Abstract
BACKGROUND
Studies of lung transplantation in the setting of donors or recipients with hepatitis C virus (HCV) have been limited but have raised concerns about outcomes associated with this infection.
METHODS
Lung transplant cases in the United Network for Organ Sharing (UNOS) database from 1994 to 2011 were analyzed for the HCV status of both donor and recipient. First, among HCV-negative recipients, those who received a lung from an HCV-positive donor (HCV+ D) were compared with those who received an HCV-negative lung (HCV− D). Donor, recipient and operative characteristics as well as outcomes were compared between groups, and overall survival was compared after adjustment for confounders. In a second analysis, HCV-positive recipients (HCV+ R) were compared with HCV-negative recipients (HCV− R). The analysis was stratified by era (1994 to 1999 and 2000 to 2011) and long-term survival was compared.
RESULTS
Of 16,604 HCV-negative patients in the UNOS database, 28 (0.2%) received a lung from an HCV+ D, with use of HCV+ D decreasing significantly over time. Overall survival (OS) was shorter in the HCV+ D group (median survival: 1.3 vs 5.1 years; p ¼ 0.002). Results were confirmed in adjusted analyses. After inclusion criteria were met, 289 (1.7%) of the lung transplant recipients were HCV+ R. These patients appeared similar to their HCV− R counterparts, except they were older and had more limited functional status. OS was significantly lower in HCV-positive individuals during the early era (median survival: 1.7 vs 4.5 years; p = 0.004), but not the recent era (median survival: 4.4 vs 5.4 years; p = 0.100). Again, results were confirmed by adjusted analysis.
CONCLUSIONS
HCV-positive status is a rare problem when considering both lung recipients and donors. Current data demonstrate significantly worse outcomes for HCV-negative patients receiving an HCV+ lung; however, since 2000, HCV+ recipients undergoing lung transplantation appear to have survival approximating that of HCV− recipients, an improvement from previous years. Recent medical advances in treatment for HCV may further improve outcomes in these groups.
Keywords: hepatitis C, immunosuppression, lung transplant, outcomes
Lung transplantation has become widely accepted for end-stage lung disease1,2 and has demonstrated both survival and quality-of-life benefits in severe pulmonary dysfunction.3–5 Use of lung transplantation is limited by a restricted supply of donors, with >400 patients removed from the waitlist each year due to death or progressive disease.6
Chronic viral infection, such as hepatitis B virus, hepatitis C virus (HCV), Epstein–Barr virus or cytomegalovirus, have been associated with complications after transplant, potentially due to more active viral disease secondary to immunosuppression.7,8 With an estimated prevalence of 1.8% in the USA,9 HCV-positive patients comprise a substantial number of potential lung transplant donors and recipients.10 Survey data have estimated that one third of lung transplant programs would not consider an HCV-seropositive recipient for transplant, whereas 45% would not consider a lung from an HCV-seropositive donor.10,11 With new, more effective therapies to cure HCV infection, such as sofosbuvir and simeprevir, both recently approved by the United States Food and Drug Administration, the outcomes of lung transplantation in the setting of HCV may be changing.12
Given potential changes in the HCV population and lack of data to guide difficult clinical decisions around lung transplantation in the setting of HCV seropositivity, we compared cases of lung transplantation with HCV+ and HCV− donors. In addition, we compared cases with HCV+ and HCV− recipients. With this analysis, we aimed to: (1) establish patterns of use for lung transplantation in the setting of HCV seropositivity; (2) provide clinicians with an understanding of the independent risk of using lung transplantation in the setting of HCV; (3) establish bench-mark outcomes in this population to set institutional and patient expectations; and (4) create a starting point and robust resource for future research in this area given the potential for improved outcomes with more effective treatments for HCV.
