Summary
Sleep is traditionally constituted of two global behavioral states, NREM and REM, characterized by quiescence and reduced responsiveness to sensory stimuli [1]. NREM sleep is distinguished by slow waves and spindles throughout the cerebral cortex, REM sleep by an ‘activated’, low-voltage fast EEG paradoxically similar to that of wake, accompanied by rapid eye movements and muscle atonia. However, recent evidence has shown that cortical activity patterns during wake and NREM sleep are not as global as previously thought. Local slow waves can appear in various cortical regions in both awake humans [2] and rodents [3-5]. Intracranial recordings in humans [6] and rodents [4, 7] have shown that NREM sleep slow waves most often involve only a subset of brain regions that varies from wave to wave rather than occurring near-synchronously across all cortical areas. Moreover, some cortical areas can transiently ‘wake up’ [8] in an otherwise sleeping brain. Yet, until now, cortical activity during REM sleep was thought to be homogenously wake-like. We show here, using local laminar recordings in freely moving mice, that slow waves occur regularly during REM sleep, but only in primary sensory and motor areas, and mostly in layer 4, the main target of relay thalamic inputs, and in layer 3. This finding may help explain why during REM sleep we remain disconnected from the environment even though the bulk of the cortex shows wakelike, paradoxical activation.
Keywords: REM sleep, local sleep, cortical layers, laminar recordings
Graphical abstract
Results & Discussion
REM sleep, so called because of the occurrence of rapid eye movements [9], is also known as “paradoxical sleep” because the activated, low-voltage fast EEG is similar to that of wake [10]. During both NREM and REM sleep subjects are disconnected from the environment and do not respond to mild stimuli. In NREM sleep the EEG shows the regular occurrence of sleep spindles and slow waves, reflecting the bistability of cortical membrane potentials [11], which can lead to cortico-cortical disconnection and fading of consciousness [12]. During REM sleep, however, cortical activity is wake-like, cortico-cortical connectivity is preserved [12], and subjects regularly dream [13]. So far, the general assumption has been that during REM sleep EEG low voltage fast activity occurs in all cortical areas and across all layers. However, this assumption has not yet been tested thoroughly. We recorded from freely moving mice implanted with electroencephalogram (EEG) electrodes in frontal and parietal cortex, electromyogram (EMG) electrodes in neck and vibrissal muscles, as well as with laminar probes in several primary and secondary/association cortical areas, to assess both global and local neuronal activity patterns during the sleep/wake cycle and after sleep deprivation. Sleep was scored in 4-sec epochs according to established criteria, and we considered all consolidated sleep episodes throughout the entire light phase, when mice sleep most of the time (Figure 1A). Global activity patterns, reflected by the EEG and EMG, were as expected (Figure 1B-C). Wake was characterized by low voltage fast activity and high muscle tone in both neck and vibrissal muscles (Figure 1B, top), and NREM was clearly dominated by slow waves (0.5- 4 Hz) and reduced muscle tone (Figure 1B, middle). By contrast, the EEG of REM sleep resembled that of wake and there was phasic vibrissal activity (Figure 1B, bottom). Accurate state detection was confirmed by EEG spectral analysis and quantification of EMG content (Figure 1C).
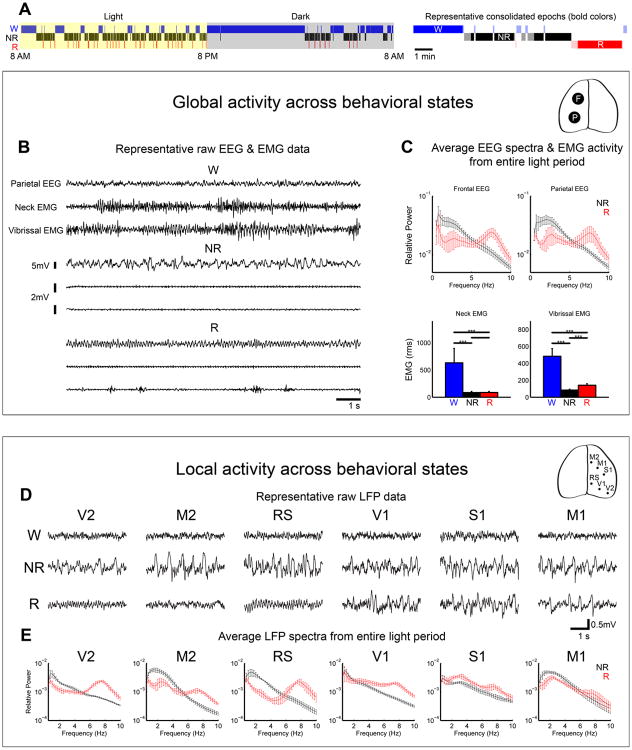
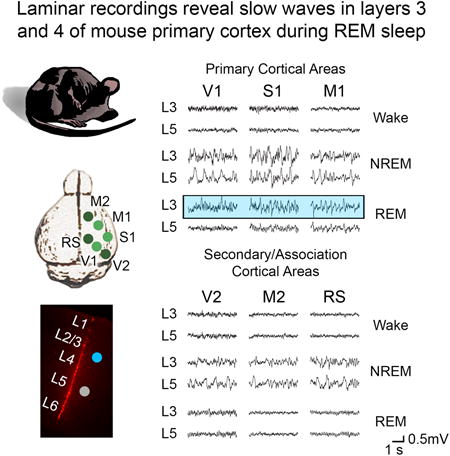
Figure 1. Occurrence of REM slow waves in primary sensory and motor cortex.
