Abstract
This study investigated whether the ability to learn word-object associations following minimal exposure (i.e., fast mapping) was associated with concurrent and later language abilities in children with ASD. Children who were poor learners at age 3½ had significantly lower receptive language abilities than children who successfully learned the new words, both concurrently (n = 59) and 2 years later (n = 53), lending ecological validity to experimental fast-mapping tasks. Fast mapping comprehension at age 3½ was associated with better language outcomes regardless of whether children had produced the new words. These findings highlight the importance of investigating processes of language learning in children with ASD. Understanding these processes will enable the development of maximally effective strategies for supporting word learning.
Keywords: Fast mapping, Word learning, Language, Cognition, Autism spectrum disorder
Introduction
Fast mapping (Carey and Bartlett 1978) refers to a word-learning process by which children initially form an association between a new word and its meaning. Fast mapping abilities relate to concurrent and later language skills in typically developing children and late talking toddlers (Ellis Weismer et al. 2011), suggesting that children who more easily learn word-object associations have a language-learning advantage. Fast mapping skills are concurrently associated with receptive and expressive language abilities in young children with ASD (McDuffie et al. 2006), but we do not yet know whether variability in fast mapping helps explain variability in later language skills among children with ASD (McDuffie et al. 2012). Investigating this issue is important because it may help to explain why some children with ASD have better language outcomes than others. Some children with ASD may have language delays, in part, because they have difficulty initially linking new words and their meanings, which has implications for intervention strategies to facilitate word learning.
Although methodological differences have led to mixed findings on word learning in children with ASD, this work has yielded at least two broad insights. First, children with ASD learn words more easily if they are not required to rely on social cues to determine word meaning (Baron-Cohen et al. 1997; Preissler and Carey 2005). Second, children with ASD who have deficits in language and cognitive skills are more likely to show deficits in word learning than children with ASD who have age-appropriate language and cognitive skills (Baron-Cohen et al. 1997; Luyster and Lord 2009; Norbury et al. 2010). These findings relate directly to two aspects of the current study design. First, because we were interested in children’s ‘baseline’ ability to associate labels and objects, our fast mapping task did not require children to rely on social cues to determine the meaning of the new words (also see McDuffie et al. 2012). Second, because our research questions focused on differences among children with ASD, we included participants with a broader range of language and cognitive skills than most previous studies of word learning in children with ASD.
Although many studies of word learning in children with ASD have focused on comprehension, Norbury et al. (2010) also tested children’s production of newly taught words and found a surprising result. Following a teaching phase, high-functioning children with ASD showed better production of novel words than typically developing children matched on age, nonverbal cognition, and vocabulary, suggesting that the children with ASD had formed more robust phonological representations. Based on this finding, Norbury and colleagues proposed that, “…phonological learning may be a compensatory mechanism that supports word learning and language development in at least some children with ASD” (p. 4018). Because the participant sample had mean receptive vocabulary and nonverbal cognitive scores in the normal range, they emphasized the need for additional research to determine whether this finding generalizes to the broader population of children with ASD.
Following from Norbury et al. (2010), the current study investigated whether children with ASD who produced newly learned words at age 3½ had better language abilities than children who did not. Profile groups were created based on comprehension and production of novel words in a fast mapping task at age 3½ (see Section “Methods”). We asked: Do language abilities differ according to profiles of fast-mapping performance among children with ASD concurrently (at age 3½) or later (at age 5½)? We predicted that ‘rich representers’ (i.e., children with successful novel word production) would have higher language abilities than ‘shallow representers’ (i.e., children with successful comprehension only), and that shallow representers would outperform ‘poor learners’ (i.e., children who showed no evidence of fast mapping comprehension or production).
Methods
Participants were 129 young children with an ASD in a longitudinal study of language development. The current study examined the second and fourth visits, when children were, on average, 3½ and 5½ years old. Children were excluded if they had uncorrected vision or hearing deficits, known chromosomal disorders, cerebral palsy, or exposure to languages other than English. Participants in the current study were also required to complete the full fast mapping task; correctly name at least one familiar object; and complete the Auditory Comprehension subscale of the Preschool Language Scale, 4th Edition (PLS-4; Zimmerman et al. 2002) at age 3½, leaving 59 children at age 3½ (51 males) and 53 children at age 5½ (48 males).
