DRC-MS). Urinary arsenic species (As3+, As5+, monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), trimethylarsine oxide (TMO), arsenobetaine (AsB), and arsenocholine (AsC)) were measured by high-performance liquid chromatography (HPLC) and ICP-DRC-MS (Verdon et al., 2009). Method limits of detection were as follows: total As, 0.6 μg/L; As3+, 1.2 μg/L; As5+, 1.0 μg/L; MMA, 0.9 μg/L; DMA, 1.7 μg/L; TMO, 1.0 μg/L; AsB, 0.4 μg/L; AsC, 0.6 μg/L. Urinary creatinine was measured using an enzymatic reaction on a Kodak Ektachem 250 clinical chemistry analyzer (Roche, Basel, Switzerland).
2.4. Data analysis
2.4.1. Preparation of diary data
Participants’ daily food and beverage consumption was compiled into a single database using the dietary information from the three-day diary. Variables were created for water consumption and for consumption of those food and beverage items (such as rice, seafood, and apple juice) that have been shown elsewhere to contribute measurably to arsenic exposure (Cullen and Reimer, 1989; deCastro et al., 2014; Rivera-Núñez et al., 2012; FDA, 2011, 2013; Nachman et al., 2013).
Water consumed directly through drinking and beverage preparation was categorized based on the source as treated or untreated, and summed across the three-day diary period. Individuals self-reported consumption using a variety of units (i.e., cups, ounces, etc.), so all units were converted to liters (L) for analysis. A standard serving of one cup (0.24 L) for a beverage was used if no amount was listed. In cases where non-standard volumes or units were used (e.g., “small glass”), NHANES measuring guides were used to estimate volume (CDC, 2010). Water consumed through food was also quantified. Food items that were identified as prepared with a treated or untreated water source were placed into one of several categories: rice, pasta/noodles, soups/stews, cereals/oatmeal, and puddings. The water content in a standard serving of the most common food items in each category was identified using the USDA National Nutrient Database for Standard Reference (USDA, 2013).
Foods that could potentially contribute to arsenic exposure were quantified, including rice, chicken, and seafood. For rice-containing items, the total gram weight and associated water weight for a single serving was identified in the National Nutrient Database for Standard Reference (USDA, 2013). The amount of dry rice in grams (g)was calculated from the total weight and water weight of each specific rice meal. All rice meals were converted first into cups of dry rice, and then multiplied by the per-cup mass of rice for the type of rice consumed, with per-cup masses as follows: white rice, 55.1 g; brown rice, 59.3 g, unspecified type (average), 57.2 g. Chicken and seafood meals were counted and totaled. Because participants were asked to abstain from eating seafood during the three-day diet period, seafood consumption was also assessed qualitatively in the staff-administered household survey. Participants were asked whether they ate any type of ocean fish, shellfish, seaweed or fresh water fish in the previous one-week or two-week period leading up to the start of the three-day diary period. For analyses, seafood consumption included any consumption of ocean fish, shellfish, or seaweed and was coded as a binary (yes vs. no) variable.
Juice consumption was categorized as apple juice, grape juice, or other juice (excluding orange, which is unlikely to contribute to arsenic exposure). Juice entries that indicated reconstitution or dilution with a water source containing >0 μg/L arsenic were also included in each participant’s water consumption totals. Again, standard portion sizes were used when volume information was not given (CDC, 2010). Further detail on all calculations used for dietary items is included in the Supplemental Information.
Times spent bathing and showering were summed over the three day diary, and converted from minutes to hours. The number of baths or showers taken over the three-day period was also summed to create separate bathing and showering event variables. If a bath or shower was indicated and no time was recorded, the average participant shower time (10 min) or average participant bath time (18 min)was substituted (N=5 children, 3 adults). If a time was entered with no accompanying indication of shower or bath, a shower or bath was assigned based on the participant’s age; participants ≤7 were assigned baths, and those ≥8 were assigned showers (N= 11 children, 5 adults).
2.4.2. Preparation of urinary arsenic data
In order to isolate exposure to inorganic arsenic, minimize the influence of dietary organoarsenic species, and reduce the influence of species where many of the samples were below the limit of detection (LOD), we created two summary exposure measures: a ‘summed’ inorganic arsenic variable, calculated by summing As3+, As5+, MMA, and DMA (ΣAsi); and a ‘subtracted’ inorganic arsenic variable, calculated by subtracting arsenobetaine (AsB) from total urinary arsenic (TAs), or [TAs–AsB]. Both of these approaches have been used elsewhere to estimate arsenic exposure (Gilbert-Diamond et al., 2011; Navas-Acien et al., 2011). All individual arsenic species measurements that were below the LOD were assigned a value equal to the species-specific LOD divided by the square root of 2 (LOD/√2) (Sanford et al., 1993). Urinary arsenic concentrations were not corrected directly for creatinine; instead, potential differences in urine dilution were accounted for by including urinary creatinine as a separate term in all regression models (Barr et al., 2005). The summed urinary arsenic measure was used to compare to national levels (i.e., NHANES urinary inorganic-related arsenic species (CDC, 2015)). Summed urinary arsenic percentiles and 95% confidence intervals for NHANES-matched age groups (6–11 years, 12–19 years, and 20 years and older) were estimated, taking into account household clustering with the SAS procedure ‘survey means’ (SAS Institute Inc., 2015).
2.4.3. Statistical analyses
Based on differences in bathing behavior (e.g. baths versus showers), creatinine levels, and urinary arsenic levels, participants were divided into three age groups: young children (1–7 years), older children (8–17 years), and adults (≥18 years). All descriptive statistics and regression analyses were stratified by these age groups.
Generalized estimating equations (GEE) were used to assess the relationships between urinary arsenic measures and untreated well water arsenic concentration, while adjusting for estimated arsenic intake from use of untreated and treated water, volumes of water consumed, bathing exposure, selected dietary sources of arsenic, creatinine levels, and age. Class and repeated subject statements were used to account for within-household clustering of study participants. Separate GEE models were constructed for each age group and for the two primary urinary arsenic measures (ΣAsi and [TAs – AsB]). Both the summed (ΣAsi) and subtracted ([TAs – AsB]) measures had non-normal distributions that were positively skewed and were natural log-transformed before regression analyses were performed.