A, left, 24-h hypnogram showing the distribution of consolidated sleep and wake episodes in one representative animal. All consolidated NREM and REM episodes during the light cycle were used for analysis (see Supplemental Experimental Procedures for details). Right, example of consolidated episodes of sleep and wake (bold colors) and the non-consolidated and transitional epochs that were excluded from the analysis (light colors). B, Representative 12-sec traces showing global activity patterns in Wake (W), NREM sleep (NR), and REM sleep (R). Parietal EEG, electroencephalogram from parietal cortex; neck and vibrissal EMG, electromyogram from neck and vibrissal musculature. C, top, spectral analysis (entire 12-h light period) of frontal and parietal EEG, showing a prominent peak in the low frequencies in NREM sleep and in the theta range (6-9 Hz) in REM sleep. EEG power was normalized by dividing the power in each frequency bin by the total power across all frequencies. Bottom, neck and vibrissal EMG content across states, showing reduced muscle tone during sleep and an increase in vibrissal EMG in REM sleep relative to NREM sleep, due to phasic muscle activity. D, Representative 4-sec local field potential (LFP) recordings from 6 cortical areas across behavioral states. Sample data for each area are from different animals. E, Spectral analysis of LFPs from superficial layers of 6 cortical areas, averaged across all consolidated sleep epochs of the 12-h light period. Abbreviations (used throughout figure legends) and number of animals for each region: V2: secondary visual cortex (n=4 mice), RS: retrosplenial cortex (n=4), M2: secondary motor cortex (n=4), V1: primary visual cortex (n=11), S1: primary somatosensory cortex (n=3), M1: primary motor cortex (n=6). Error bars are standard deviation. ***=p<0.0005.
To survey regional cortical activity across behavioral states, we examined local field potentials (LFPs) recorded from layer 3 of 6 cortical regions including primary visual, sensory, and motor areas (V1, S1, M1), secondary visual and motor areas (V2, M2) and retrosplenial cortex (RS), an association area involved in memory and spatial navigation [14] (Figure 1D-E). In both active wake (with EMG content in the top 33% of the 24-h wake EMG activity) and NREM sleep, local activity across all areas exhibited the pattern expected based on the EEG, with wake dominated by low voltage high frequency activity and NREM sleep characterized by slow waves. However, in REM sleep neural activity in some areas deviated from the expected global activity mode. Specifically, while local activity in V2, M2, and RS was wake-like, slow waves were unexpectedly present in V1, S1, and M1 (Figure 1D). Spectral analysis across all REM episodes of the light period revealed prominent SWA in these areas but not in secondary/association regions, indicating that the occurrence of slow waves is a robust phenomenon in primary cortical regions throughout REM sleep (Figure 1E). Thus sleep slow waves, the hallmark of NREM sleep, occur regularly also during REM sleep, but only in primary cortical areas. By contrast, sleep spindles, the other EEG feature of NREM sleep, were present in NREM LFP recordings of all 3 primary areas but were not detected during REM sleep (data not shown).