Best estimate clinical DSM-IV-TR diagnoses (American Psychiatric Association 2000) were determined at study entry (mean age 2½) using all available information, including the Autism Diagnostic Interview-Revised or toddler research version (Rutter et al. 2003) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2002) or ADOS Toddler Module (Luyster et al. 2009). Fifty children received a diagnosis of Autistic Disorder, and nine children received a diagnosis of Pervasive Developmental Disorder, Not Otherwise Specified. The ADOS was re-administered at each visit to confirm diagnosis. ADOS calibrated severity scores measured autism severity; Toddler Module scores were derived by recoding identical items to Module 1 algorithm scores (Gotham et al. 2009). The Visual Reception subtest of the Mullen Scales of Early Learning (Mullen 1995) assessed nonverbal cognition at age 3½. The Auditory Comprehension and Expressive Communication subscales of the PLS-4 (Zimmerman et al. 2002) assessed receptive and expressive language (e.g., semantics, syntax), respectively, at both time points. Raw scores were used in analyses because we were interested in the skills that children had acquired, not the extent of delay compared to their same-age peers.
The fast mapping task presented two familiar objects (‘apple’ and ‘cookie’), two novel objects (‘koob’ and ‘tade’), and two unlabeled foil objects in the context of a picnic with puppets (see Ellis Weismer et al. 2011). The task included three identical trials, each of which included exposure, production, and comprehension phases. In the exposure phase, the examiner labeled each familiar and novel object while holding it in the child’s line of sight (e.g., ‘Here’s a tade. Put it in the basket.’). In the production phase, children were asked to name each familiar and novel object while the examiner held it up (e.g., ‘What’s this?’). In the comprehension phase, children were asked to identify each familiar and novel object (e.g., ‘Can you get the cookie?’). Children were divided into three profile groups based on their performance (see Table 1). Rich representers (n = 23) correctly produced at least one novel word. Six rich representers produced both novel words at least once. A specific criterion for comprehension was not set for the rich representers because correctly producing a novel word was taken as evidence that children understood that word. Shallow representers (n = 14) comprehended novel words on at least 2/6 trials1 but did not produce any. Poor learners (n = 22) comprehended no more than one novel word on one trial. Although creating profile groups limited the sensitivity of the fast mapping task, we did not use a total score because the task presented only two novel words, three times each. Total scores would have thus reflected the consistency with which children answered the same questions. Table 2 presents descriptive statistics for each group at age 3½. The groups did not significantly differ in age, F(2, 56) = 2.24, p = .116, η2 = 0.07, or autism severity, F(2, 56) = 0.85, p = .431, η2 = 0.03. However, they did significantly differ in non-verbal cognition, F(2, 56) = 6.02, p = .004, η2 = 0.18. The poor learners had significantly lower nonverbal cognition than the rich representers, t(43) = −3.23, p = .003, d = 0.96, and the shallow representers, t(34) = −2.61, p = .008, d = 0.88, but rich and shallow representers did not significantly differ t(35) = 0.05, p = .965, d = 0.01.
Table 1.
Fast mapping performance according to profile group: age 3½
| Poor learners (n = 22)
|
Shallow representers (n = 14)
|
Rich representers (n = 23)
|
||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Comprehension number correct | 0.59 (0.50) | 0–1 | 2.64 (0.75) | 2–4 | 2.61 (1.80) | 0–6 |
| Production number correct | 0 (0) | – | 0 (0) | – | 2.00 (1.24) | 1–6 |
Table 2.
Participant characteristics by fast mapping profile group: age 3½
| Poor learners (n = 22)
|
Shallow representers (n = 14)
|
Rich representers (n = 23)
|
||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Age in months | 43.36 (3.13) | 38–50 | 45.64 (3.03) | 39–50 | 45.57 (4.93) | 37–53 |
| Autism severity | 7.18 (1.59) | 5–10 | 6.43 (1.40) | 4–9 | 6.87 (1.91) | 3–10 |
| Nonverbal cognition | 34.86a (6.83) | 24–48 | 41.14b (7.38) | 27–49 | 41.04b (6.00) | 27–50 |
Autism severity = ADOS calibrated severity score. Nonverbal cognition = Visual Reception raw score from the Mullen Scales of Early Learning. Means with different superscripts significantly differ. Effect sizes are presented in the text
1Defining chance success for comprehension in the fast-mapping task depends on which objects children considered as candidate word referents. Because cookie and apple were familiar objects, we assumed they would not be viewed as candidate referents for the novel words. However, we assumed that children would likely consider all four unfamiliar items as candidate referents, making chance performance 25 %. Our criterion of 2/6 (33 %) for shallow representers was thus above chance (25 %).
