Abstract
Purpose
We examined the impact of positive vascular margins in patients with pT3 clear cell renal cell carcinoma.
Materials and Methods
After excluding patients with non-vascular positive margins, metastasis, lymph node involvement, neoadjuvant therapy, or non–clear cell histology, we identified 224 patients with venous tumor invasion through our institutional database from 1999-2013. Kaplan-Meier analysis and log rank tests were used to evaluate whether positive vascular margins were associated with progression-free survival or cancer-specific survival.
Results
Forty-one patients (18%) had a positive vascular margin. Margin status was directly related to the level of invasion (p <0.0001). Compared to the negative vascular margin group, the positive group had significantly worse progression-free survival (p=0.01), but not cancer-specific survival (p=0.3). Similarly, level of vascular thrombus invasion was significantly associated with worse progression-free survival (p=0.02), but not cancer-specific survival (p=0.4). Three-year progression-free survival was worst with inferior vena cava invasion and best with segmental/muscular venous branch invasion (54% [95% CI 34–70] vs. 76% [95% CI 64–85]). Among patients with only main renal vein thrombus, vascular margin status was not associated with progression-free survival (p=0.5) or cancer-specific survival (p=0.2).
Conclusions
In patients with pT3N0/XM0 clear cell renal cell carcinoma, positive vascular margins are associated with risk for disease progression. However, the risk of relapse associated with positive vascular margin is driven by extent of vascular thrombus invasion. These findings suggest that the clinical significance of vascular margin status as currently defined in pT3 clear cell renal cell carcinoma is minimal.
Keywords: kidney neoplasms, thrombosis, neoplasm staging, prognosis, carcinoma, renal cell
Introduction
Clear cell RCC constitutes the majority of all renal carcinomas and exhibits a propensity to progress locally through growth as a tumor thrombus into successively larger caliber vessels.1 Up to 10% of all patients diagnosed with clear cell RCC have a tumor thrombus, which may extend beyond the renal vein, into the IVC and right atrium. Such disease is classified as stage T3b or T3c, depending on the thrombus level.2 The presence of tumor thrombus is associated with less favorable cancer-related outcomes.3, 4 Vascular invasion is a growth pattern reflective of tumor biology and has been suggested to offer prognostic value.5-7 Various forms of vascular invasion may be difficult to appreciate intraoperatively and potentially contribute to a PVM. Reporting vascular margin status is standard, although the clinical significance remains unclear.
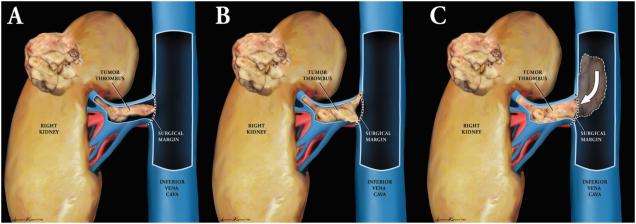
The 2012 International Society of Urologic Pathology Consensus defines a renal vein margin as positive only if there is adherent tumor at the actual margin.8 There are three scenarios in which a PVM may occur (fig. 1). First, a PVM occurs when inking the free-floating edge of an intact tumor thrombus that protrudes from the lumen of the renal vein after transection. This is the most common form of PVM and may be a result of either surgical technique or specimen processing, because the vessel wall has a tendency to retract beyond the bulging thrombus during fixation.8 Second, a PVM occurs when the tumor thrombus grows into and invades the renal vein or IVC wall at the surgical margin. Third, a PVM occurs when a tumor thrombus extends into the IVC and cannot be manipulated back toward the renal vein intraoperatively, and a cavotomy is performed to allow for the evacuation of the tumor thrombus. In this scenario, the tumor thrombus protrudes beyond the transected MRV and IVC margins.
Figure 1.
