Abstract
Galectins have emerged as potent immunoregulatory molecules that control chronic inflammation through distinct mechanisms. Galectin-8 (Gal-8), a tandem-repeat type galectin with unique preference for α2,3-sialylated glycans, is ubiquitously expressed, but little is known about its role in T cell differentiation. Here, we report that Gal-8 promotes the polyclonal differentiation of primary mouse Treg cells in vitro. We further show that Gal-8 also facilitates antigen-specific differentiation of regulatory T (Treg) cells, and that Treg cells polarized in the presence of Gal-8 express cytotoxic T lymphocyte antigen-4 (CTLA-4) and IL-10 at a higher frequency than control Treg cells, and efficiently inhibit proliferation of activated T cells in vitro. Investigation of the mechanism by which Gal-8 promotes Treg conversion revealed that Gal-8 activates TGFβ signaling and promotes sustained IL-2R signaling. Taken together, these data suggest that Gal-8 promotes the differentiation of highly suppressive Treg cells, which has implications for the treatment of inflammatory and autoimmune diseases.
Introduction
Regulatory T (Treg) cells are crucial suppressors of immune responses and are essential for maintaining immunological tolerance and homeostasis. Forkhead box P3 (Foxp3) is the master transcription factor of Treg cells and plays a central role in the development and function of these cells.1 The pivotal role of Treg cells in controlling autoimmunity is underscored by the lethal autoimmune disease in mice that lack Treg cells due to inherited mutations in Foxp3.2 Treg cells suppress effector T cell responses during infection, inflammation, and autoimmunity through several mechanisms, including expression of cytotoxic T lymphocyte antigen (CTLA)-4, which prevents T cells from receiving costimulatory signals from activated APCs.3 Treg cells also secrete the pleiotropic immunosuppressive cytokine, IL-10, to limit immune responses; this mechanism has been shown to be particularly important in preventing immunopathology in tissues that have direct contact with the external environment, such as the eye and the gut,4 and inhibiting autoimmune diseases.5 Due to the key role of Treg cells in preventing autoimmunity, there is intense interest in manipulating the signals responsible for generating and maintaining these cells. Although the generation and regulation of Treg cells is a complex and incompletely understood process, it is known that Treg cell development in the periphery depends on IL-2 and TGFβ signaling.1 Recent studies have provided evidence that members of the galectin family also have the potential to modulate the generation and stability of Treg cells.6,9
Galectins are a family of β-galactoside-binding animal lectins that regulate diverse biological processes including, but not limited to, immune homeostasis, pathogen recognition, neurogenesis, and vascular permeability.10,13 Recently, galectins have emerged as potential therapeutic agents for chronic inflammatory conditions such as autoimmunity and infection because of increasing evidence demonstrating their role in the regulation of both the innate and adaptive immune systems.10 Gal-1 and -9 enhance the frequency and immunosuppressive capacity of Treg cells,8,14 whereas Gal-3 inhibits Treg cell conversion and function.15 Gal-8 is a tandem-repeat type galectin, composed of two structurally distinct carbohydrate recognition domains (CRDs) connected by a short peptide linker. The N-terminal CRD of Gal-8 preferentially binds to α2,3-sialylated glycans, a unique specificity among galectins.16 Although the expression of Gal-8 is ubiquitous and markedly increased in response to inflammation (Chen, et al. in preparation), its role in the regulation of the immune system is poorly understood, and in particular little is known about the role of Gal-8 in the regulation of Treg cell differentiation and function. We have recently demonstrated that Gal-8 promotes Treg differentiation and ameliorates murine autoimmune uveitis pathology.17
We demonstrate here that Gal-8 enhances Treg and TH2 differentiation and kills TH17 cells. We further show that a higher percentage of Treg cells polarized in the presence of Gal-8 express the inhibitory coreceptor CTLA-4 and the immunosuppressive cytokine IL-10, as compared to control Treg cells, and that Gal-8-polarized Treg cells are potent suppressors of activated T cells in vitro. Efforts to characterize the mechanism by which Gal-8 promotes Treg cell differentiation revealed that this lectin binds to TGFβRII as well as IL-2Rβ, activating the TGFβ signaling pathway and sustaining IL-2 signaling.