For each type of water (treated and untreated), we separated arsenic intake into two terms: intake from water used for drinking and beverage preparation, and intake of water used in cooking and food preparation. For each, arsenic intake was estimated by multiplying the volume of water reported consumed in the diet diary by the corresponding water arsenic concentration. Bathing exposure was estimated as either the total time spent in baths (young children) or showers (older children and adults), as the product of time spent bathing and untreated arsenic water concentration; or as the number of bathing events over the three- day diary period. From a statistical perspective, the arsenic intake terms and the time/concentration bathing term can be viewed as interaction terms constructed from the main effect terms of water arsenic concentrations, water intakes, and bathing times. Dietary terms included: volume of apple, grape, and other juices consumed; amount of rice consumed; number of chicken meals consumed; consumption of seafood in the previous week (yes/no). For a further explanation of the regression model and terms, see Supplemental Information.
The full model described above was subjected to stepwise backward elimination, with terms removed from the model one by one in decreasing order of Z-scores, for all terms with a p-value >0.15. After the removal of each term, any substantial changes in either the coefficients or standard errors of all remaining terms were evaluated to identify potential confounders and collinear terms. Non-significant main effect terms associated with significant interaction terms were kept in the model. The final level of statistical significance was set at p < 0.05.
Once final models were complete, standard regression diagnostics were applied to assess model fit and identify potential outliers and unduly influential points. Participants associated with an elevated Cook’s D value or a relatively elevated leverage value were identified as potentially influential. These individuals were removed, models re-run, and changes in results assessed.
Sensitivity analyses were performed to evaluate the effect of untreated water use prior to the three-day diary on the relation between urinary arsenic and untreated water arsenic concentration, by excluding participants who reported any prior use of untreated water on the survey and re-running the regression model. To assess potential differences in the relationship at different exposure levels, we also created a stratified version of the final model for children age 1–7, split by untreated water arsenic concentration above and below the median level for the age group of 40 μg/L.
Finally, the GEE model results were used to construct a predictive model describing the change in urinary arsenic with increasing levels of arsenic in untreated water. Two scenarios were evaluated: one based on the reported behavior of an average study participant, and one in which the average participant’s consumption of untreated water was set to zero. The second scenario was used to estimate the potential for exposure reduction from better compliance with use of treated or bottled water. This predictive model was constructed from a pared-down regression model which excluded non-continuous gender and seafood consumption terms, along with any terms with a statistical significance of p > 0.1, unless the term was the main effect of a significant interaction term. Age-group-specific mean values were used for each explanatory variable, while the untreated water arsenic concentration was allowed to vary from 0–500 μg/L.
All statistical analyses were carried out with SAS statistical software, Version 9.3 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Study population
The initial household study size was 190 households. Five house-holds were excluded from analysis due to missing samples or survey information; 4 were excluded because their mitigated drinking water source had arsenic >10 μg/L; and 14 were dropped because they had no treatment system or bottled water substitution in place. Out of the final 167 households, there were 102 households with young children ages 1–7, 43 households with older children ages 8–17, and 163 households with adult participants (Table 1).
Table 1.
Household and participant characteristics (median [interquartile range], median {range}, number (%)).
| Age groups (years)
|
|||
|---|---|---|---|
| 1–7 | 8–17 | Adults ≥ 18 | |
| Number of households | 102 | 43 | 163 |
| Participant characteristics | |||
| Number of participants | 135 | 55 | 183 |
| Age (years) | 4 [3–5] | 11 [9–13] | 40 [34–51] |
| Female participants | 63 (47%) | 25 (45%) | 142 (78%) |
| Urinary creatinine (mg/dL) | 80.6 [53.4–109.5] | 130.0 [97.1–179.0] | 114.4 [79.9–159] |
| Participants with morning first void urine samples | 121 (90%) | 51 (93%) | 167 (91%) |
| Untreated water usage | |||
| Untreated water As concentration (μg/L) | 40.0 [18–80] | 62.0 [32–120] | 43.0 [20–85] |
| Participants reporting untreated water use | |||
| Drinking and beverages | 10 (7.4%) | 4 (7.3%) | 11 (6.0%) |
| Use in food preparation | 31 (23%) | 13 (24%) | 35 (19%) |
| Volume untreated water consumed | |||
| Drinking and beverages (L)a,b | 0 {0–0.59} | 0 {0–1.5} | 0 {0–1.6} |
| From use in food preparation (L)a,b | 0 {0–0.23} | 0 {0–0.27} | 0 {0–0.36} |
| Treated water usage | |||
| Treated water As concentration (μg/L) | <LOD [<LOD-0.9] | <LOD [<LOD-0.9] | <LOD [<LOD-0.7] |
| Treated water source | |||
| Bottled water | 89 (66%) | 37 (67%) | 118 (64%) |
| Treatment system | 35 (26%) | 15 (27%) | 54 (30%) |
| Other | 11 (8%) | 3 (5%) | 11 (6%) |
| Participants reporting treated water use | |||
| Drinking and beverages | 95 (70%) | 38 (69%) | 171 (93%) |
| Use in food preparation | 52 (39%) | 23 (42%) | 86 (47%) |
| Volume treated water consumed | |||
| Drinking and beverages (L)a | 0.24 [0–0.71] | 0.47 [0–1.2] | 2.1 [1.1–3.0] |
| From use in food preparation (L)a | 0 [0–0.09] | 0 [0–0.14] | 0 [0–0.18] |
| Dietary information | |||
| Participants reporting consuming apple juice | 39 (29%) | 6 (11%) | 7 (4%) |
| Volume apple juice consumed (L)a,b | 0 {0–1.7} | 0 {0–1.7} | 0 {0–1.4} |
| Participants reporting consuming rice | 39 (29%) | 12 (22%) | 67 (37%) |
| Amount rice consumed (dry rice g)a,b | 0 {0–142.4} | 0 {0–85.8} | 0 {0–386.1} |
| Participants reporting chicken meal consumptionc | 87 (64%) | 39 (71%) | 128 (70%) |
| Participants reporting seafood meal consumptiond | |||
| During 3-day dietary survey | 2 (1.5%) | 1 (1.8%) | 4 (2.2%) |
| 1 week before survey | 35 (26%) | 17 (31%) | 86 (47%) |
| 2 weeks before survey | 62 (46%) | 23 (42%) | 128 (70%) |
| Bathing behavior | |||
| Participants reporting taking baths | 114 (84%) | 13 (24%) | 34 (19%) |
| Time spent in bath (h)a,b | 0.5 {0–1.75} | 0 {0–1.5} | 0 {0–1.2} |
| Participants reporting taking showers | 38 (28%) | 46 (84%) | 170 (93%) |
| Time spent in shower (h)a | 0 [0–0.08] | 0.33 [0.15–0.67] | 0.4 [0.23–0.55] |
All water, apple juice, and rice consumption variables and time spent bathing are 3-day totals from the diet and bathing diary.
