To the Editor
Heterozygous germline GATA2 mutations leading to haploinsufficiency of the GATA2 transcription factor underlie a primary immunodeficiency characterized by progressive monocytopenia, dendritic cell cytopenia, and B and natural killer (NK) lymphopenia; severe viral, mycobacterial, and fungal infections; high prevalence of myelodysplastic syndrome/acute myeloid leukemia; pulmonary alveolar proteinosis; and lymphedema.1 We describe an 18-year-old female with lymphedema who developed severe blastomycosis and fatal hemophagocytic lymphohistiocytosis (HLH) associated with disseminated herpes simplex virus-1 (HSV-1) infection. Genetic testing performed posthumously revealed a novel frameshift mutation in GATA2 predicted to result in a null allele. This is the first report of severe blastomycosis and the second report of HLH in a patient with GATA2 deficiency. GATA2 deficiency is the first reported genetic disorder associated with severe blastomycosis and adds to the growing list of primary immunodeficiencies underlying HLH.
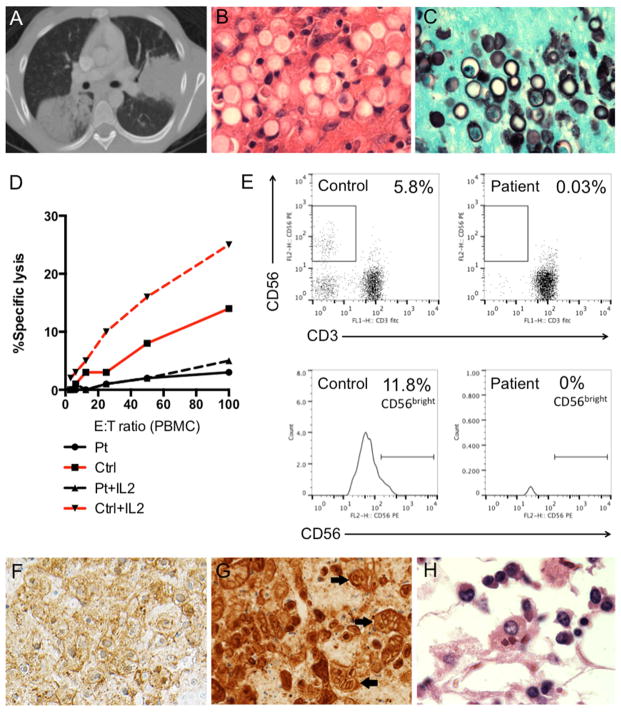
An African American woman had severe bilateral lower extremity lymphedema beginning at age 9. She was initially hospitalized at age 12 for necrotizing pneumonia with pulmonary abscess (Figure 1A). Video-assisted thoracoscopic surgery with lung biopsy identified thick-walled budding yeasts identified as Blastomyces dermatitidis on culture (Figure 1B–C). She improved on liposomal amphotericin B and was transitioned to maintenance itraconazole. During that hospitalization, she was also noted to have mild neutropenia (absolute neutrophil count: 1,400 cells/μL), lymphopenia (absolute lymphocyte count: 1,570 cells/μL), and significant monocytopenia (absolute monocyte count: 20 cells/μL). Human immunodeficiency virus testing was negative. Lymphocyte phenotyping revealed marked B lymphopenia (absolute CD19+: 23 cells/μL) and NK lymphopenia (absolute CD56+CD16+: <12 cells/μL) with relative sparing of the T-cell compartment (absolute CD3+: 1113 cells/μL, absolute CD4+: 574 cells/μL, absolute CD8+: 482 cells/μL). Serum immunoglobulin levels were normal. NK cell function was markedly reduced with minimal response to exogenous interleukin-2, and the CD56bright subset was nearly absent (Figure 1D–E). An underlying NK cell deficiency was suspected. Genetic testing for mutations in PRF1, UNC13D, and STX11 was negative.
Figure 1.
(A) Computed tomography scan of the chest demonstrating dense bilateral consolidations. (B) Lung biopsy, H&E stain (100x) and (C) Gomori methenamine silver stain (100x) showing thick-walled budding yeasts identified as Blastomyces dermatitidis. (D) NK cell functional activity measured against susceptible K562 target cells in the presence of IL-2 where indicated (dashed line) using PBMCs isolated from whole blood of the patient (black) compared to control (red). (E) Flow plots for CD56+CD3− cells showing markedly reduced NK cells and near absence of the CD56bright subset compared to control. (F) Liver (40x), hepatocytes demonstrating positivity (brown) for HSV-1 immunohistochemical stain and viral nuclear inclusions within affected cells. (G) Vagina (40x), squamous epithelial cells demonstrating strong positivity for HSV-1 (brown) and classic changes of multinucleation, nuclear molding, and margination of chromatin (arrows). (H) Bone marrow, H&E stain (100x) demonstrating hemophagocytic histiocytes.
At age 18, she presented with fevers and headache. Preliminary labs showed only her baseline leukopenia. She was started on empiric vancomycin and cefepime but remained febrile. Over the following days, she developed rapidly progressive pancytopenia (WBC: 700 cells/μL, hemoglobin: 7.0 g/dL, platelets: 14,000 cells/μL), transaminitis (AST 6821 units/L, ALT 3081 units/L), hyperferritinemia (49,900 ng/mL), and hypofibrinogenemia (<60 mg/dL). She developed worsening hypotension requiring vasopressors, and antimicrobial therapy was broadened to vancomycin, meropenem, levofloxacin, acyclovir, and liposomal amphotericin B. Dexamethasone was added for treatment of suspected HLH. HSV-1 DNA was subsequently detected in the blood. Despite appropriate antiviral therapy, she developed worsening hypotension and acidemia and progressed to cardiac arrest and death.