Results and Discussion
Gal-8 promotes Treg and TH2 cell differentiation and inhibits differentiation of TH17 cells in vitro
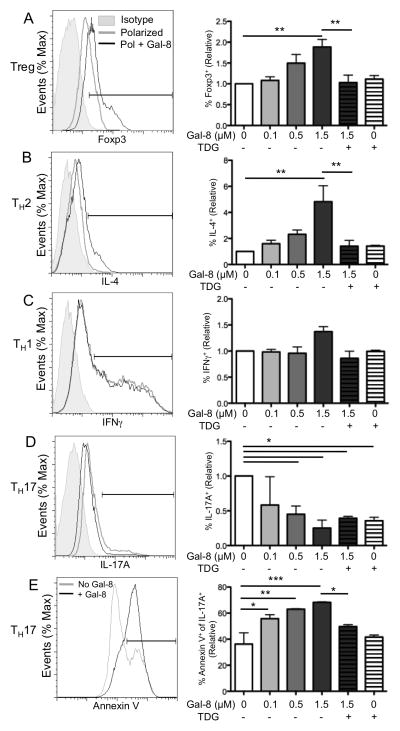
Because members of the galectin family, in particular Gal-1 and -9, have been shown to play a role in immune homeostasis,8,14 we questioned what role, if any, Gal-8 has in T cell differentiation. Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 mAbs under Treg, TH2, TH1, or TH17 polarizing conditions for 4 d in the presence and absence of Gal-8. At the end of the incubation period, we assessed CD4+ T cell polarization to Treg, TH2, TH1, and TH17 by flow cytometry for Foxp3, IL-4, IFNγ, and IL-17A, respectively. Gal-8 promoted Treg and TH2 cell differentiation in a dose-dependent manner (Fig. 1A, 1B); at the highest concentration tested, Treg and TH2 cell differentiation increased 1.9- and 4.8-fold, respectively, in the presence of Gal-8. The stimulatory effect of Gal-8 on Treg and TH2 differentiation was inhibited by TDG, a competing disaccharide that binds the CRD of galectins (Fig. 1A, B), suggesting that the effect of Gal-8 on Treg and TH2 differentiation was carbohydrate-dependent. Also, a trisaccharide, NeuAcα2-3Galβ1-4GlcNAc (α2,3-sialyllactose), which binds to the N-CRD of Gal-8,18 inhibited Treg differentiation (Fig. 2A) suggesting that the stimulatory effect of Gal-8 on Treg differentiation is mediated by binding to the 3′-sialylated glycoproteins on the CD4+ T cell surface. Gal-8 had no effect on TH1 differentiation (Fig. 1C), and reduced TH17 polarization in a dose-dependent manner (Fig. 1D). TDG alone was sufficient to inhibit TH17 differentiation (Fig. 1D). To determine whether Gal-8 inhibits TH17 cells by preventing de novo differentiation or inducing death of differentiated cells, TH17-polarized cells were incubated with varying concentrations of Gal-8 for 8 hours and death of CD4+IL-17A+ cells was measured by annexin V staining. Gal-8 killed TH17 cells in a carbohydrate-dependent manner (Fig. 1E). Differential glycosylation of T cell subsets can result in different susceptibility to galectin-mediated effects. For example, Gal-1 induces death of TH1 and TH17 cells, but not of TH2 cells because cell surface glycoproteins of TH2 cells are capped with α2–6-sialylated glycans that abrogate Gal-1 binding.19 Considering that Gal-8 expression is increased in the draining lymph nodes of Pseudomonas aeruginosa-infected corneas, and upon activation, T cells secrete Gal-8 (Sampson, Suryawanshi and Panjwani unpublished observations), it is reasonable to expect that Gal-8 is likely present at a high local concentration, and could act in an autocrine manner to promote Treg cell differentiation and inhibit TH17 responses. Because Treg cells are critically important in promoting anti-inflammatory immune responses and their frequency is higher in Gal-8-treated mice with autoimmune uveitis in vivo,17 we focused our attention on characterizing the involvement of Gal-8 in Treg cell differentiation and function.
Figure 1. Galectin-8 promotes TH2 and Treg cell differentiation, and inhibits differentiation of TH17 cells.
Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 mAb under Treg (A), TH2 (B), TH1 (C), or TH17 (D, E) polarizing conditions for 4 d in the presence of Gal-8 as indicated. Thiodigalactoside (TDG) was used as a galectin-specific inhibitor. (A–D) Intracellular expression of Foxp3, IL-4, IFNγ, and IL-17A was assessed by flow cytometry, with representative scatter plots gated on CD4+ cells shown. (E) TH17 cells were incubated with Gal-8 for 8 h, and death was measured as annexin V staining. Relative represents percentage of marker positive cells in gated population as compared to control. On overlayed histograms, % of max represents the total number of cells at a given fluorescence intensity compared to the maximum number of cells at any intensity for a given sample. Error bars represent mean ± SEM of at least three independent experiments. P values were determined by ANOVA with Tukey’s multiple comparison test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
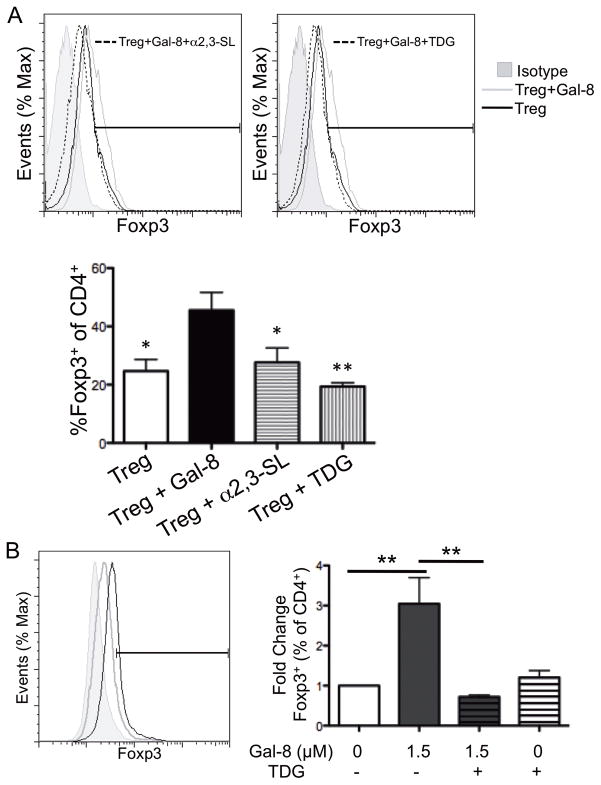
Figure 2. Galectin-8 binds α2,3-sialylated ligands on CD4+ T cells and promotes Ag-specific Treg cell differentiation.
(A) Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 and anti-CD28 mAbs under Treg polarizing conditions for 4 d in the presence of 1.5 μM Gal-8. TDG and α2,3-sialyllactose were used as galectin inhibitors. (B) CD4+ T cells were isolated from OT-II mice and cultured under Treg polarizing conditions with irradiated congenic splenocytes in the presence of OVA323–339 and Gal-8, as indicated. Relative represents percentage of marker positive cells in gated population as compared to control, and % of max represents the total number of cells at a given fluorescence intensity compared to the maximum number of cells at any intensity for a given sample. Error bars represent mean ± SEM of three independent experiments. P values were determined by ANOVA with Tukey’s multiple comparison test. *, p < 0.05;**, p < 0.01. Significance in panel A is compared to Treg cell polarization in the presence of Gal-8.
To determine whether Gal-8 promotes Ag-specific Treg cell polarization and to ensure that the observed increase in Treg polarization was not due to an artifact of stimulation with anti-CD3 and anti-CD28 mAbs, splenic OVA323–339-specific T cells from OT-II mice were incubated with OVA peptide-pulsed APCs (irradiated spleen cells) under Treg polarizing conditions in the presence or absence of Gal-8. In an Ag-specific context, the addition of 1.5 μM Gal-8 increased Treg cell differentiation three-fold in a carbohydrate-dependent manner (Fig. 2B), confirming the results observed using anti-CD3- and anti-CD28-stimulated CD4+ T cells. Thus, Gal-8 induces both Ag-specific and polyclonal Treg cell differentiation.
Gal-8-polarized Treg cells express CTLA-4 and IL-10 at high frequency and suppress T cell proliferation
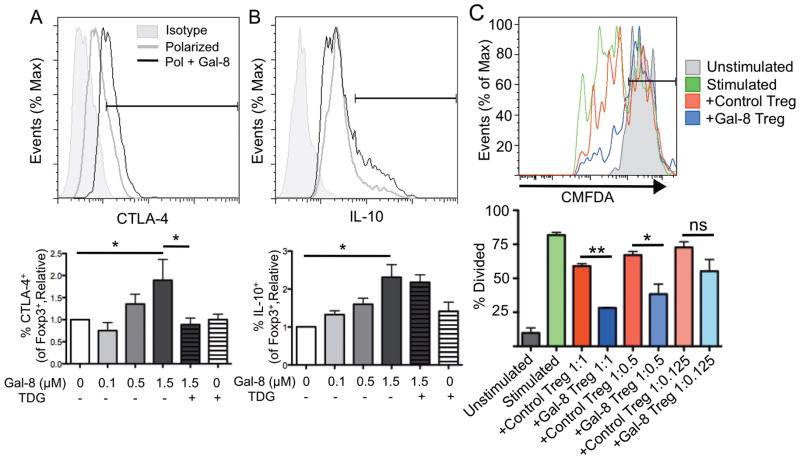
As described earlier, Treg cells suppress effector T cells. To assess whether Gal-8 influences the inhibitory function of Treg cells, we first examined the expression of the suppressive molecules CTLA-4 and IL-10 by CD4+Foxp3+ Treg cells polarized in the presence or absence of Gal-8. Gal-8 enhanced the frequency of Treg cells expressing CTLA-4 and IL-10 in a dose- and carbohydrate-dependent manner (Fig. 3A, 3B), suggesting that Treg cells polarized in the presence of Gal-8 may be endowed with a higher immunosuppressive capacity. To directly assess the suppressive ability of Gal-8-polarized Treg cells, we evaluated the ability of these cells to inhibit proliferation of activated T cells. For the suppression assay, CD4+ T cells from Foxp3mRFPIL-10eGFP mice were polarized to Treg in the presence or absence of 1.5 μM Gal-8 for 4 days and washed thoroughly to remove any unbound Gal-8. The washed Treg cells were then cocultured at indicated ratios with CD4+CMFDA+ T cells from B6 mice and APCs (irradiated splenocytes) in the presence of anti-CD3 mAb for 3 days. Proliferation was measured as dilution of CMFDA. At a ratio of 1 activated T cell to 1 Treg cell as well as 1:0.5, Gal-8-polarized Treg cells inhibited proliferation of activated T cells more efficiently than control Treg cells (Fig. 3C). Our data indicate that Treg cells polarized in the presence of Gal-8 are functional, as they are able to inhibit the proliferation of activated T cells. Gal-8 expression is upregulated in certain cancers, such as laryngeal squamous cell carcinoma and thyroid tumors20,21. Thus, it is possible that in addition to its role in angiogenesis,22 Gal-8 may promote tumor immune evasion and survival by stimulating Treg cell differentiation. Others have demonstrated that Gal-1 and Gal-9 positively regulate Treg cell function,,8,14 whereas Gal-3 negatively regulates Treg cell expansion and function.15 The current study demonstrates that Gal-8 promotes Treg cell differentiation and function as well. Although most members of the galectin family bind galactose-containing residues, variability in the CRD among galectins results in unique fine specificities for more complex galactose-containing oligosaccharides.23 As a result, each galectin may have profoundly different ligand preference based on glycosylation patterns, with consequent specific downstream effects.19,24,25 In this respect, the carbohydrate-binding affinity of the N-terminal CRD of Gal-8 is unique among members of the galectin family in that it has a high affinity for 3′-sialylated glycoconjugates.18 This unique carbohydrate specificity and structure could make Gal-8 a selective drug target.
Figure 3. Galectin-8-polarized Treg cells express CTLA-4 and IL-10 at high frequency, and suppress T cell proliferation.
(A, B) Splenic CD4+ T cells were activated under Treg polarizing conditions for 96 h with or without Gal-8 as indicated. Expression of (A) CTLA-4 and (B) IL-10 gated on CD4+Foxp3+ cells was measured by flow cytometry. (C) CD4+ T cells from Foxp3mRFPIL-10eGFP mice were polarized to Treg for 4 d in the presence or absence of Gal-8 and Treg cells were sorted as CD4+RFP+ cells. Splenic CD4+ T cells were isolated from B6 mice and stained with CMFDA, and the rest of the spleen was irradiated to use as a source of APCs. Proliferation of unstimulated CD4+CMFDA+ cells, or cells stimulated for 3 d in the presence or absence of control or Gal-8-polarized Treg cells was assessed as dilution of CMFDA. Ratios are activated T cells: Treg cells. Relative represents percentage of marker positive cells in gated population as compared to control, and % of max represents the total number of cells at a given fluorescence intensity compared to the maximum number of cells at any intensity for a given sample. Error bars represent mean ± SEM of at least three independent experiments. P values were determined by ANOVA with Tukey’s multiple comparison test. *, p < 0.05; **, p < 0.001; ns, not significant.
Gal-8 activates the TGFβR signaling pathway and promotes sustained IL-2R signaling
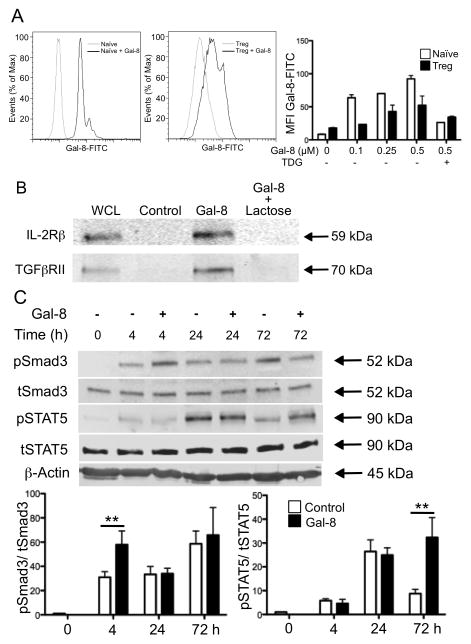
Having established that Gal-8 promotes Treg cell differentiation in vitro, we performed studies to characterize the mechanism by which Gal-8 promotes Treg cell conversion. First, we tested whether Gal-8 binds to cell surface glycoproteins of naïve T cells and/or Treg cells. Splenic CD4+CD62LhiCD44lo naïve T cells and in vitro-polarized Treg cells were incubated with FITC-conjugated Gal-8 and mean fluorescence intensity (MFI) was measured by flow cytometry. Although Gal-8 bound both cell types, naïve T cells stained with a higher intensity at all doses of Gal-8 (Fig. 4A), indicating that Gal-8 ligands are highly expressed on naïve T cells. Binding of Gal-8 to naïve T cells as well as Treg cells was inhibited by TDG, suggesting that the lectin binding to both cell types was carbohydrate-dependent.