Methods
UNOS database
UNOS has maintained the Standard Transplant Analysis and Research files of waitlisted transplant candidates, recipients and donors in the USA since 1987.13 UNOS assures data quality through trained data abstracters and managers, quality checks and on-site auditing, and these prospective data are used to provide risk-adjusted performance measures.14
Study population
Using the UNOS database, all lung transplant cases from 1987 through 2011 were identified, with cases of pediatric and multiple-organ transplantation excluded. Cases with missing HCV donor or recipient status were also excluded, after which only cases from 1994 to 2011 remained. Two data sets were created for analysis. The first, designed to analyze cases by HCV donor status, classified cases as HCV+ D or HCV− D. For the second analysis, cases were classified by recipient status (HCV+ R vs HCV− R). HCV status was based on serology, as UNOS did not provide data on viral load or state of infection. Demographic, comorbidity, procedural and outcomes data on both recipients and donors were collected. In addition, rare cases of HCV+ donor lungs transplanted into HCV+ recipients (n = 7) were excluded from the main analyses but included in a sub-analysis comparing HCV+ R, HCV+ D and the 7 HCV+ R/D cases.
Outcomes
The primary outcome was overall survival, defined as time from transplantation. Secondary outcomes included post-operative complications: airway dehiscence; dialysis; infection; stroke; or re-operation. We also examined 1- and 5-year survival, as well as cause of death. Due to significant improvements in the treatment of HCV since 2000, survival analysis was stratified into 2 temporal groups: 1994 to 1999 and 2000 to 2011.
Statistical methods
The Cochran–Armitage trend test was used to evaluate significant changes in utilization rates over time. A descriptive analysis examining institutional use of HCV cases was also performed. In both the analysis by HCV donor status and HCV recipient status, baseline donor and recipient characteristics, procedural details and clinical outcomes were summarized with frequency counts and percentages for categorical data and median and interquartile range (IQR) for continuous data. Comparisons were made using Pearson’s chi-square test and Fisher’s exact test for categorical data, and analysis of variance (ANOVA) for continuous data. Overall survival was assessed using Kaplan–Meier methods and the log-rank test.
To account for confounders (era and recipient medical condition at time of transplant) among patients undergoing lung transplantation from an HCV+ D vs HCV− D, we used Cox proportional hazards models. As a sensitivity analysis, a propensity score analysis with 3:1 matching was used after eliminating centers not performing a single HCV+ D transplant. In the analysis of HCV+ vs HCV− recipients, overall survival was compared using Kaplan–Meier survival curves and confirmed with Cox proportional hazards models to account for possible confounders, including donor diabetes, donor smoking history, recipient age, primary diagnosis, body mass index, functional status, cytomegalovirus mismatch, transplant era, transplant center volume and use of BOLT. p < 0.05 was considered statistically significant. Analyses were performed using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of 16,604 transplant recipients without HCV, 28 recipients (0.2%) were transplanted with lungs from an HCV+ D. This use decreased significantly over time, from 0.73% during 1994 to 1996 to 0.06% from 2000 to 2011 (p < 0.001 for temporal trend in annual rate). HCV+ D had higher rates of tobacco abuse (64.3% vs 20.0%; p < 0.001) and trended toward older age (median: 36 vs 30 years; p = 0.072), but were otherwise similar. Recipients from the HCV+ D group were more likely to be on life support, in the hospital or in the intensive care unit (Table 1).