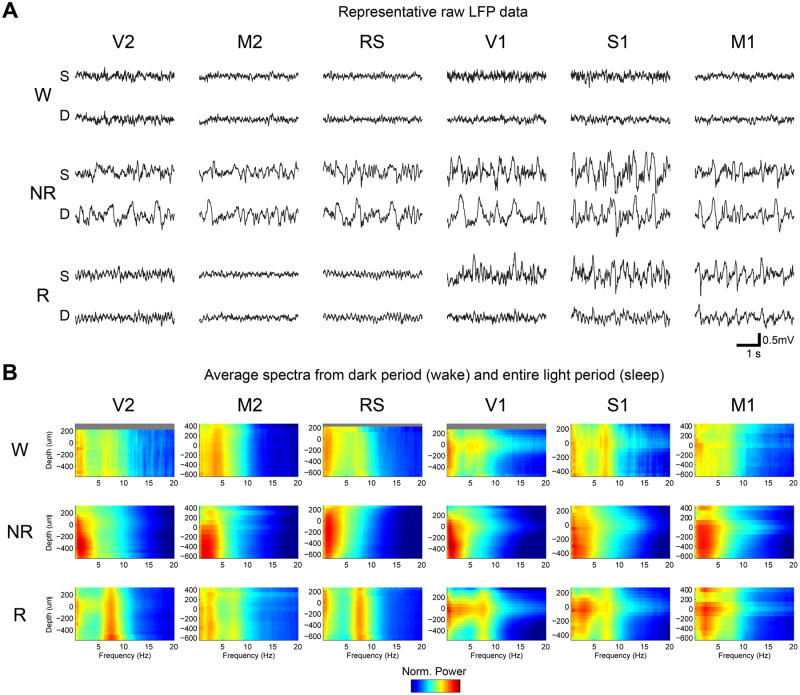
Previous studies of local intracortical activity patterns during REM sleep reported wake-like activated patterns but not sleep slow waves [11, 15], perhaps because they often probed association cortex [11] and/or because unit intracortical recordings are usually biased toward deep layers [15]. Taking advantage of laminar probes, we examined activity not only across multiple cortical areas, but also across all cortical layers (Figure S1). Examples of simultaneously recorded LFPs from superficial (layer 3) and deep (layer 5) layers are shown in Figure 2A. During active wake, the characteristic activated pattern was found in both superficial and deep layers of all recorded areas (Figure 2A, top row). Similarly during NREM sleep slow waves were present across all areas and layers (Figure 2A, middle row). However, in REM sleep the wake-like, activated pattern was observed in both superficial and deep layers in secondary/association areas, whereas in primary sensory cortical areas slow waves were recorded in superficial layers, but not in deep layers, which instead exhibited an activated pattern (Figure 2A, bottom row). In M1, slow waves were also present in deep layers but were of smaller amplitude than in superficial layers. Spectral analysis across all layers, averaged across animals, confirmed these findings: SWA was low in wake and high across all layers during NREM sleep, with peak power in the deep layers (Figure 2B, top and middle rows). During REM sleep, SWA was low in secondary/association areas across layers. In primary sensory areas (S1, V1), SWA peaked in layer 4 and decreased in power with distance from layer 4 (Figure 2B, bottom row), and in M1, SWA peaked near the bottom of layer 3 and extended into deep layers. This analysis confirmed that REM SWA was specific to these regions and further suggested that it was generated by a mechanism in the middle (layer 4) and superficial (mainly layer 3) layers. Similar results were found by analyzing sleep in the dark period (Figure S2), as well as using a different normalization method (Figure S3). Thus, we found two dissociations in the pattern of cortical activity during REM sleep, between primary and secondary/association areas, and within primary sensory areas, between deep and middle/superficial layers. In sum, slow waves do occur during REM sleep, but only in primary areas, and only or mainly in middle/superficial layers, especially layer 4.
Figure 2. REM slow waves are present in superficial but not deep layers of primary cortical areas.
A, Representative 4-sec LFP recordings from superficial (S, layers 1-4) and deep (D, layers 5-6) layers during sleep and wake across cortical areas. Sample data for each area are from different animals. B, Group spectrograms in V2 (W=2 mice, sleep=4 mice), M2 (W=3, sleep=4), RS (W=3, sleep=4), V1 (W=5, sleep=11), S1 (W=1, sleep=3) and M1 (W=2, sleep=6). Only mice with a sufficient quantity of wake data without movement artifacts were included, and in all cases mice used for sleep analysis contributed both artifact-free NREM and REM sleep data. Wake spectral data are averaged across consolidated wake epochs in the dark cycle, when mice are mostly awake, and sleep spectral data are averaged across all consolidated sleep epochs in the light cycle, when mice mostly sleep (similar results were obtained when analyzing sleep epochs in the dark cycle, Figure S2). LFP power was normalized by dividing the power in each frequency bin on each channel by the total power across all frequencies and channels on the 16-channel probe. Results did not change when spectral data were normalized to the mean total power across all probes (Figure S3). Zero indicates the depth at which the gamma peak occurs, and superficial and deep layers correspond to depths ≥ 100 um and ≤ -100 um, respectively (see Supplemental Experimental Procedures and Figure S1 for details). Grey bars indicate layers/depths from which recordings were not available (usually layer 1).