Figure 4. Galectin-8 activates TGFβR and IL-2R signaling pathways.
(A) Gal-8 binding to the surface of naïve T cells and Treg cells was assessed by incubation with Gal-8-FITC. % of max represents the total number of cells at a given fluorescence intensity compared to the maximum number of cells at any intensity for a given sample. Mean fluorescence intensity (MFI) is averaged across two independent experiments, and error bars are mean ± SEM. (B) Splenic CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs, lysed, and incubated with agarose beads alone (Control), or Gal-8-conjugated beads in the presence or absence of the competitive inhibitor lactose. Bound fractions and whole cell lysate were analyzed by western blotting for Gal-8-binding proteins. Blots are representative of three independent experiments with similar results. (C) CD4+ T cells were polarized toward a Treg cell profile in the presence or absence of Gal-8, lysates were collected at indicated times, probed for pSTAT5 and pSmad3, and each normalized to the appropriate non-phosphorylated control. Error bars represent mean ± SEM of at least three independent experiments. P values were determined by ANOVA with Tukey’s multiple comparison test. **, p < 0.01.
Naïve T cells require IL-2 and TGFβ in conjunction with proper Ag stimulation to differentiate into Treg cells. It is well established that the expression of Foxp3, the master regulator of Treg cells, requires IL-2R- and TGFβR-dependent phosphorylation of STAT5 and Smad3, respectively.1 To determine whether TGFβR and/or IL-2R are Gal-8-binding partners, lysates of CD4+ T cells were incubated with Gal-8-conjugated agarose beads in the presence or absence of 0.1M β-lactose, a galectin inhibiting sugar. Bound proteins were examined along with total cell lysates by western blot using antibodies directed against IL-2R and TGFβR. Both IL-2Rβ (59 kDa) and TGFβRII (70 kDa) were detected in the bound fraction of Gal-8-conjugated beads, but not in control beads (Fig. 4B), indicating that both IL-2R and TGFβR are Gal-8-binding proteins. Binding of IL-2R and TGFβR to Gal-8 was inhibited by β-lactose, demonstrating that Gal-8 interacts with IL-2R and TGFβR in a carbohydrate-dependent manner. Although it is likely that Gal-8 binds to additional receptors on the T cell surface, in the present study we focused on IL-2R and TGFβR signaling because of the well characterized role of these receptors in Treg cell differentiation.1
To determine whether Gal-8 activates signaling pathways downstream of TGFβR and/or IL-2R, CD4+ T cells were incubated under Treg polarizing conditions for 0, 4, 24, or 72 hours in the presence or absence of 1.5 μM Gal-8. At the end of the incubation period, whole cell lysates were prepared and the phosphorylation status of Smad3 and STAT5 was evaluated by western blot analysis using phospho-specific antibodies. Gal-8 markedly increased phosphorylation of Smad3 at 4 h, which then decreased to the same extent as seen in control cells. In control cells, phosphorylation of STAT5 peaked at 24 h and declined to basal levels by 72 h. Exposure to Gal-8 resulted in sustained STAT5 phosphorylation over time; at 72 hours, STAT5 phosphorylation was as high as that detected at 24 h of incubation (Fig. 4C). These results suggest that Gal-8 activates TGFβR signaling and sustains IL-2R signaling. The multivalency of galectins allows them to cross-link many cell surface receptors, including growth factor receptors and integrins, to regulate signal transduction pathways.27 These postulated supramolecular galectin-ligand lattices have been shown to inhibit endocytosis, and the increased surface retention time can have a dramatic effect on receptor signaling.27,28 Alternatively, galectins may directly ligate and activate their receptors independent of lattice formation. In this regard, Gal-9 promotes apoptosis of murine TH1 cells through direct ligation of Tim-3,29 and Gal-1 inhibits deactivation of microglia and prevents immune-mediated neurodegeneration by ligating microglial CD45 phosphatase. A recent study has shown that Gal-9 also supports Treg conversion by modulating TGFβ signaling.9 It is known that in some cases, multiple galectins can influence the same function in a given cell type, although the mechanism, signaling pathways and glycan specificity may substantially differ for each family member. For example, Gal-1, -3, and -9 mediate the death of Jurkat cells by binding to distinct cell surface ligands.30,31 How Gal-8 binding modulates IL-2R and TGFβR dynamics remains to be explored.