At autopsy, she had disseminated HSV-1 infection involving the lungs, liver, and vagina (Figure 1F–G). The bone marrow was hypocellular with decreased trilineage hematopoiesis and abundant hemophagocytosis (Figure 1H). The cause of death was HLH in the setting of her previously recognized NK cell deficiency and widespread HSV-1 infection. GATA2 deficiency was suspected given her history of lymphedema, characteristic cytopenias, NK cell dysfunction, and severe fungal and herpesvirus infections. Genetic testing performed posthumously confirmed a frameshift mutation in intron 4 of GATA2 (c.871+2_3insT), which is predicted to result in nonsense-mediated decay leading to a null allele.
GATA2 mutations underlie at least five previously described disorders: monocytopenia and mycobacterial infections (MonoMAC) syndrome; dendritic cell, monocyte, B and NK lymphoid (DCML) deficiency; familial myelodysplastic syndrome/acute myeloid leukemia; Emberger syndrome; and classical NK cell deficiency.1 These syndromes are now recognized as overlapping phenotypes of the same genetic disorder.1 Our patient had multiple clinical features strongly suggestive of GATA2 deficiency, including lymphedema, monocytopenia and B and NK lymphopenia, NK cell dysfunction with near absence of the CD56bright subset, and severe fungal and herpesvirus infections.2 Blastomycosis and aggressive HLH further expand the spectrum of infectious and inflammatory complications of GATA2 deficiency.
Blastomyces dermatitidis is a thermally dimorphic fungus endemic to the Mississippi, Ohio, and Missouri river valleys that typically causes a mild respiratory infection in immunocompetent hosts. In contrast, immunocompromised hosts may develop necrotizing pneumonia or disseminated infection with spread to the skin, bones, or genitourinary tract. Disseminated blastomycosis has been reported in patients with idiopathic CD4 lymphocytopenia, but has not been previously described in association with a recognized genetic defect.3 GATA2 deficiency is thus the first reported genetic disorder associated with severe blastomycosis. This association complements prior reports of disseminated histoplasmosis and coccidioidomycosis in patients with GATA2 deficiency, and suggests a significant defect in host defense against dimorphic fungi.1,4 The molecular mechanisms accounting for this fungal susceptibility are unclear, but likely involve dysfunction of the interleukin-12/interferon-gamma axis, which is the lesioned pathway in the majority of cases of primary immunodeficiency predisposing to dimorphic fungal infection.5,6
HLH is a life-threatening syndrome of hyperinflammation caused by genetic mutations affecting the cytolytic function of T and NK cells (primary HLH) or in response to various infections, rheumatologic disorders, or malignancy (secondary HLH). Infection with Epstein-Barr virus (EBV) and other herpesviruses is a common trigger of HLH and in some cases may indicate an underlying primary immunodeficiency.7 This is exemplified by X-linked lymphoproliferative disease (XLP) and the growing number of primary immunodeficiencies characterized by impaired control of EBV infection.8 Similar to XLP, GATA2 deficient patients have marked NK cell dysfunction and susceptibility to herpesvirus infection, which may lead to aggressive HLH in some cases.1,2 A previously reported patient with GATA2 deficiency developed HLH in the setting of marked EBV viremia and an EBV-driven T-cell lymphoproliferative disorder.9 In contrast to our patient’s fatal course, that patient was successfully treated with etoposide and dexamethasone followed by hematopoietic stem cell transplantation (HSCT).9 These reports suggest that GATA2 deficiency should be considered in the differential diagnosis for herpesvirus-associated HLH and that early HSCT is critical.
This case further expands the spectrum of infectious and inflammatory complications of GATA2 deficiency. In particular, this case further confirms the importance of GATA2 in host defense against dimorphic fungi and identifies blastomycosis as an infection that can signify primary immunodeficiency. Additionally, this case further supports the critical role of NK cells in the immune response to herpesvirus infections, dysfunction of which may lead to HLH.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- EBV
Epstein-Barr virus
- HLH
Hemophagocytic lymphohistiocytosis
- HSCT
Hematopoietic stem cell transplantation
- HSV-1
Herpes simplex virus-1
- NK
Natural killer
- XLP
X-linked lymphoproliferative disease
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–21. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56bright subset. Blood. 2013;121:2669–77. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siderits RH, Ouattara O, Marcus A, Gao HG, Deng HB, Godyn J. Case study documenting the diagnosis of idiopathic CD4+ lymphocytopenia in a patient with atypical fungal infection (disseminated blastomycosis) by FNA of adrenal mass. Cytojournal. 2010;5:7–13. doi: 10.4103/1742-6413.67106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imran T, Cui C. GATA2 transcription factor deficiency predisposing to severe disseminated coccidioidomycosis. Frontiers in Immunology Conference Abstract: 15th International Congress of Immunology; Milan, Italy. 2013. [Google Scholar]
- 5.Zerbe CS, Holland SM. Disseminated histoplasmosis in persons with interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2005;141:e38–41. doi: 10.1086/432120. [DOI] [PubMed] [Google Scholar]
- 6.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol. 2013;131:1624–34. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faitelson Y, Grunebaum E. Hemophagocytic lymphohistiocytosis and primary immune deficiency disorders. Clin Immunol. 2014;155:118–25. doi: 10.1016/j.clim.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Parvaneh N, Filipovich AH, Borkhardt A. Primary immunodeficiencies predisposed to Epstein-Barr virus-driven haematological diseases. Br J Haematol. 2013;162:573–86. doi: 10.1111/bjh.12422. [DOI] [PubMed] [Google Scholar]
- 9.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20:1940–8. doi: 10.1016/j.bbmt.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]