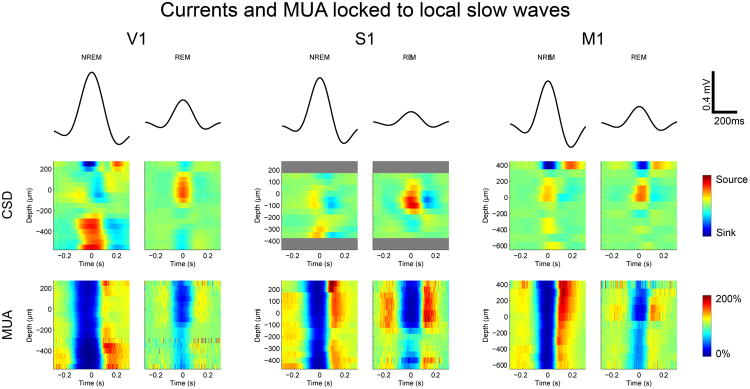
NREM sleep slow waves are due to the alternation, every second or so, of neuronal ON periods, characterized by a depolarized membrane potential (UP state) and neuronal firing, and OFF periods, during which the membrane potential hyperpolarizes (DOWN state) and neurons cease to fire [11, 16]. To clarify the neuronal substrates underlying REM sleep SWA, we analyzed current source density (CSD) and multiunit activity (MUA) locked to individual slow waves. In V1, NREM sleep slow waves were associated with sources (positive current leaving neurons) in the deep layers and OFF periods across all layers (Figure 3), consistent with previous findings in cat frontal, parietal and occipital cortex [17]. By contrast, during REM sleep slow waves were associated with sources in the middle/superficial layers, which also showed the strongest decrease in MUA, while firing in deep layers was slightly reduced but remained tonic (Figure 3). Similar results were obtained for NREM and REM sleep slow waves in S1 and M1 (Figure 3). In all three primary areas, REM slow waves were smaller than those in NREM sleep, likely reflecting the fact that only a spatially-restricted subset of neurons in the cortical column participated in the underlying OFF period. Thus, in primary areas, REM sleep slow waves are associated with current sources and neuronal OFF periods in middle/superficial layers but not, or much less so, in deep layers.
Figure 3. Neuronal dynamics underlying NREM and REM slow waves.
Top, mean LFP from all slow waves (SWs) used for analysis from NREM and REM sleep (entire 12-h light period) in V1 (n=9 mice), S1 (n=3), and M1 (n=6). Middle, bottom, CSD and MUA locked to the peak of individual slow waves and averaged across slow waves. For MUA, firing rate was normalized by the baseline firing rate measured between -1 and -0.5 sec before the slow wave peak. Sinks and sources represent positive current entering and leaving cells, respectively. Grey bars indicate layers/depths for which CSD/MUA data were not available.
We also recorded from pairs of regions (V1/M1, S1/M1) in the same animal, to test whether REM sleep slow waves were synchronized across the 3 primary areas or remained local. The latter was true in all cases, that is, we found that individual REM slow waves in one area were not associated with decreased MUA in the other area (data not shown). Moreover, REM sleep slow waves were not associated with decreased MUA in secondary/association areas (data not shown). Altogether, these findings reveal a novel type of local slow wave that occurs in primary cortical regions and is characterized by a decoupling of activity patterns in the middle/superficial and deep compartments. Previous research in slices [18] and in vivo [19] had shown that superficial layers do not always participate in UP states; the present results extend these findings by revealing that middle/superficial layers can have OFF periods even in states when deep layers fire tonically.