Overall, these results suggest that Gal-8 promotes Treg cell differentiation through a multifaceted mechanism involving sustained STAT5 phosphorylation and increased Smad3 phosphorylation. It would be important to investigate in the future whether Gal-8 could potentially serve as a valuable therapeutic intervention for a broad range of autoimmune and chronic inflammatory conditions that are mediated by TH17 cells and ameliorated by the action of Treg cells. In this respect, recent studies in our lab have shown that Gal-8 promotes Treg differentiation in vivo and reduces pathology in a murine model of autoimmune uveitis,17 supporting our hypothesis that Gal-8 functions as an anti-inflammatory modulator of the immune system.
Methods
Mice
C57BL/6J (B6) and OT-II mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Foxp3mRFPIL-10eGFP mice were a gift from J. Weinstock.32 All animal study protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Reagents, Ags, and Abs
The galectin competitive inhibitors (D-galactopyranosyl)-β-D-thiogalactopyranoside (TDG) and α2,3-sialyllactose were purchased from Carbosynth (Compton, UK), and β-lactose was purchased from Fisher Scientific (Waltham, MA, USA). Ovalbumin (OVA)323-339 was purchased from Invivogen (San Diego, CA, USA). The following antibodies were purchased from eBioscience (San Diego, CA, USA), as conjugated to FITC, PE, or allophycocyanin: IFNγXMG1.2), IL-17A (eBio17B7), Foxp3 (FJK-16S), CTLA-4 (UC10-4B9), IL-10 (JES5-16E3), and isotype controls. The following antibodies were purchased from BD (East Rutherford, NJ, USA), as conjugated to FITC, PE, V450, or APC: CD4 (RM4-5), CD44 (IM7), CD62L (MEL-14), CD103 (M290), IL-4 (11B11), and isotype controls. Antibodies and recombinant cytokines for T cell polarization were purchased from BioLegend (San Diego, CA, USA). Recombinant human glutathione S-transferase (GST) tagged Gal-8 was produced and purified from bacteria expressing GST-Gal-8 as previously described.33
In vitro CD4+ T cell differentiation
CD4+ T cells were isolated from spleens by negative selection using the CD4 T Lymphocyte Enrichment Set (BD). Cells were stimulated for 96 h with 1 μg/ml anti-CD3 (145-2C11; BD) and 2 μg/ml anti-CD28 (37.51; BD) for polyclonal activation. Cells were differentiated to TH1 by supplementation with 10 ng/ml IL-12 and 10 μg/ml anti-IL-4 mAb (11B11). TH2 differentiation was induced by supplementation with 10 ng/ml IL-4 and 10 μg/ml anti-IFNγ mAb (XMG1.2). For TH17 conversion, cells were supplemented with 20 ng/ml IL-6, 10 ng/ml IL-23, 1 ng/ml TGFβ1, and 10 ng/ml IL-1β in the presence of 10 μg/ml anti-IFNγ mAb and 10 μg/ml anti-IL-4 mAb. To induce Treg polarization, cells were incubated with 10 ng/ml TGFβ1, 1 ng/ml IL-2, and 1 ng/ml all-trans retinoic acid (Sigma, St. Louis, MO, USA). For Ag-specific activation, CD4+ T cells from OT-II mice were incubated with 20 μM OVA323-339 in the presence of γ-irradiated CD4− splenocytes under Treg polarizing conditions as above. For assessment of Gal-8 binding, naïve CD4+ T cells (CD4+CD62LhiCD44lo) were prepared by FACS from spleens of B6 mice using an Influx Cell Sorter (BD).
Flow cytometry
Flow cytometry was performed as described previously19 on a FACS Calibur (BD), and data were analyzed with FlowJo software (Tree Star, Ashland, OR, USA). Gates were set based on appropriate isotype controls.
In vitro Treg cell suppression assay
CD4+ T cells were isolated from the spleens of Foxp3mRFPIL-10eGFP mice and polarized to Treg in the presence or absence of 1.5 μM Gal-8. At the end of the polarization, Treg cells were sorted to 99% purity as RFP+ cells using an Influx Cell Sorter. CD4+ T cells were isolated from the spleen of B6 mice and labeled with CellTracker Green CMFDA (Molecular Probes, Eugene, OR, USA). CMFDA-labeled T cells (6 x 104) were incubated with irradiated splenocytes (6 x 105) and 1 μg/ml anti-CD3 for 72 h in the presence or absence of control or Gal-8-polarized sorted Treg cells (at 1 activated T cell:1 Treg, 1:0.5, and 1:0.25). Proliferation of labeled activated T cells was assessed as dilution of CMFDA by flow cytometry.