Three sources of positive vascular margins in T3 disease. (A) Free-floating thrombus protrudes from vein lumen following transection (B) Microscopic vascular wall invasion from tumor thrombus growing beyond the transection margin. (C) Free-floating thrombus extends into the IVC and cannot be manipulated back toward the MRV. A cavotomy is performed to allow for the evacuation of the tumor thrombus.
The purpose of our study was to determine the prognostic value of PVMs in regard to predicting oncologic outcomes in patients with pT3 clear cell RCC.
Materials and Methods
Following institutional review board approval, patients with pT3 renal tumors who underwent open or minimally invasive radical nephrectomy were retrospectively identified from January 1999 to June 2013. Prior to surgery, all patients underwent routine metastatic work-up, which included physical examination, cross-sectional radiographic imaging, and blood testing. On pathologic assessment, only patients with evidence of tumor thrombus invasion into the muscular/segmental venous branch, MRV, or IVC were included. Excluded from the study were those patients with metastatic disease at the time of diagnosis, positive surgical margins other than PVMs, node-positive disease, neoadjuvant therapy, and non-clear cell histology. In total, 224 patients were eligible for the final analysis.
For patients with IVC thrombus on perioperative imaging, all visible tumor thrombus was surgically excised, including resection of vena caval wall if wall invasion was suspected. We did not routinely perform intraoperative frozen sections to assess renal vein margin status. PVMs were identified from pathology reports using the standard pathology criteria that define a PVM by grossly visible tumor protruding beyond the margins of MRV or IVC wall transection.8
Routine follow-up included serial chest and abdominal imaging. Clinical trials in the adjuvant setting were offered to eligible patients with pT3 clear cell RCC at our institution. PFS was defined as time to first discovery of local recurrence or distant metastasis. Local recurrence was defined as recurrence of disease at the site of vascular excision, within or adjacent to the renal fossa. Distant metastasis was defined as recurrence of disease other than local recurrence. CSS was calculated as time from surgery to death due to disease or last-known follow-up, whichever occurred earlier.
Baseline categorical variables were compared between patient groups using Fisher’s exact test and continuous variables using rank sum tests. Kaplan-Meier analyses were used to estimate the three-year probability of PFS and CSS based on vascular margin status and extent of tumor thrombus involvement. Differences in PFS and CSS were determined through log-rank test. Pathologic stage was defined according to the 2010 American Joint Committee on Cancer classification.2 Statistical analyses were performed using Stata® version 13 (StataCorp, College Station, Texas) with p-values <0.05 considered statistically significant.
Results
Table 1 summarizes the patient characteristics. PVMs were present in 41 patients (18%) and were associated with male gender, right-sided tumor, larger tumor size, increased level of tumor thrombus invasion, advanced pathologic stage of disease, and ipsilateral adrenal resection. PVM and negative vascular margin patients did not differ significantly in the adjuvant therapy rate (p>0.9). The median follow-up for surviving patients was 3.2 years (IQR: 1.6 – 5.6).
Table 1.