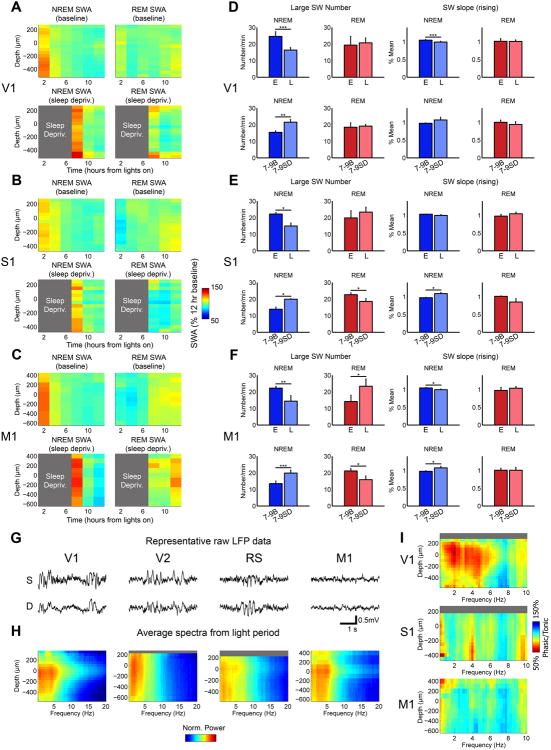
Finally, we sought to characterize other similarities and differences between REM sleep SWA and SWA occurring in other behavioral states. NREM sleep SWA is well-known to increase with prior wake duration [20], a result confirmed in our data: NREM sleep SWA was highest at sleep onset and after sleep deprivation, and declined with sleep across the light cycle (Figure 4A-C). However, REM sleep SWA did not increase with sleep pressure and remained stable across the light cycle in V1, while in S1 and M1 it actually increased toward the end of the light cycle, when sleep pressure is lowest, although the trend was not statistically significant (Figure 4A-C). Similarly, other slow wave parameters such as number and slope, which also reflect sleep pressure in NREM sleep [21], were not regulated homeostatically in REM sleep (Figure 4D-F). Increasing evidence suggests that the homeostatic regulation of NREM SWA may reflect the build-up of stronger synaptic connections among cortical neurons during extended wake, leading to higher synchrony in the occurrence of ON and OFF periods during subsequent sleep [22]. Other evidence shows that while most intracortical synapses across all layers remain plastic in the adult brain, this may not be the case for the thalamocortical synapses of layer 4 [23]. Thus, we speculate that REM SWA does not appear to reflect sleep/wake history because it peaks in layer 4, where at least some synapses may be less responsive to plastic changes during wake.
Figure 4. Additional differences between REM SWA and SWA in other behavioral states.
A-F. NREM and REM slow waves are regulated differently: during NREM sleep SWA and number and slope of slow waves peak in early baseline sleep, increase with sleep deprivation, and decline in the course of sleep, but none of these changes occur in REM sleep. Relative SWA (2-hr bins, expressed as % of 12-hr baseline light period for each state) in NREM and REM sleep during the baseline light period and after sleep deprivation (indicated by grey areas) in V1 (A, n=9 baseline, n=4 sleep deprivation), S1 (B, n=3 baseline, n=3 sleep deprivation) and M1 (C, n=6 baseline, n=4 sleep deprivation). D. Number of large amplitude slow waves (top 20th percentile) in V1 during early and late sleep in baseline (E vs. L, n=11) and during the first three hours of sleep following sleep deprivation (hours 7-9 after lights on), which were compared with the same circadian time in baseline (7-9B vs. 7-9SD, n=5). Early and late sleep are defined as the first three and last three hours of the baseline 12-hour light period, respectively. Rising slope of large amplitude slow waves in early and late sleep and during the first three hours of sleep following SD in V1. E,F. Same as in D, from S1 (E, baseline, n=3; sleep deprivation, n=3) and M1 (F, baseline, n=6; sleep deprivation, n=4). G,H. SWA in quiet wake involves deep layers and occurs in V2 and RS. Representative 4-sec LFP recordings from superficial (S) and deep (D) layers of V1, V2, RS, and M1 (G) and power spectra (H) during quiet wake from V1 (n=10), V2 (n=3), RS (n=3), and M1 (n=4). Quiet wake was defined as wake epochs with neck EMG content in the bottom 33 percentile of all wake across 24 hrs, and the epochs analyzed in H were specifically from bouts of consolidated wake in the light period. Grey bars indicate layers/depths for which spectral data were not available. I. Phasic motor activity in REM sleep does not abolish REM SWA in V1 and S1. Ratio of spectral activity in phasic REM sleep epochs (high vibrissal EMG) and tonic REM sleep epochs (low vibrissal EMG) in V1 (n=9), S1 (n=3), and M1 (n=4). Grey bars indicate layers/depths for which spectral data were not available. * p<0.05, ** p<0.005, ***p<0.0005.