Assessment of Gal-8 binding to cell surface
Naïve T cells or Foxp3+ in vitro-polarized Treg cells were incubated with varying concentrations of FITC-conjugated Gal-8 (0.1 to 0.5 μM) for 1 h at 4°C, and binding of Gal-8 to the cell surface was measured by flow cytometry.
Affinity precipitation assay
Affinity precipitation with Gal-8-conjugated agarose beads was performed as described previously.34 Briefly, primary CD4+ T cell lysates were incubated with agarose-conjugated Gal-8 overnight at 4°C. Non-specific binding proteins were removed by washing, bound proteins were eluted by boiling the beads in Laemmli sample buffer, and assessed by western blotting.
Western blot analysis
Cell lysates were electrophoresed on SDS-PAGE gels (4–15% gradient polyacrylamide gels, BioRad, Hercules, CA, USA) and blots of the gels were probed with the following antibodies: anti-IL-2Rβ Novus, Littleton, CO, USA), anti-TGFβRII, anti-pSTAT5 (Tyr694), anti-STAT5, anti-pSmad3 (Ser423/Ser425), anti-Smad3, and anti-β-actin (all Cell Signaling, Beverly, MA, USA). The secondary Ab used were anti-rabbit IgG IRDye 800CW and anti-mouse IgG IRDye 680LT (LI-COR, Lincoln, NE, USA). Membranes were imaged using the Odyssey Infrared Imager and quantified using Image Studio v2.0 software (LI-COR).
Statistical analysis
Statistical analysis was performed using ANOVA with Tukey’s test for multiple comparisons. P-values were considered statistically significant at p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
Acknowledgments
This work was generously supported by National Eye Institute Grants R01EY007088 (NP), R01EY009349 (NP), Mass Lions Eye Research fund (NP), New England Corneal Transplant Fund (NP) and an unrestricted award from Research to Prevent Blindness (NP). We thank Dr. Joel Weinstock (Tufts University) for Foxp3mRFPIL-10eGFP mice. We greatly appreciate Drs. Andrea Soza and Alfonso González for generously providing the Gal-8 construct for preparation of rGal-8. We would also like to thank the Flow Cytometry Core for help with sorting, and Drs. David Thorley-Lawson (Tufts University) and Michael J. Ophir (Harvard University) for critical reading of the manuscript.
Abbreviations used
- B6
C57BL/6J
- CTLA-4
cytotoxic T lymphocyte antigen-4
- CRD
carbohydrate recognition domain
- Foxp3
Forkhead box P3
- Gal
galectin
- OVA
ovalbumin
- TDG
(D-Galactopyranosyl)-β-D-thiogalactopyranoside
- TH
T helper type
- Treg cell
regulatory T cell
Footnotes
Authorship
J.F.S. and A.S. contributed to the conception, design and performance of the work. J.F.S. prepared the manuscript. W.S.C. contributed to the performance. G.A.R. and N.P. contributed to the conception and design of the study. N.P. helped with the writing of the manuscript. All authors approved the final version of the manuscript.
Conflict of interest disclosure
All authors declare no financial conflict of interest with the submission of this manuscript.
All authors declare no conflict of interest.