Patient and disease characteristics, N=224
| Positive vascular margins (N=41) |
Negative vascular margins (N=183) |
p Value | |
|---|---|---|---|
| Age at surgery, years | 62 (56, 66) | 64 (58, 71) | 0.09 |
| Male | 36 (88) | 120 (66) | 0.005 |
| Right sided tumor | 34 (83) | 96 (52) | 0.0004 |
| ASA* Score | 0.5 | ||
| 1 | 0 (0) | 2 (1.1) | |
| 2 | 19 (46) | 65 (36) | |
| 3 | 21 (51) | 111 (61) | |
| 4 | 0 (0) | 5 (2.7) | |
| Unknown | 1 (2.4) | 0 (0) | |
| Procedures | 0.2 | ||
| Open radical nephrectomy | 37 (90) | 155 (85) | |
| Open nephroureterectomy | 1 (2.4) | 0 (0) | |
| Laparoscopic radical | 1 (2.4) | 14 (7.7) | |
| nephrectomy | |||
| Robotic radical nephrectomy | 2 (4.9) | 14 (7.7) | |
| Tumor size, cm | 9.1 (7.0, 12.2) | 7.5 (6.0, 9.5) | 0.003 |
| Level of tumor thrombus invasion |
<0.0001 | ||
| Segmental/muscular venous branch |
0 (0) | 109 (60) | |
| MRV | 12 (29) | 74 (40) | |
| IVC | 29 (71) | 0 (0) | |
| Pathologic stage | <0.0001 | ||
| T3a | 12 (29) | 183 (100) | |
| T3b | 26 (63) | 0 (0) | |
| T3c | 3 (8) | 0 (0) | |
| Regional Lymphadenectomy | 30 (73) | 135 (74) | >0.9 |
| Ipsilateral adrenal resection | 34 (83) | 115 (63) | 0.017 |
| Tumor Fuhrman grade | 0.29 | ||
| 2 | 7 (17) | 46 (25) | |
| 3 | 23 (56) | 105 (57) | |
| 4 | 11 (27) | 32 (17) | |
| Adjuvant therapy | 5 (12) | 23 (12) | >0.9 |
All values are median (interquartile range) for continuous variables and frequency (percentage) for categorical variables.
American Society of Anesthesiologists
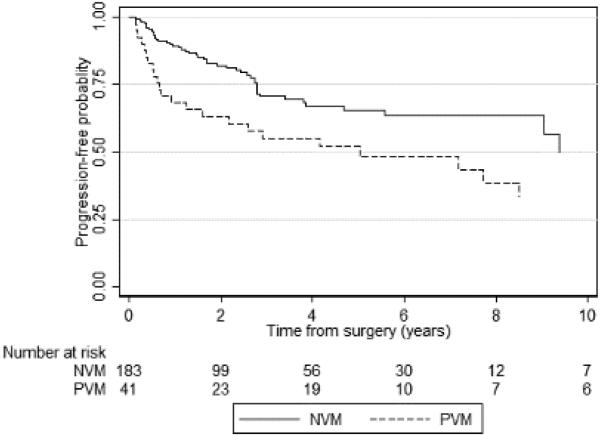
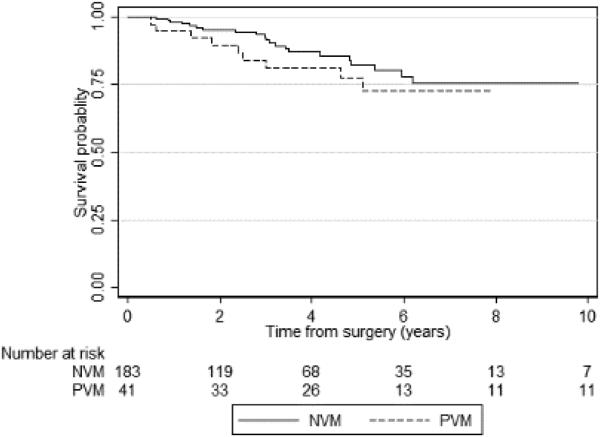
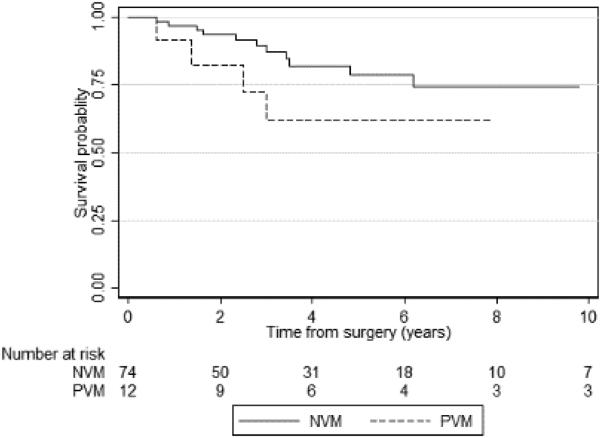
Kaplan-Meier curves for PFS and CSS according to vascular margin status are shown in Figures 2A and 2B, respectively. At 3 years, the PFS probability was 71% (95% CI 62–78) for patients with NVMs and 55% (95% CI 38–69) for patients with PVMs. PVMs were associated with increased disease progression (p=0.01). CSS at 3 years was slightly worse in patients with a PVM (81%) than in those with an NVM (92%), but the difference was not statistically significant (p=0.3).