Slow waves have also been described in rodents during quiet wake [3-5], as well as during the performance of a reaching task [4], though little is known about their laminar dynamics. We found that in freely moving mice SWA also occurred in quiet wake (with EMG content in the bottom 33% of the 24-h wake EMG activity), but unlike during REM sleep, slow waves during quiet wake usually spanned all layers and were present in secondary areas (V2 and RS; Figure 4G,H). These results suggest that, from an electrophysiological perspective, slow waves in quiet wake more closely resemble those in NREM sleep than those in REM sleep. SWA in quiet wake and REM sleep also showed differential regulation by motor activity: wake SWA was reduced with increased motor activity (quiet wake in Figure 4G; active wake in Figure 2B), while REM sleep SWA peaked during periods with high phasic vibrissal muscle activity (“phasic REM”) (Figure 4I), at least in V1.
We report here that cortical neuronal activity during REM sleep is not homogenously wakelike, as was previously thought, but is characterized by the occurrence of local slow waves and associated neuronal OFF periods in the middle/superficial layers of primary cortical areas. This finding demonstrates that local activity patterns in the cerebral cortex can deviate from the global activity mode in all major behavioral states, as was recently shown for wake [4], NREM sleep [8], and now for REM sleep. The spatially restricted nature of REM slow waves may explain why REM SWA has not previously been reported: LFPs recorded in either the deep or the most superficial layers (layers 1-2) of primary areas would likely miss slow waves originating in layer 4. Moreover, REM slow waves may go undetected at the scalp level since they are not present in layers 1-2 and since EEG captures activity of a broader neuronal network that may include nearby secondary/association areas.
The mechanisms responsible for the local occurrence of slow waves during REM sleep are currently unknown. However, it is known that REM sleep is characterized by a combination of high acetylcholine and low noradrenaline levels [24]. Cholinergic stimulation can directly hyperpolarize layer 4 spiny stellate cells in primary areas (A1, S1, V1) [25], decrease layer 4 firing [26], and reduce sensory evoked responses in this layer [27, 28], while noradrenaline, but not acetylcholine, is sufficient to activate layer 4 [29]. Thus, local slow waves in layer 4 of primary areas may be facilitated by the high ratio between acetylcholine and noradrenaline. The absence of other neuromodulators could also contribute, such as orexin or histamine [30]. However, orexinergic and histaminergic projections mainly target deep layers and at least for orexin, post-synaptic signaling seems to be confined to layer 6 [31, 32]. Thalamic mechanisms could also play a role, since relay thalamic nuclei provide the main input to layer 4 in primary areas. However, thalamic recordings in REM sleep show wake-like electrophysiological properties [33-35], and thalamic lesions do not disrupt wake activity patterns in layer 4 of awake rats [29]. Finally, since REM slow waves in M1 were more likely to spread to deep layers, it is possible that slow waves in this area are caused by an altogether different mechanism.
What is the possible functional role of local REM sleep slow waves in the middle and superficial layers of primary cortices? In other behavioral states, the occurrence of slow waves and the underlying OFF periods have behavioral consequences. In awake rats, for instance, OFF periods occurring during a reaching attempt interfere with task performance [4]. In NREM sleep, the bistable dynamics between ON and OFF periods that characterizes slow waves impairs cortical information transmission and cortico-cortical effective connectivity [12, 36]. NREM sleep slow waves may also be responsible for sensory disconnection, since higher SWA at the onset of NREM sleep is associated with longer OFF periods [15] and higher arousal thresholds [37, 38]. Until now, the profound sensory disconnection of REM sleep had remained a paradox [13], since all cortical neurons were thought to be tonically activated and cortico-cortical connectivity is preserved [39]. The present findings suggest two possible mechanisms. First, the peak occurrence of slow waves and associated neuronal OFF periods in layer 4 of primary cortical areas may gate stimulus-evoked signaling right upon entering cortex, since inputs from relay thalamic nuclei target primarily layer 4. Consistent with this possibility, hyperpolarization of layer 4 cells decreases angular tuning in mouse S1 [40]. Also consistent with this hypothesis, sensory-evoked activity decreases [41] and arousal thresholds increase [37] especially during phasic REM sleep, which is when we observed a surge in slow waves. Second, the OFF periods in the superficial layers of primary cortices, which originate the major feedforward output to the rest of the cortex, may gate cortico-cortical interactions between primary and secondary regions.