Where one or more age groups’ median and interquartile range were all equal to 0, the median and range are presented.
Chicken meal consumption indicates participants that consumed any chicken or meals with chicken during the 3-day diary period.
Seafood meal consumption includes individuals that reported consuming a seafood meal in their 3-day diet diary and data from the household survey where participants indicated whether they ate seafood in the previous 1 or 2 weeks.
3.2. Water samples
Arsenic levels in untreated well water ranged from 0.5–640 μg/L (Table 1). Bottled water usage was the most common treated drinking water source, with 66% of all households relying on bottled water, and 28% employing a POU filter system (Table 1). In comparison, only 6% of households reported their treated water source as ‘Other.’ Treated water arsenic concentrations ranged from<LOD-9.4 μg/L, with an average concentration of <1 μg/L for all age groups.
3.3. Water use and estimated arsenic intake
Fewer than 10% of participants reported consuming untreated water directly through drinking and beverage consumption on their three-day diet diaries (Table 1). Approximately 20% of participants reported use of untreated water to prepare meals such as rice, pasta, or soups (Table 1). Seven households (4%) reported prior, habitual use of untreated water for drinking and beverage preparation on the household survey, but no use during the three-day diary period. Forty-eight households (28%) reported prior, habitual use of untreated water for cooking in the household survey, but did not report any use during the three-day diary period.
Among participants reporting untreated water use during the three-day diary period, median estimated arsenic intake was on the order of 2 to 6 μg per day from drinking and beverage preparation, and just under 1 μg per day from cooking (Table 2). Several individuals had substantial daily intakes (13 to 24 μg per day). High arsenic intake resulted in some instances from small amounts of water use (~0.05 L/day) of high-arsenic water (410 to 640 μg/L), and in other instances from more regular use (~0.5 L/day) of low-arsenic water (14 to 16 μg/L).
Table 2.
Daily arsenic intake (μg/day) from untreated water use for individuals who reported untreated water use (median {range}).
| Untreated water use | Age groups and number of individuals
|
|||||
|---|---|---|---|---|---|---|
| 1–7 | n | 8–17 | n | Adults ≥ 18 | n | |
| Drinking and beverages | 2.76 {0.51–13.2} | 10 | 1.63 {0.62–8.20} | 4 | 6.31 {1.68–24.3} | 11 |
| Use in food preparation | 0.90 {0.10–19.2} | 31 | 0.75 {0.09–2.21} | 13 | 0.85 {0.01–13.5} | 35 |
| Total untreated water use | 1.01 {0.10–19.2} | 36 | 0.95 {0.09–8.68} | 15 | 1.18 {0.01–24.3} | 42 |
3.4. Bathing behavior
Young children were more likely to report taking baths than showers, and spent the most time in the bath out of the three age groups (Table 1). Older children took mostly showers, and spent the most time showering, as compared to both young children and adults. Adults’ bathing and showering habits were similar to those of older children (Table 1).
3.5. Dietary intake
Items identified in the three-day diet diary that had the potential to contribute to arsenic exposure included rice, chicken, apple juice, and seafood. Rice consumption was reported by all age groups, with >20% of children and almost 40% of adult participants consuming rice (Table 1). Chicken consumption was common during the diary period, with more than half of the population consuming one or more chicken meals. Young children were the largest consumers of apple juice (Table 1). Older children reported some apple juice consumption, while only a small percent of adults reported consuming apple juice (Table 1). Although participants had been asked to abstain from eating any fish or seafood during the three-day diet diary period, one or more individuals in each age group reported seafood consumption during the three-day diary period (Table 1). Results from the household survey indicated that nearly a third of the children and almost half of the adults ate some type of seafood within the week prior to the three-day diet diary, with a larger fraction indicating they ate seafood within the previous two weeks (Table 1).
3.6. Urinary arsenic levels
Nearly all participants had detectable levels of total arsenic in their urine. The most commonly measurable urinary arsenic species were the methylated metabolites MMA and DMA, and arsenobetaine (Table 3). The inorganic species, As3+ and As5+, were often below the LOD (Table 3). Young children had the largest proportion of individuals with detectable MMA levels, a proportion that was significantly greater than the adult population and borderline significant (p=0.065)when compared to older children (Table 3). Young children also had significantly higher DMA levels than older children and adults. Arsenobetaine, an organoarsenic species present in seafood, was commonly detected in adults, while fewer than half of children in either age group had detectable levels (Table 3). The difference in arsenobetaine levels between age groups was consistent with reported seafood consumption from the household survey.
Table 3.
Urinary arsenic levels.
| Urinary measure (μg/L) | Age groups
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–7 (n = 135)
|
8–17 (n = 55)
|
Adults ≥ 18 (n = 183)
|
||||||||||||||||
| % > LOD | Median | (Range) | 75th | 90th | 95th | % > LOD | Median | (Range) | 75th | 90th | 95th | % > LOD | Median | (Range) | 75th | 90th | 95th | |
| Total As | 100.0% | 12.20 | (1.87–63.4) | 19.90 | 32.01 | 43.6 | 100.0% | 11.79 | (2.94–38.8) | 17.41 | 19.72 | 23.2 | 99.5% | 12.10 | (0.42–147.2) | 20.40 | 35.70 | 54.6 |
| As3+ | 18.5% | <LOD | (0.85–3.50) | <LOD | 1.70 | 2.1 | 20.0% | <LOD | (0.85–3.20) | <LOD | 1.70 | 1.8 | 16.4% | <LOD | (0.85–5.50) | <LOD | 1.50 | 2.0 |
| As5+ | 9.6% | <LOD | (0.71–41.5) | <LOD | <LOD | 1.4 | 7.3% | <LOD | (0.71–1.70) | <LOD | <LOD | 1.1 | 6.0% | <LOD | (0.71–5.40) | <LOD | <LOD | 1.3 |
| MMA | 48.9%a | <LOD | (0.64–8.40) | 2.10 | 3.10 | 3.6 | 34.6%ab | <LOD | (0.64–7.80) | 1.80 | 2.80 | 4.0 | 33.3%b | <LOD | (0.64–7.90) | 1.80 | 2.90 | 3.3 |
| DMA | 97.0% | 7.50c | (1.20–33.7) | 11.20 | 17.70 | 23.9 | 94.6% | 6.70d | (1.20–25.1) | 8.30 | 10.50 | 15.0 | 93.4% | 5.10d | (1.20–47.6) | 7.50 | 12.50 | 15.4 |
| Arsenobetaine | 43.7%a | <LOD | (0.28–12.2) | 1.20 | 3.30 | 6.8 | 43.6%a | <LOD | (0.28–11.6) | 1.20 | 3.70 | 8.7 | 78.7%b | 2.10 | (0.28–101.9) | 6.90 | 13.30 | 22.6 |
| Summed | – | 10.26c | (3.39–48.2) | 15.10 | 22.81 | 32.3 | – | 8.89d | (3.39–34.2) | 11.66 | 15.41 | 21.4 | – | 7.39d | (3.39–62.8) | 10.99 | 17.30 | 21.3 |
| Subtracted | – | 11.22c | (1.40–61.8) | 17.31 | 30.51 | 42.2 | – | 10.02cd | (2.34–38.1) | 13.50 | 19.10 | 22.9 | – | 8.25d | (0.04–65.0) | 14.10 | 21.50 | 34.3 |
Summed urinary measure = (As3+ +As5+ + MMA + DMA). Subtracted urinary measure=(total As–arsenobetaine). <LOD represents urinary arsenic measures where the majority of samples were less than the limit of detection. Measures with different letters indicate significant difference (p < 0.05) between age groups, where ab indicates % > LOD comparisons and cd indicates urinary arsenic level comparisons.