Table 1.
Recipient Characteristics and Procedural Details by Donor HCV Status
| Overall | HCV+ donor | HCV− donor | p-value | |
|---|---|---|---|---|
| N | 16,604 | 28 (0.2%) | 16,576 (99.8%) | |
| Recipient characteristics | ||||
| Age, years (IQR) | 56 (45 to 61) | 52 (38 to 61) | 56 (45 to 61) | 0.236 |
| Male | 9,108 (54.9%) | 16 (57.1%) | 9,092 (54.9%) | 0.957 |
| Ethnicity | 0.881 | |||
| White | 14,341 (86.4%) | 26 (92.9%) | 14,315 (86.4%) | |
| Black | 1,251 (7.5%) | 2 (7.1%) | 1,249 (7.5%) | |
| Asian | 736 (4.4%) | 0 (0%) | 736 (4.4%) | |
| Other/missing | 276 (1.7%) | 0 (0%) | 276 (1.7%) | |
| BMI, kg/m2 (IQR) | 24.2 (20.6 to 27.8) | 24 (20 to 27) | 24 (21 to 28) | 0.507 |
| Primary diagnosis | 0.284 | |||
| Obstructive | 7,610 (45.8%) | 11 (39.3%) | 7,599 (45.8%) | |
| Restrictive | 5,979 (36%) | 9 (32.1%) | 5,970 (36%) | |
| CF/immunodeficiency | 2,269 (13.7%) | 5 (17.9%) | 2,264 (13.7%) | |
| Pulmonary vascular | 746 (4.5%) | 3 (10.7%) | 743 (4.5%) | |
| Diabetes | 2,081 (13.1%) | 2 (7.4%) | 2,079 (13.1%) | 0.569 |
| ADL requiring assistance | 10,867 (69.9%) | 17 (81%) | 10,850 (69.9%) | 0.345 |
| Lung allocation score (IQR) | 38.9 (34.3 to 47.9) | 45 (40 to 49) | 39 (34 to 48) | 0.939 |
| Pre-operative oxygen requirement, liters (IQR) | 2 (2 to 3) | 3 (2 to 4) | 2 (2 to 3) | 0.158 |
| 6-minute walk test <150 feet | 957 (11%) | 4 (16.7%) | 953 (11%) | 0.330 |
| Operative characteristics | ||||
| Type of lung transplant | 0.345 | |||
| Bilateral sequential | 9,087 (54.7%) | 12 (42.9%) | 9,075 (54.7%) | |
| Single left | 3,898 (23.5%) | 7 (25%) | 3,891 (23.5%) | |
| Single right | 3,619 (21.8%) | 9 (32.1%) | 3,610 (21.8%) | |
| HLA mismatch ≥5 | 8,215 (58.5%) | 8,195 (58.5%) | 20 (76.9%) | 0.088 |
| Life support at transplantation | 920 (5.5%) | 5 (17.9%) | 915 (5.5%) | 0.018 |
| Medical condition before transplant | 0.041 | |||
| Not hospitalized | 14,778 (89%) | 21 (75%) | 14,757 (89%) | |
| Hospitalized not in ICU | 1,004 (6%) | 4 (14.3%) | 1,000 (6%) | |
| In ICU | 822 (5%) | 3 (10.7%) | 819 (4.9%) | |
| Waitlist days (IQR) | 161 (46 to 441) | 133 (36 to 213) | 162 (46 to 441) | <0.001 |
| Ischemic time, hours (IQR) | 4.7 (3.6 to 5.8) | 4 (3 to 6) | 5 (4 to 6) | 0.993 |
| Center volume, total cases over study period (IQR) | 524 (312 to 695) | 655 (474 to 686) | 524 (312 to 695) | 0.328 |
| Transplant period | <0.001 | |||
| 1994 to 1990 | 3,889 (23.4%) | 20 (71.4%) | 3,869 (23.3%) | |
| 2000 to 2011 | 12,715 (76.6%) | 8 (28.6%) | 12,707 (76.7%) |
ADL, activities of daily living; BMI, body mass index; CF, XXXXXXXXXX; HCV, hepatitis C virus; HLA, human leukocyte antigen; ICU, intensive care unit; IQR, interquartile range.
Unadjusted analysis revealed minimal differences in short-term outcomes; however, overall survival was dramatically lower in the HCV+ D group (median survival: 1.3 vs 5.1 years; p = 0.002; Figure 1A). Adjusted Cox models reinforced the significantly higher mortality among HCV+ D patients (hazard ratio [HR] 1.66; 95% confidence interval [CI] 1.10 to 2.49; p = 0.015). Decreased overall survival in the HCV+ D group remained after propensity matching (median survival: 1.3 vs 4.8 years; p = 0.026; Figure 1B). When examining cause of death, the HCV+ D group had higher rates of hepatitis infection (8.3% vs 0.1%, p < 0.001; Table 2). The type of hepatitis infection was not defined in the database.
Figure 1.