Figure 2A.
Kaplan-Meier graph of progression-free survival according to vascular margin status (p=0.01). PVM – positive vascular margin; NVM – negative vascular margin.
Figure 2B.
Kaplan-Meier graph of cancer-specific survival according to vascular margin status (p=0.31). PVM – positive vascular margin; NVM – negative vascular margin.
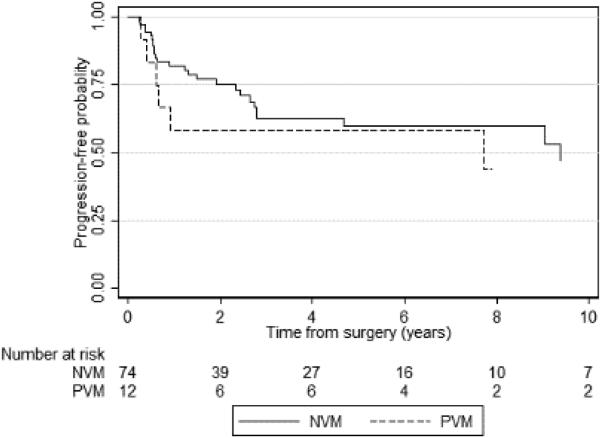
Given that the level of vascular thrombus invasions was significantly different between the positive and negative vascular margin groups, we hypothesized that PFS reflected vascular thrombus involvement rather than vascular margin status. Therefore, we performed a secondary analysis looking at the level of thrombus involvement in conjunction with vascular margin status. Extent of venous thrombus involvement was significantly associated with worse PFS (p=0.02), but not CSS (p=0.4). Three-year probability of PFS was 76% (95% CI 64–85) for margin-negative patients with invasion only of the segmental/muscular venous branch, compared with 63% (95% CI 49–74) for margin-negative patients with MRV invasion, 58% (95% CI 27–80) for margin-positive patients with MRV invasion, and 54% (95% CI 34–70) for margin-positive patients with IVC invasion. Since the only variation in vascular margin status was seen among the 86 patients with MRV invasion (ie. there were no margin-positive patients with only segmental/muscular venous branch invasion, and no margin-negative patients with IVC invasion), a multivariate analysis involving pathologic stage and level of thrombus involvement was not performed. Instead, oncologic outcomes were assessed in patients with only MRV invasion, and we found that PVMs were not associated with a worse PFS (p=0.5) or CSS (p=0.2) compared to negative margins (figs. 3A and 3B).
Figure 3A.
Kaplan-Meier graph of progression-free survival according to vascular margin status in patients with MRV invasion only (p=0.5). PVM – positive vascular margin; NVM – negative vascular margin.
Figure 3B.
Kaplan-Meier graph of cancer-specific survival according to vascular margin status in patients with MRV invasion only (p=0.2). PVM – positive vascular margin; NVM – negative vascular margin.
During the follow-up period, 71 patients developed disease recurrence, including 6 (8%) with local recurrences. Patients with PVMs had a higher 3-year local recurrence rate (5%, vs 1% for those with negative margins), but this did not reach statistical significance (p=0.08). Of the 6 patients with local recurrences, 3 patients had vascular recurrences in the IVC; all three of these patients had undergone IVC thrombectomy with PVMs at the initial surgery. One patient underwent repeat resection of the IVC recurrence 7 years after initial surgery and is free of disease 8 years after the second surgery. The second patient developed IVC recurrence 2 months after initial surgery and was treated with sunitinib followed by bevacizumab. Abdominal imaging 2.5 years later demonstrated resolution of tumor thrombus without evidence of distant metastasis. The third patient developed IVC recurrence 6 months after initial surgery and has been on sunitinib for the last 7 years with stable disease. All three patients were alive at their most recent follow-up.