The two methods for quantifying total inorganic urinary arsenic concentration (ΣAsi and [TAs–AsB])were highly correlated (R2=0.86). [TAs – AsB] urinary arsenic levels were marginally higher than Σ Asi for all age groups (Table 3). Both measures displayed a slight decreasing trend across age groups, with young children having significantly higher levels than older children or adults (Table 3).
3.7. Regression results
3.7.1. Relation of untreated water arsenic to urinary arsenic
Due to the high proportion of non-detects among the summed species, the subtracted [TAs – AsB] measure was used as the primary urinary arsenic measure for regression analyses. Results from regression analyses with the summed (ΣAsi) urinary arsenic measure were similar to results with the subtracted exposure measure (Supplemental Information).
Urinary arsenic, as ln[TAs–AsB], increased with increasing untreated arsenic water concentration for all age groups. This association remained statistically significant after controlling for volume of untreated water consumed, arsenic intake from reported use of untreated water, treated water arsenic concentration, treated water consumption, age, gender, urinary creatinine, and consumption of selected dietary items (Table 4). In this full model, the arsenic intake term based on reported use of untreated water for drinking and beverage preparation was also positively associated with urinary arsenic for all three age groups. The corresponding intake term for use of untreated water in cooking was only significant for the adult age group.
Table 4.
Regression model parameter estimates (95% confidence intervals) for children ages 1–7 and 8–17 and adults ≥18.
| Parameter | 1–7 (n = 135)
|
8–17 (n = 55)
|
≥18 (n = 183)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p value | Estimate | (95% CI) | p value | Estimate | (95% CI) | p value | |
| Untreated water [As] (μg/L) | 0.0021 | (0.0008, 0.0034) | 0.0013 | 0.0013 | (0.0007, 0.0018) | <.0001 | 0.0014 | (0.0006, 0.0022) | 0.0010 |
| Untreated water consumption (L) | −0.7872 | (−1.7116, 0.1422) | 0.0969 | −1.6286 | (−2.5035, −0.7537) | 0.0003 | −0.1435 | (−0.6390, 0.3520) | 0.5703 |
| Untreated water from cooking (L) | 1.1372 | (−0.5421, 2.8165) | 0.1844 | −0.7605 | (−2.1996, 0.6786) | 0.3003 | −1.5169 | (−3.3288, 0.2951) | 0.1009 |
| Untreated water [As] × untreated water consumption (μg) | 0.0205 | (0.0028, 0.0382) | 0.0235 | 0.0957 | (0.0509, 0.1405) | <.0001 | 0.0224 | (0.0036, 0.0411) | 0.0194 |
| Untreated water [As] × untreated water from cooking (μg) | 0.0117 | (−0.0044, 0.0278) | 0.1538 | −0.0008 | (−0.0690, 0.0675) | 0.9828 | 0.0395 | (0.0139, 0.0650) | 0.0025 |
| Treated water [As] (μg/L) | 0.1037 | (0.0254, 0.1820) | 0.0095 | 0.0602 | (0.0096, 0.1108) | 0.0197 | 0.0798 | (0.0335, 0.1260) | 0.0007 |
| Treated water consumption (L) | −0.0478 | (−0.2183, 0.1227) | 0.5826 | 0.2219 | (0.1029, 0.3409) | 0.0003 | 0.0557 | (−0.0028, 0.1142) | 0.0619 |
| Treated water from cooking (L) | −1.1925 | (−2.0308, −0.3541) | 0.0053 | −1.1882 | (−1.6792, −0.6972) | <.0001 | −0.1321 | (−0.5772, 0.3129) | 0.5606 |
| Apple juice consumption (L) | 0.1988 | (−0.0900, 0.4875) | 0.1773 | 0.4430 | (0.2023, 0.6837) | 0.0003 | – | – | – |
| Rice meal consumption (g dry rice) | 0.0071 | (0.0040, 0.0102) | <.0001 | 0.0104 | (0.0060, 0.0147) | <.0001 | 0.0027 | (0.0009, 0.0044) | 0.0029 |
| Seafood consumption (previous week) | 0.0165 | (−0.1772, 0.2102) | 0.8675 | 0.1382 | (−0.1469, 0.4233) | 0.3421 | 0.2655 | (0.0915, 0.4396) | 0.0028 |
Results for ln-transformed subtracted urinary arsenic adjusted for gender, age, and creatinine. All consumption variables represent 3-day totals.
3.7.2. Relation of bathing exposure to urinary arsenic
Bathing exposure to untreated water was not associated with urinary arsenic, regardless of how it was modeled. For young children, the group that spent the most time in the bath, neither total time spent bathing over the three-day diary period nor the interaction parameter of time spent bathing multiplied by untreated water arsenic concentration were significant predictors of urinary arsenic (Table 5). The number of bathing events was also not a significant explanatory variable for urinary arsenic. In the case of older children and adults, who spent more time showering, none of the shower-related terms were significant predictors of exposure (Table 5).
Table 5.