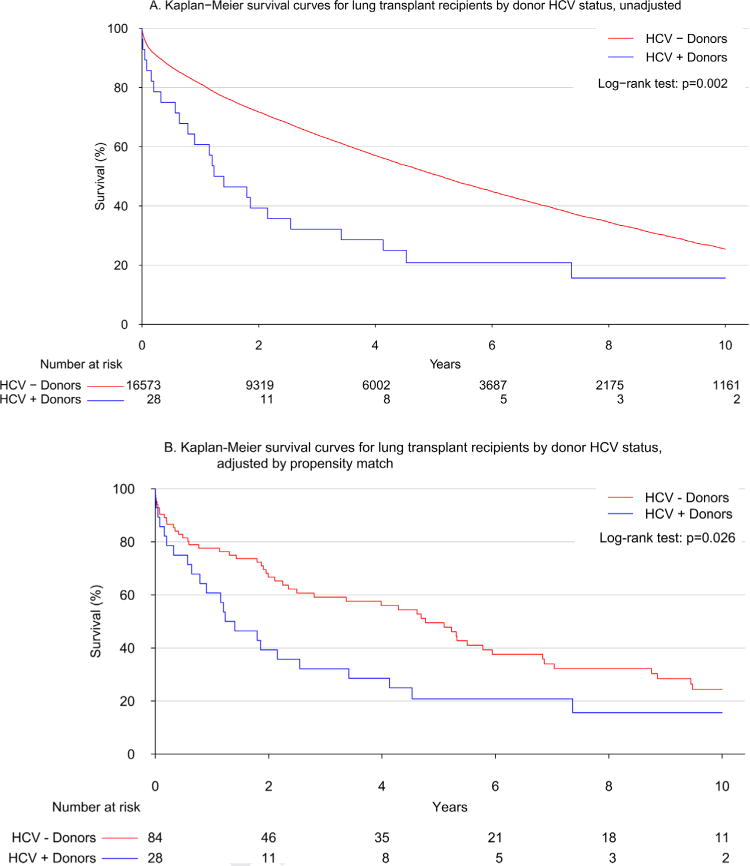
(A) Unadjusted Kaplan-Meier survival curve after lung transplantation by donor HCV status. Overall survival was significantly shorter in cases of lung transplantation from HCV+ donors by the log-rank test (p = 0.002). (B) Kaplan-Meier survival curve after lung transplantation by donor HCV status in the propensity-matched cohort. Overall survival was significantly shorter in cases of lung transplantation from HCV+ donors after propensity matching according to log-rank test (p = 0.026).
Table 2.
Outcomes After Lung Transplant by Donor HCV Status
| Overall | HCV+ donor | HCV− donor | p-value | |
|---|---|---|---|---|
| N | 16,604 | 28 (0.2%) | 16,576 (99.8%) | |
| Post-operative airway dehiscence | 212 (1.3%) | 0 (0%) | 212 (1.3%) | 0.999 |
| Post-operative dialysis | 889 (5.4%) | 2 (7.1%) | 887 (5.4%) | 0.663 |
| Post-operative infection requiring antibiotics | 4,315 (44%) | 13 (48.1%) | 4,302 (44%) | 0.812 |
| Post-operative stroke | 366 (2.2%) | 1 (3.7%) | 365 (2.2%) | 0.458 |
| Any other post-operative reoperation | 1,793 (18.3%) | 2 (7.4%) | 1,791 (18.3%) | 0.210 |
| Long-term survival | ||||
| 1-year survival, % (95% CI) | 81.2% (80.6–81.8%) | 60.7% (45.1–81.8%) | 81.2% (80.6–81.8%) | |
| 5-year survival, % (95% CI) | 50.6% (49.7–51.5%) | 20.8% (10.0–43.4%) | 50.6% (49.8–51.5%) | |
| Median survival, years (95% CI) | 5.1 (5.0–5.3%) | 1.3 (0.8–4.1%) | 5.1 (5.0–5.3%) | 0.002 |
| Cause of death | <0.001 | |||
| Graft failure | 1,586 (20.5%) | 5 (20.8%) | 1,581 (20.5%) | 0.999 |
| Pulmonary | 1,475 (19.1%) | 3 (12.5%) | 1,472 (19.1%) | 0.603 |
| Infection | 1,934 (25%) | 7 (29.2%) | 1,927 (25%) | 0.812 |
| Infection—hepatitis | 6 (0.1%) | 2 (8.3%) | 4 (0.1%) | <0.001 |
| Liver failure | 55 (0.7%) | 1 (4.2%) | 54 (0.7%) | 0.157 |
| Cardio- or cerebrovascular | 735 (9.5%) | 3 (12.5%) | 732 (9.5%) | 0.493 |
| Malignancy | 628 (8.1%) | 1 (4.2%) | 627 (8.1%) | 0.717 |
| Multiple-organ failure | 454 (5.9%) | 1 (4.2%) | 453 (5.9%) | 0.999 |
| Other | 869 (11.2%) | 1 (4.2%) | 868 (11.2%) | 0.511 |
p-values represent result of Pearson’s chi-square test or Fisher’s exact test as appropriate, with the exception of median survival, which was compared using the log-rank test.
p-values for cause of death represent the overall chi-square test, whereas the individual p-values compare each cause as a binary variable. The type of infectious hepatitis was not defined.