Discussion
In surgical oncology, complete tumor resection is determined by the gross and microscopic assessment of surgical margins. This is considered a critical component of care in diseases such as locally advanced RCC, where curative outcomes are possible in 40%–50% of patients and for which there are limited effective alternatives to surgery.9-12 In some cases, microscopic assessment of surgical margin status may contradict gross evaluation from surgical assessment, bringing into question the clinical value and significance of these observations. In a study of 32 patients with radical nephrectomy and IVC thrombectomy, Zini et al. demonstrated that renal vein wall invasion is associated with a higher risk of recurrence and decreased CSS.13 Clearing this vascular margin is a priority for the surgeon operating with curative intent, though the accuracy of vascular margin assessment and the measures to identify success are not well defined. Histologic evaluation by microscopy is considered by many to be the gold standard. However, the assessment of vascular margins may be meaningless in the evaluation of a free-floating thrombus in the vessel lumen, which does not represent a surgical margin but a blood “tissue” margin. Such a “margin” cannot be surgically controlled except by completely extracting the thrombus from the lumen and removing it from further contact with the blood. Our study demonstrated that PVMs were associated with worse progression of disease, but this is likely a result of higher pathologic stage and more advanced vascular thrombus invasion observed in this cohort. These findings suggest that the prognostic value of PVMs using the current definition is minimal.
One issue related to PVMs is the risk of local recurrence and whether a repeat surgical resection is necessary if a PVM is identified after surgery. In a series of 256 patients with non-metastatic RCC who underwent radical nephrectomy and thrombectomy, the investigators at MD Anderson reported that the incidence of PVMs was near 20% and PVMs were associated with higher risks of local recurrences;14 however, most patients (98.2%) with locally recurrent disease also had systemic disease, suggesting that an aggressive resection of the vascular structures may be of little value. PVMs in that study were defined as microscopic invasions of tumor thrombus into the vein wall at resection site rather than a grossly visible tumor at the vein margin. Our study also supports this finding of a higher local recurrence rate in patients with PVMs, however this was not a significant finding. Additionally, both studies demonstrate that the recurrence in most patients with PVMs are distant metastases, suggesting that early repeat resection of PVMs is unlikely to provide clinical benefit. Instead, the finding of PVMs might suggest stratification for adjuvant therapy, clinical trials or close follow-up for recurrence and the initiation of early salvage treatment if desired.15-17
Isolated IVC recurrence is rare after tumor thrombectomy even with the presence of a PVM. Logically, it is likely that the source of such a recurrence is unresected microscopic invasion of the IVC wall present at the time of surgery.18 However, with the current means of assessing vascular margin status, PVMs are not a strong predictor for IVC recurrence. A study by Kato et al. evaluated outcomes of IVC resections with PVMs for urologic malignancies and showed that none had developed IVC recurrence.19 Similarly, none of the 47 patients with PVMs treated for RCC at MD Anderson Cancer Center developed IVC recurrences at a median follow-up of 36.7 months.14 The current study reports isolated IVC recurrences in 3 patients (7.3%) with PVMs. All 3 patients had complete resection of grossly visible disease at the initial surgery and also had disease extending above the hepatic vein, providing ample surface contact along the vessel for implantation to occur without clear indication as to precise location.20 The lack of strong association between PVM and IVC recurrence would not support the routine practice of re-resection in this setting.
In the current series, patients with PVMs were more likely to have a high level of thrombus invasion and systemic relapse of disease. However, their prognosis follows the existing pathologic staging criteria for pT3 RCC. In the subset of patients with only MRV thrombus, we demonstrated that vascular margin status was not associated with an inferior oncologic outcome. These observations suggest that patients with vascular thrombus, regardless of vascular margin status, represent a shared biology on a limited portion of the spectrum of localized disease without need for further sub-stratification beyond the current staging system.