Parameter estimates (95% confidence intervals) for bathing and showering for children ages 1–7 and 8–17 and adults ≥18.
| Parameter | 1–7 (n = 135)
|
8–17 (n = 55)
|
≥18 (n = 183)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | (95% CI) | p value | Estimate | (95% CI) | p value | Estimate | (95% CI) | p value | |
| Bathing hours | 0.0480 | (−0.1687, 0.2647) | 0.6643 | - | – | – | – | – | – |
| Untreated water [As] × bathing hours | 0.0006 | (−0.0020, 0.0032) | 0.6351 | - | – | – | – | – | – |
| Number of baths | −0.0224 | (−0.1046, 0.0597) | 0.5922 | - | – | – | – | – | – |
| Showering hours | – | – | – | −0.2254 | (−0.4468, −0.0040) | 0.0460 | −0.0263 | (−0.3456, 0.2929) | 0.8715 |
| Untreated water [As] × showering hours | – | – | – | −0.0011 | (−0.0032, 0.0010) | 0.2848 | −0.0012 | (−0.0032, 0.0009) | 0.2570 |
| Number of showers | – | – | – | −0.0393 | (−0.1182, 0.0396) | 0.3291 | −0.0145 | (−0.0756, 0.0465) | 0.6410 |
Results from the full model with ln-transformed subtracted urinary arsenic adjusted for gender, age, and creatinine. Time spent bathing or showering and number of baths and showers are 3-day totals.
3.7.3. Relation of dietary items to urinary arsenic
Rice consumption was positively and strongly associated with urinary arsenic for all age groups (Table 4). Apple juice consumption was only significant for older children. Seafood consumption reported in the previous week was significantly associated with urinary arsenic for adults, but not for children (Table 4). Chicken consumption was not a significant predictor of urinary arsenic in any age group (Supplementary Information).
3.7.4. Sensitivity analyses and stratified models
Two approaches were used to assess whether the relationship between urinary arsenic and untreated water arsenic might be related to either unreported use of untreated water or use prior to the three day diary period. Regression analyses were re-run after excluding individuals who reported any use of untreated water in either the three-day diet diary or the household survey. When these individuals were excluded, the untreated water arsenic parameter estimate was reduced more than 3-fold for adults and was no longer significant (Table 6). The parameter estimate was also no longer significant for older children, though the magnitude remained relatively unchanged. In contrast, for young children, neither the parameter estimate nor the statistical significance changed. We further explored the model for young children by stratifying it by the median untreated water arsenic level of 40 μg/L. After this stratification, the untreated water arsenic parameter remained unchanged in magnitude and significance at higher water arsenic levels. However, the parameter estimate was substantially reduced and no longer significant for the group with untreated water arsenic <40 μg/L (Table 6).
Table 6.
Remaining residual exposure from untreated water for participants that did not report any untreated water consumption in either the 3-day diet diary or household survey and children ages 1–7 grouped by median untreated water arsenic concentration.
| Comparison between all participants and participants that reported no untreated water consumption | Untreated water [As] (μg/L) parameter
|
||
|---|---|---|---|
| Estimate | (95% CI) | p value | |
| All children 1–7 (n = 135) | 0.0021 | (0.0008, 0.0034) | 0.0013 |
| No untreated water consumption children 1–7 (n = 56) | 0.0023 | (0.0006, 0.0040) | 0.0088 |
| All children 8–17 (n = 55) | 0.0013 | (0.0007, 0.0018) | <.0001 |
| No untreated water consumption children 8–17 (n = 25) | 0.0010 | (−0.0007, 0.0027) | 0.2439 |
| All adults ≥18 (n = 185) | 0.0014 | (0.0006, 0.0022) | 0.0010 |
| No untreated water consumption adults ≥ 8 (n = 95) | 0.0004 | (−0.0006, 0.0015) | 0.4219 |
| All children 1–7 untreated water As level stratification | |||
| Children 1–7 household untreated water [As] < 40 μg/L (n = 66) | −0.0014 | (−0.0133, 0.0105) | 0.8202 |
| Children 1–7 household untreated water [As] ≥ 40 μg/L (n = 69) | 0.0022 | (0.0005, 0.0039) | 0.0123 |
Results from the ln-transformed subtracted urinary arsenic model adjusted for gender, age, and creatinine.
3.7.5. Predictive analysis
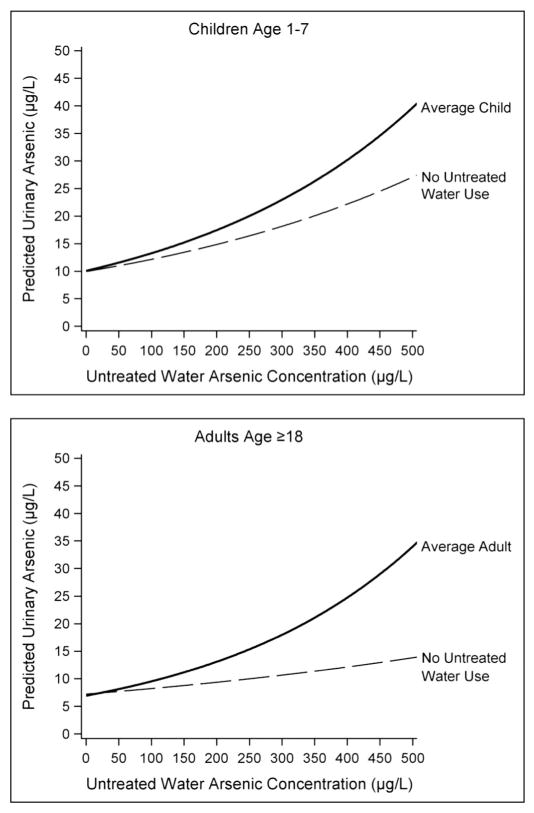
Regression models for young children and adults were used to predict the relationship between increasing untreated water arsenic concentration and urinary arsenic levels for the ‘average’ individual within the study population. The models predicted considerable increases in urinary arsenic with increasing untreated water arsenic concentration for both age groups, with urine levels approaching 40 μg/L at an untreated water level of 500 μg/L (Fig. 1). When the predictive models were run with untreated water consumption set to zero, the increase in urinary arsenic with increasing untreated water arsenic was substantially reduced – though the effect was smaller for young children than for adults (Fig. 1). For example, as untreated water arsenic concentration ranged from 100 to 500 μg/L, the predicted reduction in urinary arsenic due to total avoidance of untreated water ranged from 8% to 32% for young children, and 14% to 59% for adults.
Fig. 1.