In the analysis based on recipient HCV status, 16,672 lung transplants from HCV-negative donors were available, and 1.7% of these recipients (n = 289) were HCV+. There were no significant changes in use of lung transplant in HCV+ R over time, going from 1.2% of all lung transplant cases from 1994 to 1996 to 1.4% from 2009 to 2011 (p = 0.794 for temporal trend in annual rate). Donor characteristics appeared similar between the groups (Table 3), but HCV+ R subjects were younger, more often black, and more likely to have independent functional status.
Table 3.
Donor, Recipient and Procedural Characteristics by Recipient HCV Status
| Overall | HCV+ recipient | HCV− recipient | p-value | |
|---|---|---|---|---|
| N | 16,672 | 289 (1.7%) | 16,383 (98.3%) | |
| Donor characteristics | ||||
| Age, years (IQR) | 30 (20 to 44) | 32 (20 to 45) | 30 (20 to 44) | 0.555 |
| BMI, kg/m2 (IQR) | 24.1 (21.6 to 27.1) | 25 (22 to 27) | 24 (22 to 27) | 0.340 |
| Tobacco abuse | 3,290 (19.9%) | 51 (17.8%) | 3,239 (19.9%) | 0.416 |
| Diabetes | 736 (4.4%) | 13 (4.5%) | 723 (4.4%) | 0.999 |
| PaO2, mm Hg (IQR) | 438 (333.4 to 504) | 445 (358 to 506) | 438 (333 to 504) | 0.618 |
| Recipient characteristics | ||||
| Age, years (IQR) | 56 (45 to 61) | 53 (43 to 59) | 56 (46 to 62) | 0.023 |
| Male | 9,147 (54.9%) | 170 (58.8%) | 8,977 (54.8%) | 0.192 |
| Ethnicity | <0.001 | |||
| White | 14,396 (86.3%) | 228 (78.9%) | 14,168 (86.5%) | |
| Black | 1,259 (7.6%) | 41 (14.2%) | 1,218 (7.4%) | |
| Asian | 740 (4.4%) | 14 (4.8%) | 726 (4.4%) | |
| Other/missing | 277 (1.7%) | 6 (2.1%) | 271 (1.7%) | |
| BMI, kg/m2 (IQR) | 24.2 (20.6 to 27.8) | 24 (20 to 28) | 24 (21 to 28) | 0.293 |
| Primary diagnosis | 0.191 | |||
| Obstructive | 7,649 (45.9%) | 146 (50.5%) | 7,503 (45.8%) | |
| Restrictive | 6,008 (36%) | 88 (30.4%) | 5,920 (36.1%) | |
| CF/immunodeficiency | 2,273 (13.6%) | 44 (15.2%) | 2,229 (13.6%) | |
| Pulmonary vascular | 742 (4.5%) | 11 (3.8%) | 731 (4.5%) | |
| Diabetes | 2,097 (13.1%) | 35 (12.4%) | 2,062 (13.1%) | 0.793 |
| ADL with assistance | 10,901 (69.8%) | 170 (62.5%) | 10,731 (69.9%) | 0.008 |
| Lung allocation score (IQR) | 38.9 (34.3 to 47.9) | 37 (34 to 44) | 39 (34 to 48) | 0.111 |
| Pre-operative oxygen requirement, liters (IQR) | 2 (2 to 3) | 2 (2 to 4) | 2 (2 to 3) | 0.345 |
| 6-minute walk test <150 feet | 961 (11.1%) | 17 (10.5%) | 944 (11.1%) | 0.916 |
| Operative characteristics | ||||
| Type of lung transplant | 0.210 | |||
| Bilateral sequential | 9,122 (54.7%) | 169 (58.5%) | 8,953 (54.6%) | |
| Single left | 3,914 (23.5%) | 69 (23.9%) | 3,845 (23.5%) | |
| Single right | 3,636 (21.8%) | 51 (17.6%) | 3,585 (21.9%) | |
| HLA mismatch ≥5 | 8,248 (58.5%) | 139 (57.4%) | 8,109 (58.5%) | 0.731 |
| Life support at transplantation | 920 (5.5%) | 14 (4.8%) | 906 (5.5%) | 0.706 |
| Medical condition before transplant | 0.817 | |||