Results
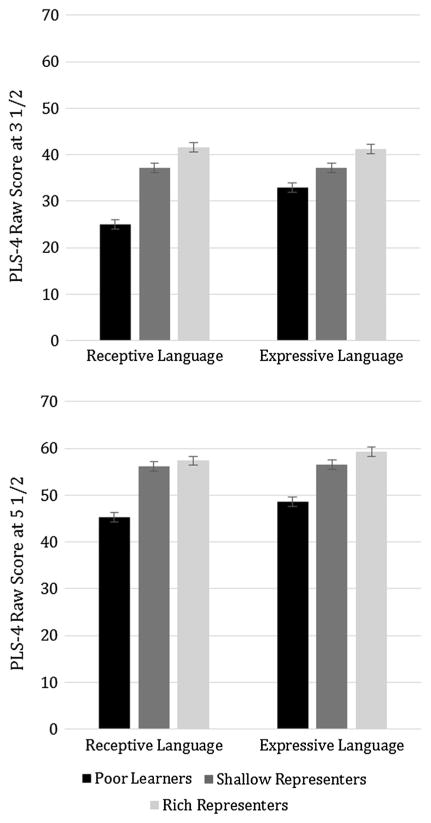
We conducted one-way ANOVAs with fast-mapping profile group as the between-subjects factor and language (receptive or expressive raw scores on the PLS-4) as the dependent variable. ANOVAs were followed by planned pairwise comparisons with Fisher’s LSD, using one-tailed p values based on our predictions. At age 3½, receptive language differed across the groups, F(2, 56) = 13.98, p < .001, η2 = .33 (see Table 3 and the top panel of Fig. 1). Poor learners had lower receptive language scores than shallow representers, t(34) = −3.43, p = .001, d = 1.13, and rich representers, t(43) = −5.40, p < .001, d = 1.61, but rich and shallow representers did not differ, t(35) = −1.19, p = .100, d = 0.40. Expressive language also differed by group at age 3½, F(2, 54) = 3.78, p = .029, η2 = .12. Poor learners had significantly lower expressive language than rich representers, t(42) = −2.64, p = .004, d = .79, but expressive language did not significantly differ between rich and shallow representers, t(33) = −1.33, p = .077, d = 0.48, or between shallow representers and poor learners, t(33) = −1.08, p = .185, d = 0.37.
Table 3.
Receptive and expressive language by fast mapping profile group
| Poor learners
|
Shallow representers
|
Rich representers
|
||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Age 3 ½ | ||||||
| PLS-AC Raw | 26.41a (7.97) | 17–45 | 37.21b (10.94) | 22–55 | 41.52b (10.57) | 22–61 |
| PLS-EC Raw | 34.64a (6.60) | 22–51 | 37.15a,b (6.82) | 25–48 | 41.18b (9.60) | 27–58 |
| Age 5 ½ | ||||||
| PLS-AC Raw | 46.28a (12.40) | 24–62 | 55.92b (8.53) | 32–62 | 57.29b (5.07) | 44–62 |
| PLS-EC Raw | 50.17a (11.66) | 36–67 | 55.77a,b (11.05) | 38–66 | 59.95b (7.73) | 45–67 |
PLS-AC and PLS-EC = Auditory Comprehension and Expressive Communication subscales from the Preschool Language Scale, 4th Edition; Raw = raw score. Means with differing superscripts significantly differ. Effect sizes are presented in the text
Age 3 ½ sample sizes: Poor learners, n = 22; Shallow representers, n = 14; Rich representers, n = 23. Age 5 ½ sample sizes: Poor learners, n = 19; Shallow representers, n = 13; Rich representers, n = 21
Fig. 1.
Receptive and expressive language at age 3½ and age 5½ by fast mapping profile group. PLS-4 = Preschool Language Scale, 4th Edition. Error bars represent one standard error. Top panel At age 3½, poor learners had significantly lower receptive language skills than the other two groups; shallow and rich representers did not significantly differ. For expressive language, only the poor learners and rich representers significantly differed. Bottom panel At age 5½, poor learners had significantly lower receptive language skills than the other two groups; shallow and rich representers did not significantly differ. For expressive language, only the poor learners and rich representers significantly differed
At age 5½, receptive language also differed significantly by profile group, F(2, 49) = 8.02, p = .001, η2 = .25. Poor learners had lower receptive language than shallow representers, t(29) = −2.42, p = .003, d = 0.91, and rich representers, t(37) = −3.73, p < .001, d = 1.16, but rich and shallow representers did not differ, t(32) = −0.59, p = .336, d = 0.20. Expressive language also significantly differed by group at age 5½, F(2, 49) = 4.58, p = .015, η2 = .16 (see the bottom panel of Fig. 1). Poor learners had lower expressive language scores than rich representers, t(37) = −3.13, p = .002, d = 0.99, but not shallow representers, t(29) = −1.35, p = .067, d = 0.49. Shallow representers and rich representers also did not differ, t(32) = −1.30, p = .123, d = 0.44.