Predicted urinary arsenic levels for average household behavior with comparison to no untreated water use. Predicted urinary arsenic levels from pared-down regression models for individual age groups. Mean age-specific variable values used for average behavior with increasing household untreated water arsenic concentrations. No untreated water use represents average behavior with all untreated water consumption variables set to zero.
4. Discussion
Arsenic contamination of groundwater is a global concern for regions in both developed and developing nations. The World Health Organization has identified arsenic as one of the top 10 chemicals of major public health concern (WHO, 2012). In Maine, a state where more than half the population relies on private well water, arsenic levels above 10 μg/L are common. To mitigate exposure to arsenic, Maine families most commonly use POU treatment systems at the kitchen sink or switch to bottled water (Flanagan et al., 2015a). The effectiveness of these mitigation strategies under typical use, and over a wide range of well water arsenic levels, has not been evaluated. The overarching goal of our cross-sectional study was to assess arsenic exposure in these settings, with a special focus on families with young children who may have exposures that are qualitatively different from those of adults. The primary findings of our study were fourfold: 1) participants were not perfectly compliant in using their POU-treated or bottled water supplies, and their use of untreated well water was associated with higher levels of arsenic exposure; 2) even after accounting for this consumption of untreated water, the concentration of arsenic in well water was associated with higher levels of urinary arsenic, most likely indicating an unmeasured exposure pathway or measurement error in measured exposure pathways; 3) bathing was not a significant exposure pathway for either child or adult study participants; and 4) several dietary items, most notably rice and rice-containing foods, contributed significantly to participants’ overall arsenic exposure, especially for children.
Our finding that occasional consumption of untreated water was significantly associated with higher urinary arsenic (Table 4) may seem unsurprising, but presents reasons for concern. The fact that participants reported any use of untreated water challenges the prevailing assumption that private well owners with elevated arsenic in well water will be consistently compliant with using POU systems or bottled water. In our study population, use of untreated water for cooking was more common than use for drinking and beverage preparation. Yet estimated arsenic intake was greater for use of untreated water for drinking and beverages versus cooking, and was more consistently associated with urinary arsenic.
To assess the importance of this reported use of untreated water, we conducted a predictive analysis (Fig. 1), and found that for the average study participant, eliminating all consumption of untreated water via drinking, beverage preparation, and cooking would result in a substantial reduction in arsenic exposure for adults and a moderate reduction for young children. For example, at a concentration of 200 μg/L arsenic in untreated water, total elimination of untreated water use would result in a predicted reduction in urinary arsenic of ~30% for adults and ~15% for children. This predictive analysis is consistent with empirical observations in the published literature. Josyula et al. (2006) reported mean urinary arsenic levels were reduced 21% following bottled water intervention in a population served by water with moderately elevated arsenic ~(20 μg/L), but reduced 34% among those reporting exclusive use of bottled water for drinking, beverage preparation, and cooking. These findings reinforce the importance of promoting compliance with a POU treatment system or bottled water mitigation strategy.
After controlling for documented arsenic intake from use of untreated water, we still found a positive association between levels of arsenic in untreated well water and urinary arsenic. Surprisingly, the association was strongest for young children. This observation suggests either another exposure pathway, or that the exposure pathways we accounted for via survey and diary were measured imperfectly. One possibility is that three-day diaries may not be representative of participant water use prior to our study. Data from our household survey indicate that over 30% of participants who reported no use of untreated water for cooking during the three-day diary period, reported some use prior to enrolling in our study. Urinary arsenic is reported to have a half-life of 2–4 days (NRC, 1999; Zheng et al., 2002), so it is possible that untreated water used just prior to the three-day diary could contribute to measured urinary arsenic levels and result in an association with untreated water arsenic. Our sensitivity analyses supported this possibility for adults, but not young children. There was no longer an association between urinary arsenic and untreated water arsenic when the regression model was re-run excluding adult participants reporting any prior use of untreated water on the household survey. In contrast, the association for young children remained with similar magnitude and statistical significance, suggesting either that their exposure was from a still-unidentified pathway, or that diet diaries by adult proxy failed to capture unsupervised or incidental consumption of untreated water.
We would expect unsupervised or incidental untreated water exposure among children to contribute most strongly to urinary arsenic when water arsenic levels are high. For example, one study participant had an estimated arsenic intake of 19 μg/day from consuming just an ounce (30 mL) of water per day with very high arsenic. Our stratified analyses with young children support this possibility, with the absence of an association between urinary arsenic and untreated water arsenic at water arsenic <40 μg/L, while the association remained unchanged at concentrations ≥40 μg/L (Table 6).
The stratified analysis suggests that a POU treatment system or bottled water substitution are effective exposure control strategies for only moderately elevated arsenic levels. Spayd et al. (2015) reported that use of a POU treatment system was less effective at reducing urinary arsenic than use of a whole-house point-of-entry (POE) treatment system in a study of NJ residents with average untreated water levels of 40–45 μg/L. A POE system removes the reliance on behavior to avoid exposure to untreated water. This convenience comes at a cost. Bottled water use can cost several hundred dollars per year (Sargent-Michaud et al., 2006). A POU treatment system can cost several hundred to over a thousand dollars to install and about $100 per year for ongoing maintenance; a POE treatment system can cost $2000 to $3000 to install and $200 to $300 per year in operational costs (New Jersey Geological Survey, 2005). High treatment costs are a known barrier to acting on high arsenic water for some families (Flanagan et al., 2015b).
Bathing exposure for children is a common concern among parents, and the lack of empirical data to address this concern was a motivation for our study. Our results suggest that bathing is not an important contributor to urinary arsenic for either children or adults. Dermal uptake or inhalation exposure should be directly proportional to the time spent bathing and the water arsenic concentration (EPA, 2004). Yet neither the total time spent bathing over a three-day period, nor an interaction term between time spent bathing and untreated water arsenic concentration were associated with urinary arsenic for young children who primarily took baths, or older children and adults who showered (Table 5). We also did not see any relationship between urinary arsenic and the number of bathing events, which might be a better metric for episodic play-related behavior. Our findings indicate that any exposure associated with bathing is small in magnitude.