Based on the lack of an association between margin status and survival outcomes in the currently published experience on vascular margins, the clinical utility of vascular margin status should be called into question, and the definition more strictly refined. As suggested by Zini and colleagues, a more telling sign of invasive tumor phenotype comes from microscopic evaluation for direct tumor invasion into the vessel wall at the cut surface of the blood vessel margin.13 In fact, the American Joint Commission on Cancer has recently designated tumors with direct caval wall invasion into the pT3c category along with tumor thrombus above the diaphragm, supporting the notion that prognostic impact relies not only on thrombus level, but also on local invasive features.2 Other pathological characteristics such as tumor thrombus consistency have been suggested to reflect metastatic potential. One such characteristic is friable tumor thrombus, which two recent studies demonstrated to be an independent predictor of worse overall survival.21, 22 Compared to solid tumor thrombus, friable tumor thrombus was demonstrated to have lower levels of connective tissue and of E-cadherin, which is responsible for cell-to-cell adhesion, highlighting the enhanced metastatic potential associated with friable thrombus.22 The useful characteristic of thrombus consistency is a subjective quality however and difficult to standardize which could be a limitation to its widespread use though notable for the association with quantitative biomarkers of adhesion. Other biomarkers such as circulating tumor cells or tumor DNA may also play a future role in early assessment of the risk for recurrence, while augmented intraoperative tumor visualization with fluorescence may offer better real-time evaluation of surgical margin status.
This retrospective study has several limitations. The vascular margin status was collected retrospectively rather than by reassessment of specimens by a single pathologist. Therefore, this study may be affected by variability in the interpretations of margin status. However, all specimens were reviewed by experienced genitourinary pathologists who followed a standardized protocol for RCC at our institution. Another limitation is that we did not identify the patients with microscopic venous wall invasions in the PVM group; identifying these patients may provide further insight into whether venous wall invasion is truly associated with poor prognosis. A prospective trial with standardized pathologic assessment of vascular margins at the time of radical nephrectomy and tumor thrombectomy could provide better characterization of the impact of PVMs on cancer outcomes in patients with pT3 clear cell RCC.
Conclusions
In the setting of pT3 clear cell RCC, a PVM is associated with higher risk of disease progression, but not of disease-specific death. However, the risk of disease progression associated with PVMs is driven by the extent of vascular thrombus invasion. These findings suggest that there is minimal clinical significance for reporting vascular margin status of the tumor thrombus using the current definition. These findings also support future studies to further refine the definition of vascular margins in patients with pT3 clear cell RCC.
Acknowledgments
Funding/support: None
Abbreviations
- RCC
renal cell carcinoma
- PVM
positive vascular margin
- IVC
inferior vena cava
- MRV
main renal vein
- PFS
progression-free survival
- CSS
cancer-specific survival
Footnotes
Disclosure of Potential Conflicts of Interest: None
References
- 1.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th Springer; New York: 2010. [Google Scholar]
- 3.Leibovich BC, Cheville JC, Lohse CM, et al. Cancer specific survival for patients with pT3 renal cell carcinoma-can the 2002 primary tumor classification be improved? J Urol. 2005;173:716. doi: 10.1097/01.ju.0000151830.27750.d2. [DOI] [PubMed] [Google Scholar]