Given that nonverbal cognition may contribute to variability in language skills, analyses were repeated statistically controlling for nonverbal cognition at age 3½ using ANCOVA. There were no significant group differences in expressive language at age 3½, F(2, 53) = 1.59, p = .214, , or age 5½, F(2, 48) = 1.79, p = .178, . However, group differences in receptive language were significant after controlling for nonverbal cognition at age 3½, F(2, 55) = 7.70, p = .001, , and age 5½, F(2, 48) = 3.42, p = .041, . At age 3½, rich representers had significantly higher receptive language than poor learners, t(43) = −4.05, p <.001, d = 1.21, and shallow representers, t(35) = −1.89, p = .029, d = 0.64; the difference between poor learners and shallow representers was marginal, t(34) = −1.69, p = .052, d = 0.58. At age 5½, poor learners had significantly lower receptive language than rich representers, t(37) = −2.64, p = .007, d = 0.85, and shallow representers, t(29) = −1.83, p = .039, d = 0.67; shallow and rich representers did not differ, t(32) = −0.53, p = .298, d = 0.19.
Discussion
This study assessed the relationship between fast mapping at age 3½, and concurrent and later language abilities in children with ASD. Based on a proposal by Norbury et al. (2010), we predicted that children who produced new words (i.e., rich representers) would have better language skills than children who only comprehended the words (i.e., shallow representers), who in turn would have better language skills than children who showed no evidence of fast mapping (i.e., poor learners). These predictions were only partially supported. As predicted, language abilities differed by fast-mapping performance concurrently and 2 years later. Children who were poor learners at age 3½ had lower expressive language skills than rich representers and lower receptive language skills than rich and shallow representers at both time points. These findings could not be entirely explained by limited task compliance or by the inability to produce spoken words because all participants had completed the task and named a familiar object. The fact that early fast mapping related to later receptive and expressive language lends ecological validity to experimental fast-mapping tasks, suggesting that that the difficulties children with ASD experience during the initial stages of word learning are meaningful, in addition to their known difficulties with lexical integration (Henderson et al. 2014).
Contrary to our predictions, rich representers did not have better language skills than shallow representers at either time point, demonstrating no clear advantage for children who produced novel words over children who only comprehended them. Assuming that novel word production reflects the specificity of phonological representations, this finding is inconsistent with the hypothesis that phonological learning is a compensatory mechanism that supports language learning in young children with ASD. However, it is possible that this compensatory mechanism develops gradually and is thus more evident in older children with ASD—or in the subgroup of children with age-appropriate language skills (Norbury et al. 2010). Additionally, shallow representers may have failed to produce the novel words not because they had weaker phonological representations, but because they were affected by factors such as motor limitations (Leonard et al. 2015) or the social demands of the task (McDuffie et al. 2012). Given the complicated nature of spoken language in this population, it would be beneficial to develop alternative measures of phonological representations in young children with ASD (e.g., eye-gaze processing measures; Venker and Kover 2015).
Interestingly, group differences in expressive language were no longer significant when nonverbal cognition was statistically controlled, demonstrating that cognitive ability may have accounted, at least in part, for expressive language differences in the poor learners (also see McDuffie et al. 2012). In contrast, group differences in receptive language remained significant even after accounting for nonverbal cognition, highlighting the relationship between fast mapping and receptive language. Rich representers had significantly higher receptive language than shallow representers at age 3½ only after nonverbal cognition was controlled. That is, the concurrent receptive language abilities of the shallow representers were not as strong as would be expected taking into account their nonverbal cognitive abilities compared to the rich representers. This finding aligns with the direction of our hypothesis; however, there are limitations to using ANCOVA to account for nonverbal cognitive ability across groups, particularly in terms of interpretation (Dennis et al. 2009; Miller and Chapman 2001). Overall, our findings point to complex relationships among fast mapping, nonverbal cognition, and language abilities in ASD.