Our final finding concerns the contribution of specific foods to urinary arsenic. The primary reason for measuring dietary arsenic in the present study was to control for these exposures as we explored any remaining water-related exposures. Yet the issue of dietary arsenic exposure is of increasing concern. Dietary arsenic has been reported to be the major source of inorganic arsenic exposure when water arsenic is low (Kurzius-Spencer et al., 2013). Rice consumption, in particular, has been reported to be associated with increased urinary arsenic levels in U.S. populations (Davis et al., 2012; deCastro et al., 2014; Gilbert-Diamond et al., 2011). We also found that increased rice consumption resulted in higher urinary arsenic levels for all age groups (Table 3), and our parameter estimates for adult rice consumption are in close agreement with those reported by Gilbert-Diamond et al. (2011) when expressed as dry grams per day (0.007 g/day versus 0.009 g/day). We believe our findings may represent the first data on the contribution of rice consumption to urinary arsenic for children less than 6 years of age. Davis et al. (2012) reported that rice was a significant predictor of arsenic in children’s diets for children 6 years of age and older. In our study, rice intake was a stronger and a more significant predictor of urinary arsenic for both child age groups, compared to adults.
Our study had several limitations. While diet diaries are generally considered the “gold standard” of dietary assessment (Thompson and Byers, 1994), there are some limitations with their use in our study. Diaries for young children in this study were completed by an adult proxy, and care-givers may not have been aware of all untreated water ingestion by children. As noted above, consumption of untreated water prior to the three-day diary could contribute to our measured urinary arsenic levels, given a biological half-life of 2–4 days. Either could explain larger parameter estimates for the effect of untreated water arsenic on urinary arsenic for young children (after controlling for estimated arsenic intake), and both were explored in sensitivity and stratified analyses.
An additional limitation of the diet diary is that it was not designed to precisely quantify water intake from individual foods that were prepared with water, such as pasta, soups, or rice. Water volumes associated with these foods were estimated based on standard reference volumes from the USDA National Nutrient Database. Consequently, uncertainty in the estimates of water consumption from food preparation was unavoidable. It is difficult to assess how these uncertainties affect the estimates of association. Typically misclassification errors will bias toward the null, although this is not always the case (Flegal et al., 1991).
A limitation with the “subtracted”, [TAs – AsB], urinary arsenic exposure biomarker is that it may include arsenic species related to diet rather than well water, such as arsenosugars. The summed arsenic exposure measure (sum of As3+, As5+, MMA, and DMA) has the benefit of including only those arsenic species associated with well water (As3+ and As5+) and their methylated metabolites (MMA and DMA). However, replacing the large number of censored non-detect values for As3+ and As5+ with a values equal to the LOD/√2 introduces other potential bias, and reduces power to detect associations in a regression framework (Hughes, 2000).
Finally, our study population cannot be viewed as a random sample of the general populations of Maine, and thus cannot be used to make population estimates. Our study population purposefully represents households with a known arsenic exposure source, so it is therefore not surprising that urinary arsenic levels from our study population are substantially higher than a representative sampling of the general U.S. population reported by NHANES (Table 7). Our study population also reflects households that have both taken action to mitigate exposure and were willing to participate in a study requiring a three-day diet and bathing diary, urine collection, and home visit. We therefore suspect our study population is likely to be more conscientious about using treated water than the general Maine population on private well water.
Table 7.
Summed inorganic-related urinary arsenic (As3+, As5+, MMA, and DMA) level comparisons with 2003–2004 NHANES results (median (95% confidence interval)).
| Population | Age groups
|
||
|---|---|---|---|
| 6–11 years | 12–19 years | 20 years or older | |
| Maine study | 10.3 (8.79–11.7) | 6.89 (6.40–10.5) | 7.39 (6.92–8.42) |
| NHANES 2003–2004a | 6.10 (5.80–6.90) | 6.10 (5.90–7.00) | 6.00 (5.30–6.20) |
NHANES 2003–2004 results are the closest period available to the Maine study dates.
Our findings add to the challenges confronting public health practitioners promoting private well water safety. Recent surveys of residents of central Maine (a region known to have a high prevalence of wells with elevated arsenic) have documented that 41% of respondents had never tested their well water for arsenic; that more than 30% of respondents known to have elevated arsenic well water had yet to take any action to mitigate exposure when surveyed years later; and that, of those that had taken steps to reduce exposure, 15% were found to have treatment systems that were failing to provide drinking water with arsenic less than 10 μg/L (Flanagan et al., 2015a, b). Thus, in addition to promoting well water testing, encouraging treatment of contaminated water and emphasizing the importance of maintaining treatment systems so that they continue to function effectively, public health practitioners must also stress the importance of consistently using treated or bottled water for exposure reduction, especially when arsenic levels are above 40 μg/L, and especially when the household contains young children.
5. Conclusions
We have found clear evidence of water-related exposure among families relying on POU treatment systems or supplemental use of bottled water to mitigate water arsenic exposure. This exposure resulted in part from the occasional use of untreated water for beverage preparation or for cooking, and in part from unquantified water-related pathways. Our findings suggest that private well owners relying on these two common mitigation strategies need to practice vigilance in avoiding use of untreated water. This exposure avoidance behavior becomes increasingly important as well as water arsenic level increase above 40 μg/L, and is especially important in households with young children whose ability or willingness to refrain from ingestion of untreated water may be less consistent.
Supplementary Material
HIGHLIGHTS.
Common mitigation strategies to prevent well water arsenic exposure were assessed.
These strategies were less able to prevent exposure when arsenic levels were >40 μg/L.
Bathing was not a significant arsenic exposure source for children or adults.
Untreated water use explained more arsenic exposure in adults than children.
Complete compliance with a mitigation strategy is important in reducing exposure.
Acknowledgments
We would like to acknowledge the integral role of Deborah Moll in the study design and study logistics. We thank Tom Crosby and Cheryl Soucy for laboratory water analyses, Deborah Rice for helpful discussions on dietary data and associated exposure measures, Doug Thompson for assistance with statistical analyses, Erin Guay and Emily Gilbertson for field work, and Raquel Sabogal and Carlos Bell for assistance with the development of study materials and data entry. We also thank Carl Verdon, Mark Fresquez, Dana Henahan, Jeff Jarrett, Jennifer Buzzel and Pam Olive at the CDC for laboratory urine analyses. This work was jointly supported by funding from the Maine Department of Health and Human Services and the CDC.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scitotenv.2015.11.136.
References
- Ayotte JD, Montgomery DL, Flanagan SM, Robinson KW. Arsenic in groundwater in eastern New England: occurrence, controls, and human health implications. Environ Sci Technol. 2003;37:2075–2083. doi: 10.1021/es026211g. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Measuring guides for the dietary recall interview. [Last accessed on 02/19/15];National Health and Nutrition Examination Survey. 2010 http://www.cdc.gov/nchs/nhanes/measuring_guides_dri/measuringguides.htm.