- 4.Feifer A, Savage C, Rayala H, et al. Prognostic impact of muscular venous branch invasion in localized renal cell carcinoma cases. J Urol. 2011;185:37. doi: 10.1016/j.juro.2010.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Salamanca JI, Huang WC, Millan I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol. 2011;59:120. doi: 10.1016/j.eururo.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol. 2004;171:598. doi: 10.1097/01.ju.0000108842.27907.47. [DOI] [PubMed] [Google Scholar]
- 7.Kim HL, Zisman A, Han KR, et al. Prognostic significance of venous thrombus in renal cell carcinoma. Are renal vein and inferior vena cava involvement different? J Urol. 2004;171:588. doi: 10.1097/01.ju.0000104672.37029.4b. [DOI] [PubMed] [Google Scholar]
- 8.Trpkov K, Grignon DJ, Bonsib SM, et al. Handling and staging of renal cell carcinoma: the International Society of Urological Pathology Consensus (ISUP) conference recommendations. Am J Surg Pathol. 2013;37:1505. doi: 10.1097/PAS.0b013e31829a85d0. [DOI] [PubMed] [Google Scholar]
- 9.Bissada NK, Yakout HH, Babanouri A, et al. Long-term experience with management of renal cell carcinoma involving the inferior vena cava. Urology. 2003;61:89. doi: 10.1016/s0090-4295(02)02119-2. [DOI] [PubMed] [Google Scholar]
- 10.Glazer AA, Novick AC. Long-term followup after surgical treatment for renal cell carcinoma extending into the right atrium. J Urol. 1996;155:448. [PubMed] [Google Scholar]
- 11.Haddad AQ, Wood CG, Abel EJ, et al. Oncologic outcomes following surgical resection of renal cell carcinoma with inferior vena caval thrombus extending above the hepatic veins: a contemporary multicenter cohort. J Urol. 2014;192:1050. doi: 10.1016/j.juro.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 12.Kaag MG, Toyen C, Russo P, et al. Radical nephrectomy with vena caval thrombectomy: a contemporary experience. BJU Int. 2011;107:1386. doi: 10.1111/j.1464-410X.2010.09661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zini L, Destrieux-Garnier L, Leroy X, et al. Renal vein ostium wall invasion of renal cell carcinoma with an inferior vena cava tumor thrombus: prediction by renal and vena caval vein diameters and prognostic significance. J Urol. 2008;179:450. doi: 10.1016/j.juro.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 14.Abel EJ, Carrasco A, Karam J, et al. Positive vascular wall margins have minimal impact on cancer outcomes in patients with non-metastatic renal cell carcinoma (RCC) with tumour thrombus. BJU Int. 2014;114:667. doi: 10.1111/bju.12515. [DOI] [PubMed] [Google Scholar]
- 15.Husch T, Reiter MA, Mager R, et al. Treatment of Locally Advanced Renal Cell Carcinoma. Eur Urol. 2012;11:66. [Google Scholar]
- 16.Tobert CM, Uzzo RG, Wood CG, et al. Adjuvant and neoadjuvant therapy for renal cell carcinoma: a survey of the Society of Urologic Oncology. Urol Oncol. 2013;31:1316. doi: 10.1016/j.urolonc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Zhu Y, Zhang C, et al. Sorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrence. Urol Oncol. 2013;31:1800. doi: 10.1016/j.urolonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Minervini A, Salinitri G, Lera J, et al. Solitary floating vena caval thrombus as a late recurrence of renal cell carcinoma. Int J Urol. 2004;11:239. doi: 10.1111/j.1442-2042.2003.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Tanaka T, Kitamura H, et al. Resection of the inferior vena cava for urological malignancies: single-center experience. Int J Clin Oncol. 2013;18:905. doi: 10.1007/s10147-012-0473-x. [DOI] [PubMed] [Google Scholar]
- 20.Blute ML, Leibovich BC, Lohse CM, et al. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94:33. doi: 10.1111/j.1464-410X.2004.04897.x. [DOI] [PubMed] [Google Scholar]
- 21.Bertini R, Roscigno M, Freschi M, et al. Impact of venous tumour thrombus consistency (solid vs friable) on cancer-specific survival in patients with renal cell carcinoma. Eur Urol. 2011;60:358. doi: 10.1016/j.eururo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Weiss VL, Braun M, Perner S, et al. Prognostic significance of venous tumour thrombus consistency in patients with renal cell carcinoma (RCC) BJU Int. 2014;113:209. doi: 10.1111/bju.12322. [DOI] [PubMed] [Google Scholar]