In sum, our results suggest that SWA in REM sleep may promote disconnection, as it does in NREM sleep. The higher levels of SWA in NREM sleep may result in greater disconnection in this phase of sleep, consistent with the observation that arousal thresholds in deep NREM sleep are higher than in REM sleep [38, 42], and may also disrupt conscious experience in this state. Instead, the focal occurrence of REM SWA specifically in the layers of primary sensory cortex involved in feedforward signaling, combined with activation in secondary/association areas, may bias the balance between bottom-up and top-down cortical signaling in favor of top-down, potentially accounting for both sensory disconnection and dreaming [13].
Experimental Procedures
All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and facilities were reviewed and approved by the IACUC of the University of Wisconsin-Madison, and were inspected and accredited by AAALAC. Mice were implanted with laminar probes (NeuroNeuxs, A1×16, 50 or 100 um site spacing) and custom-made EMG and EEG probes. Baseline and sleep deprivation recordings were performed in freely moving mice using a PZ amplifier and RZ2 system (Tucker-Davis Technologies). Sleep scoring was performed using SleepSign (Kissei Comtec). Data analyses were performed using custom Matlab (MathWorks), FieldTrip [43], and the CSDPlotter toolbox [44]. Detailed methods are provided in Supplemental Materials.
Supplementary Material
Acknowledgments
Funded by NIMH grant R01MH099231 to CC and GT, NINDS grant P01NS083514 to CC and GT, Wisconsin Distinguished Rath Graduate Fellowship to CMF, NIGMS T32 GM008962 to CMF, HFSP long-term fellowship LT000263/2012-L to SH, SciMed GRS Fellowship to AVR, and NRSA NIGMS T32 GM007507 to AVR. The authors thank Drs. Vladyslav Vyazovskiy and Yuval Nir for input on original data analysis.
G. Tononi is involved in a research study in humans supported by Philips Respironics. This study is not related to the work presented in the current manuscript.
Footnotes
Conflicts of interest: The other authors have indicated no financial conflicts of interest.
Supplemental Materials: Supplemental Materials include three figures and Supplemental Experimental Procedures.
Author Contributions. C.M.F., S.H. and A.V.R. conducted the experiments, C.M.F analyzed the data, C.M.F., C.C., and G.T. designed the experiments and wrote the paper.
Contributor Information
Chadd M. Funk, Email: cmfunk@wisc.edu.
Sakiko Honjoh, Email: sakiko.honjoh@wisc.edu.
Alexander V. Rodriguez, Email: avrodriguez@wisc.edu.
References
- 1.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardi G, Siclari F, Yu X, Zennig C, Bellesi M, Ricciardi E, Cirelli C, Ghilardi MF, Pietrini P, Tononi G. Neural and Behavioral Correlates of Extended Training during Sleep Deprivation in Humans: Evidence for Local, Task-Specific Effects. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:4487–4500. doi: 10.1523/JNEUROSCI.4567-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nature neuroscience. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 4.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett C, Arroyo S, Hestrin S. Subthreshold mechanisms underlying state-dependent modulation of visual responses. Neuron. 2013;80:350–357. doi: 10.1016/j.neuron.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain research. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Nobili L, Ferrara M, Moroni F, De Gennaro L, Russo GL, Campus C, Cardinale F, De Carli F. Dissociated wake-like and sleep-like electro-cortical activity during sleep. NeuroImage. 2011;58:612–619. doi: 10.1016/j.neuroimage.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Aserinsky E, Kleitman N. Regularly occuring periods of eye motility, and concominant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 10.Jouvet M. Paradoxical Sleep--a Study of Its Nature and Mechanisms. Progress in brain research. 1965;18:20–62. doi: 10.1016/s0079-6123(08)63582-7. [DOI] [PubMed] [Google Scholar]
- 11.Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 12.Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 13.Nir Y, Tononi G. Dreaming and the brain: from phenomenology to neurophysiology. Trends in cognitive sciences. 2010;14:88–100. doi: 10.1016/j.tics.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 15.Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. doi: 10.1016/s0306-4522(97)00319-9. [DOI] [PubMed] [Google Scholar]