- CDC. National Health and Nutrition Examination Survey Fourth National Report on Human Exposure to Environmental Chemicals. 2015 Updated Tables, February 2015. http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf.
- Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro BR, Caldwell KL, Jones RL, Blount BC, Pan Y, Ward C, Mortensen ME. Dietary sources of methylated arsenic species in urine of the United States population. NHANES 2003–2010 PLoS One. 2014;9(9):e108098. doi: 10.1371/journal.pone.0108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Determination of trace elements in waters and wastes by inductively coupled plasma — mass spectrometry. Environmental Monitoring Systems Laboratory. Office of Research and Development. U.S. Environmental Protection Agency; 1994. Method 200.8. [Google Scholar]
- EPA. Risk assessment guidance for superfund volume I. Part E. Supplemental Guidance for Dermal Risk Assessment. U.S. Environmental Protection Agency; 2004. EPA/540/R/99/005. [Google Scholar]
- FDA. Arsenic in apple juice analytical results, 2005–2011. [Last accessed on 02/05/2015];Toxic elements food and foodware program. 2011 http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm273328.htm.
- FDA. [Last accessed on 02/19/2015];Analytical results from inorganic arsenic in rice and rice products sampling. 2013 Sep; 2013 http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm319870.htm.
- Flanagan SV, Marvinney RG, Johnston RA, Yang Q, Zheng Y. Dissemination of well water arsenic results to homeowners in Central Maine: influences on mitigation behavior and continued risks for exposure. Sci Total Environ. 2015a;505:1282–1290. doi: 10.1016/j.scitotenv.2014.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan SV, Marvinney RG, Zheng Y. Influences on domestic well water testing behavior in a Central Maine area with frequent groundwater arsenic occurrence. Sci Total Environ. 2015b;505:1274–1281. doi: 10.1016/j.scitotenv.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Keyl PM, Nieto FJ. Differential missclassification arising from nondifferential errors in exposure measurment. Am J Epidemiol. 1991;134:1233–1244. doi: 10.1093/oxfordjournals.aje.a116026. [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MD. Analysis and design issues for studies using censored biomarker measurements with an example of viral load measurements in HIV clinical trials. Stat Med. 2000;19:3171–3191. doi: 10.1002/1097-0258(20001215)19:23<3171::aid-sim619>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Josyula AB, McClellen H, Hysong TA, Kurzius-Spencer M, Poplin GS, Sturup S, Burgess JL. Reduction in urinary arsenic with bottled-water intervention. J Health Popul Nutr. 2006;24:298–304. [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, Burgess JL, Harris RB, Hartz V, Roberge J, Huang S, Hsu CH, O’Rourke MK. Contribution of diet to aggregate arsenic exposures-an analysis across populations. J Expo Sci Environ Epidemiol. 2014;24:156–162. doi: 10.1038/jes.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, O’Rourke MK, Hsu CH, Hartz V, Harris RB, Burgess JL. Measured versus modeled dietary arsenic and relation to urinary arsenic excretion and total exposure. J Expo Sci Environ Epidemiol. 2013;23:442–449. doi: 10.1038/jes.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiselle MC, Marvinney RG, Smith AE. Spatial distribution of arsenic in groundwater in Maine. Geol Soc Am. 2001;33:1. [Google Scholar]
- Maine Tracking Network. Environmental and Public Health Tracking. Maine Center for Disease Control and Prevention; 2014. [Last accessed on 2/8/2015]. https://data.mainepublichealth.gov/tracking/ [Google Scholar]
- Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: a U.S.-based market basket sample. Environ Health Perspect. 2013;121:818–824. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Francesconi KA, Silbergeld EK, Guallar E. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US. Environ Res. 2011;111:110–118. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Jersey Geological Survey. arsenic water treatment for residential wells in New Jersey. [Last accessed on 2/5/2015];Information circular. 2005 http://www.nj.gov/dep/pwta/Arsenic_Treatment.pdf.
- NRC. Arsenic in Drinking Water. National Research Council (US) Subcommittee on Arsenic in Drinking Water. National Academies Press (US); Washington (DC): 1999. [PubMed] [Google Scholar]
- Peters SC. Arsenic in ground waters in the Northern Appalachian Mountain belt: a review of patterns and processes. J Contam Hydrol. 2008;99:8–21. doi: 10.1016/j.jconhyd.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Rivera-Núñez Z, Meliker JR, Meeker JD, Slotnick MJ, Nriagu JO. Urinary arsenic species, toenail arsenic, and arsenic intake estimates in a Michigan population with low levels of arsenic in drinking water. J Expo Sci Environ Epidemiol. 2012;22:182–190. doi: 10.1038/jes.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryker SJ. Mapping arsenic in groundwater — a real need, but a hard problem. Geotimes News Mag Earth Sci. 2001;46:3. [Google Scholar]
- Sanford RT, Pierson CT, Crovelli RA. An objective replacement method for censored geochemical data. Math Geol. 1993;25:59–80. [Google Scholar]
- Sargent-Michaud J, Boyle KJ, Smith AE. Cost effective arsenic reductions in private well water in Maine. J Am Water Resour Assoc. 2006;42:9. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 14.1 User’s Guide. SAS Institute Inc; Cary, NC: 2015. The SURVEYMEANS procedure. [Google Scholar]
- Spayd SE, Robson MG, Buckley BT. Whole-house arsenic water treatment provided more effective arsenic exposure reduction than point-of-use water treatment at New Jersey homes with arsenic in well water. Sci Total Environ. 2015;505:1361–1369. doi: 10.1016/j.scitotenv.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FE, Byers T. Dietary assessment resource manual. J Nutr. 1994;124:2245S–2317S. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- USDA. National nutrient database for standard reference release 26. Agricultural Research Service. National Agricultural Library; 2013. [Last accessed on 02/19/15]. http://ndb.nal.usda.gov. [Google Scholar]
- Verdon CP, Caldwell KL, Fresquez MR, Jones RL. Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: a CDC population biomonitoring method. Anal Bioanal Chem. 2009;393:939–947. doi: 10.1007/s00216-008-2537-3. [DOI] [PubMed] [Google Scholar]
- WHO. [Last accessed on 11/13/15];Arsenic fact sheet N°372. 2012 http://www.who.int/mediacentre/factsheets/fs372/en/
- Zheng Y, Wu J, Ng JC, Wang G, Lian W. The absorption and excretion of fluoride and arsenic in humans. Toxicol Lett. 2002;133:77–82. doi: 10.1016/s0378-4274(02)00082-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.