The current study had several strengths—a fast mapping task that assessed comprehension and production, a relatively heterogeneous participant sample, and a longitudinal design—but it also had some limitations. The fast mapping task had limited sensitivity, which may have impacted our results. Future studies may measure fast mapping latency or number of trials correct (McDuffie et al. 2006). The current study did not address causality; future work is needed to determine whether better fast mapping leads to better language learning, vice versa, or both. The PLS-4 is an omnibus language measure, so we could not test whether fast mapping was more robustly associated with specific aspects of language (e.g., vocabulary; McDuffie et al. 2012). We used raw scores because we were interested in children’s absolute abilities, but these scores present psychometric challenges because they are not measured on equal interval scales.
The findings of this study highlight the importance of investigating processes of language learning in children with ASD. Studying these processes will help us understand why some children learn language more easily than others and will enable the development of maximally effective strategies for supporting word learning. Another important issue for future studies to investigate is how nonverbal cognitive abilities influence fast mapping and language acquisition.
Acknowledgments
This work was supported by R01DC012513 (Ellis Weismer, PI), T32 DC005359 (Ellis Weismer, PI), and P30HD003352 (Mailick, PI). Thanks to Amy Esler, Corey Ray-Subramanian, Nan Huai, Courtney Karasinski, and Heidi Sindberg for their clinical expertise. Sincere thanks to the children and families who took part in this study.
Footnotes
Author contributions SEW conceived of and designed the original task, secured grant funding for this work, and helped coordinate the study. CEV and STK conceived of the current study, performed the statistical analysis, and interpreted the findings. CEV drafted the manuscript. STK and SEW provided feedback on the manuscript. All authors read and approved the final manuscript.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Baron-Cohen S, Baldwin DA, Crowson M. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child Development. 1997;68(1):48–57. [PubMed] [Google Scholar]
- Carey S, Bartlett E. Acquiring a single new word. Proceedings of the Stanford Child Language Conference. 1978;15:17–29. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covatiate in cognitive studies of neurodevelopmental disorders. Journal of the International Neurological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481.Why. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis Weismer S, Venker CE, Evans JL, Moyle MJ. Fast mapping in late-talking toddlers. Applied Psycholinguistics. 2011 doi: 10.1017/S0142716411000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3.Standardizing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L, Powell A, Gareth Gaskell M, Norbury C. Learning and consolidation of new spoken words in autism spectrum disorder. Developmental Science. 2014 doi: 10.1111/desc.12169. [DOI] [PubMed] [Google Scholar]
- Leonard HC, Bedford R, Pickles A, Hill EL. Predicting the rate of language development from early motor skills in at-risk infants who develop autism spectrum disorder. Research in Autism Spectrum Disorders. 2015;13–14:15–24. doi: 10.1016/j.rasd.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule: ADOS. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The Autism Diagnostic Observation Schedule—Toddler Module: A new module of a standardized diagnostic measure for autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(9):1305–1320. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster R, Lord C. Word learning in children with autism spectrum disorders. Developmental Psychology. 2009;45(6):1774–1786. doi: 10.1037/a0016223.Word. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Kover ST, Hagerman R, Abbeduto L. Investigating word learning in fragile X syndrome: A fast-mapping study. Journal of Autism and Developmental Disorders. 2012;43(7):1676–1691. doi: 10.1007/s10803-012-1717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Yoder P, Stone W. Fast-mapping in young children with autism spectrum disorders. First Language. 2006;26(4):421–438. doi: 10.1177/0142723706067438. [DOI] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning manual. Minneapolis, MN: AGS edition ed; 1995. [Google Scholar]
- Norbury CF, Griffiths H, Nation K. Sound before meaning: word learning in autistic disorders. Neuropsychologia. 2010;48(14):4012–4019. doi: 10.1016/j.neuropsychologia.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Preissler MA, Carey S. The role of inferences about referential intent in word learning: Evidence from autism. Cognition. 2005;97(1):B13–B23. doi: 10.1016/j.cognition.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism diagnostic interview-revised. Los Angeles: Western Psychological Service; 2003. [Google Scholar]
- Venker CE, Kover ST. An open conversation on using eye-gaze methods in studies of neurodevelopmental disorders. Journal of Speech, Language, and Hearing Research. 2015 doi: 10.1044/2015_JSLHR-L-14-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman I, Steiner V, Pond R. Preschool language scale. 4. San Antonio, TX: The Psychological Corporation; 2002. [Google Scholar]