References
- 1.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4-/- mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci U S A. 2007;104:13756–13761. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo LV, Xu H, Chan CC, Wiggert B, Caspi RR. IL-10 has a protective role in experimental autoimmune uveoretinitis. Int Immunol. 1998;10:807–814. doi: 10.1093/intimm/10.6.807. [DOI] [PubMed] [Google Scholar]
- 6.Toscano MA, Commodaro AG, Ilarregui JM, Bianco GA, Liberman A, Serra HM, et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176:6323–6332. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 7.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73:1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 8.Seki M, Oomizu S, Sakata K-M, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, et al. Immunity. Vol. 41. Elsevier Inc; 2014. Galectin-9-CD44 Interaction Enhances Stability and Function of Adaptive Regulatory T Cells; pp. 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinovich GA, Toscano MA. Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 11.Quattroni P, Li Y, Lucchesi D, Lucas S, Hood DW, Herrmann M, et al. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell Microbiol. 2012;14:1657–1675. doi: 10.1111/j.1462-5822.2012.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishibashi S, Kuroiwa T, Sakaguchi M, Sun L, Kadoya T, Okano H, et al. Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp Neurol. 2007;207:302–313. doi: 10.1016/j.expneurol.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Wu M-H, Ying N-W, Hong T-M, Chiang W-F, Lin Y-T, Chen Y-L. Galectin-1 induces vascular permeability through the neuropilin-1/vascular endothelial growth factor receptor-1 complex. Angiogenesis. 2014;17:839–849. doi: 10.1007/s10456-014-9431-8. [DOI] [PubMed] [Google Scholar]
- 14.Cedeno-Laurent F, Opperman M, Barthel SR, Kuchroo VK, Dimitroff CJ. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J Immunol. 2012;188:3127–3137. doi: 10.4049/jimmunol.1103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fermino ML, Dias FC, Lopes CD, Souza MA, Cruz AK, Liu F-T, et al. Galectin-3 negatively regulates the frequency and function of CD4(+) CD25(+) Foxp3(+) regulatory T cells and influences the course of Leishmania major infection. Eur J Immunol. 2013;43:1806–1817. doi: 10.1002/eji.201343381. [DOI] [PubMed] [Google Scholar]
- 16.Ideo H, Seko A, Ishizuka I, Yamashita K. The N-terminal carbohydrate recognition domain of galectin-8 recognizes specific glycosphingolipids with high affinity. Glycobiology. 2003;13:713–723. doi: 10.1093/glycob/cwg094. [DOI] [PubMed] [Google Scholar]
- 17.Sampson JF, Hasegawa E, Mulki L, Suryawanshi A, Jiang S, Chen W-S, et al. Galectin-8 ameliorates murine autoimmune ocular pathology and promotes a regulatory T cell response. PLoS One. doi: 10.1371/journal.pone.0130772. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ideo H, Matsuzaka T, Nonaka T, Seko A, Yamashita K. Galectin-8-N-domain recognition mechanism for sialylated and sulfated glycans. J Biol Chem. 2011;286:11346–11355. doi: 10.1074/jbc.M110.195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toscano MA, Bianco GA, Ilarregui JM, Croci DO, Correale J, Hernandez JD, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 20.Dong GW, Kim J, Park JH, Choi JY, Il Cho S, Lim SC. Galectin-8 expression in laryngeal squamous cell carcinoma. Clin Exp Otorhinolaryngol. 2009;2:13–19. doi: 10.3342/ceo.2009.2.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savin S, Cveji D, Jankovi M, Isi T, Paunovi I, Tati S. Evaluation of galectin-8 expression in thyroid tumors. Med Oncol. 2009;26:314–318. doi: 10.1007/s12032-008-9122-7. [DOI] [PubMed] [Google Scholar]
- 22.Delgado VMC, Nugnes LG, Colombo LL, Troncoso MF, Fernandez MM, Malchiodi EL, et al. Modulation of endothelial cell migration and angiogenesis: a novel function for the “tandem-repeat” lectin galectin-8. FASEB J. 2011;25:242–254. doi: 10.1096/fj.09-144907. [DOI] [PubMed] [Google Scholar]
- 23.Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim Biophys Acta. 2002;1572:232–254. doi: 10.1016/s0304-4165(02)00311-2. [DOI] [PubMed] [Google Scholar]
- 24.Stowell SR, Arthur CM, Mehta P, Slanina KA, Blixt O, Leffler H, et al. Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem. 2008;283:10109–10123. doi: 10.1074/jbc.M709545200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo Y, Chammas R, Bellis SL. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J Biol Chem. 2008;283:22177–22185. doi: 10.1074/jbc.M8000015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tribulatti MV, Cattaneo V, Hellman U, Mucci J, Campetella O. Galectin-8 provides costimulatory and proliferative signals to T lymphocytes. J Leukoc Biol. 2009;86:371–380. doi: 10.1189/jlb.0908529. [DOI] [PubMed] [Google Scholar]
- 27.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signalling. Biochem Soc Trans. 2008;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 30.Lu L-H, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, et al. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem. 2007;141:157–172. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
- 31.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu F-T, et al. Galectin-3 and Galectin-1 Bind Distinct Cell Surface Glycoprotein Receptors to Induce T Cell Death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 32.Hang L, Blum AM, Setiawan T, Joseph P, Stoyanoff KM, Weinstock JV. Heligmosomoides polygyrus bakeri Infection Activates Colonic Foxp3 + T Cells Enhancing Their Capacity To Prevent Colitis. J Immunol. 2015;191:1927–1934. doi: 10.4049/jimmunol.1201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diskin S, Chen W-S, Cao Z, Gyawali S, Gong H, Soza A, et al. Galectin-8 promotes cytoskeletal rearrangement in trabecular meshwork cells through activation of Rho signaling. PLoS One. 2012;7:e44400. doi: 10.1371/journal.pone.0044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markowska AI, Jefferies KC, Panjwani N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem. 2011;286:29913–29921. doi: 10.1074/jbc.M111.226423. [DOI] [PMC free article] [PubMed] [Google Scholar]