- 18.Krause BM, Raz A, Uhlrich DJ, Smith PH, Banks MI. Spiking in auditory cortex following thalamic stimulation is dominated by cortical network activity. Front Syst Neurosci. 2014;8:170. doi: 10.3389/fnsys.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata S, Harris KD. Laminar structure of spontaneous and sensory-evoked population activity in auditory cortex. Neuron. 2009;64:404–418. doi: 10.1016/j.neuron.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deboer T. Behavioral and electrophysiological correlates of sleep and sleep homeostasis. Curr Top Behav Neurosci. 2015;25:1–24. doi: 10.1007/7854_2013_248. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy VV, Riedner BA, Cirelli C, Tononi G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep. 2007;30:1631–1642. doi: 10.1093/sleep/30.12.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 24.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 25.Eggermann E, Feldmeyer D. Cholinergic filtering in the recurrent excitatory microcircuit of cortical layer 4. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11753–11758. doi: 10.1073/pnas.0810062106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakata S, Harris KD. Laminar-dependent effects of cortical state on auditory cortical spontaneous activity. Front Neural Circuits. 2012;6:109. doi: 10.3389/fncir.2012.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donoghue JP, Carroll KL. Cholinergic modulation of sensory responses in rat primary somatic sensory cortex. Brain research. 1987;408:367–371. doi: 10.1016/0006-8993(87)90407-0. [DOI] [PubMed] [Google Scholar]
- 28.Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain research. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 29.Constantinople CM, Bruno RM. Effects and mechanisms of wakefulness on local cortical networks. Neuron. 2011;69:1061–1068. doi: 10.1016/j.neuron.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John J, Wu MF, Boehmer LN, Siegel JM. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayer L, Serafin M, Eggermann E, Saint-Mleux B, Machard D, Jones BE, Muhlethaler M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, Ye Z, Houston CM, Zecharia AY, Ma Y, Zhang Z, Uygun DS, Parker S, Vyssotski AL, Yustos R, et al. Wakefulness Is Governed by GABA and Histamine Cotransmission. Neuron. 2015;87:164–178. doi: 10.1016/j.neuron.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steriade M, Iosif G, Apostol V. Responsiveness of thalamic and cortical motor relays during arousal and various stages of sleep. Journal of neurophysiology. 1969;32:251–265. doi: 10.1152/jn.1969.32.2.251. [DOI] [PubMed] [Google Scholar]
- 34.Fourment A, Hirsch JC. Synaptic potentials in cat's lateral geniculate neurons during natural sleep with special reference to paradoxical sleep. Neuroscience letters. 1980;16:149–154. doi: 10.1016/0304-3940(80)90335-3. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch JC, Fourment A, Marc ME. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain research. 1983;259:308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- 36.Pigorini A, Sarasso S, Proserpio P, Szymanski C, Arnulfo G, Casarotto S, Fecchio M, Rosanova M, Mariotti M, Russo GL, et al. Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Ermis U, Krakow K, Voss U. Arousal thresholds during human tonic and phasic REM sleep. J Sleep Res. 2010;19:400–406. doi: 10.1111/j.1365-2869.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 38.Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16:467–477. [PubMed] [Google Scholar]
- 39.Massimini M, Ferrarelli F, Murphy M, Huber R, Riedner B, Casarotto S, Tononi G. Cortical reactivity and effective connectivity during REM sleep in humans. Cognitive neuroscience. 2010;1:176–183. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012;490:397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- 41.Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Auer DP, Pollmacher T, Czisch M. Functional microstates within human REM sleep: first evidence from fMRI of a thalamocortical network specific for phasic REM periods. The European journal of neuroscience. 2007;25:863–871. doi: 10.1111/j.1460-9568.2007.05314.x. [DOI] [PubMed] [Google Scholar]
- 42.Rechtschaffen A, Hauri P, Zeitlin M. Auditory awakening thresholds in REM and NREM sleep stages. Percept Mot Skills. 1966;22:927–942. doi: 10.2466/pms.1966.22.3.927. [DOI] [PubMed] [Google Scholar]
- 43.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettersen KH, Devor A, Ulbert I, Dale AM, Einevoll GT. Current-source density estimation based on inversion of electrostatic forward solution: effects of finite extent of neuronal activity and conductivity discontinuities. J Neurosci Methods. 2006;154:116–133. doi: 10.1016/j.jneumeth.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.