| Not hospitalized | 14,844 (89%) | 260 (90%) | 14,584 (89%) | |
| Hospitalized not in ICU | 1,005 (6%) | 17 (5.9%) | 988 (6%) | |
| In ICU | 823 (4.9%) | 12 (4.2%) | 811 (5%) | |
| Waitlist days (IQR) | 161 (46 to 441) | 148 (54 to 401) | 162 (46 to 442) | 0.586 |
| Ischemic time, hours (IQR) | 4.7 (3.6 to 5.8) | 4 (3 to 6) | 5 (4 to 6) | 0.002 |
| Center volume, total cases over study period (IQR) | 524 (312 to 695) | 423 (252 to 655) | 524 (312 to 695) | 0.001 |
| Year of transplant | 0.236 | |||
| 1994 to 1999 | 3,891 (23.3%) | 59 (20.4%) | 3,832 (23.4%) | |
| 2000 to 2011 | 12,781 (76.7%) | 230 (79.6%) | 12,551 (76.6%) |
PaO2, partial pressure of oxygen. Refer to Table 1 for all other abbreviations.
In the unadjusted analysis, most short-term post-operative outcomes were similar (Table 4). In the pre-2000 period, survival was significantly worse in the HCV+ R group (median survival: 1.3 vs 4.5 years; p = 0.004). 2000 to 2011, however, survival between groups was similar (median survival: 4.4 vs 5.4 years, p = 0.100; Figure 2). In stratified, multivariable adjusted Cox proportional hazards models, recipients who were HCV+ before 2000 had significantly worse mortality (HR 2.5; 95% CI 1.2 to 5.2; p = 0.01), whereas the hazard for mortality for HCV+ R after 2000 was similar to their HCV− R counterparts (HR 1.2; 95% CI 0.97 to 1.4; p = 0.09). In the overall model, there was a significant interaction between HCV+ status and study period, indicating a significant change in the association of HCV status with survival from the pre-2000 to post-2000 period (p = 0.02). Compared with the post-2000 cohort, the pre-2000 point estimates for graft failure (20% vs 15%; p = 0.57) and infection rates (34% vs 30%; p = 0.73) were mildly increased in HCV+ R; however, no statistically significant differences were found within this limited sample size.
Table 4.
Outcomes After Lung Transplant by Recipient HCV Status
| Overall | HCV+ recipient | HCV− recipient | p-value | |
|---|---|---|---|---|
| N | 16,672 | 289 (1.7%) | 16,383 (98.3%) | |
| Post-operative airway dehiscence | 217 (1.3%) | 5 (1.8%) | 212 (1.3%) | 0.433 |
| Post-operative dialysis | 893 (5.4%) | 19 (6.6%) | 874 (5.4%) | 0.441 |
| Post-operative stroke | 369 (2.2%) | 8 (2.8%) | 361 (2.2%) | 0.682 |
| Post-operative infection | ||||
| requiring antibiotics | 4,317 (44%) | 95 (51.6%) | 4,222 (43.9%) | 0.042 |
| Any other post-operative reoperation | 1,798 (18.3%) | 41 (22.3%) | 1,757 (18.2%) | 0.188 |
| Long-term survival | ||||
| 1-year survival, % (95% CI) | 81.2% (80.6–81.8%) | 78.2% (73.5–83.3%) | 81.3% (80.7–81.9%) | |
| 5-year survival, % (95% CI) | 50.6% (49.7–51.4%) | 42.1% (36.0–49.1%) | 50.7% (49.8–51.6%) | |
| Median survival, years (95% CI) | 5.1 (5.0–5.3) | 3.8 (2.9–4.9) | 5.1 (5.0–5.3) | 0.005 |
| Cause of death | 0.368a | |||
| Graft failure | 1,590 (20.5%) | 25 (16.6%) | 1,565 (20.6%) | 0.267 |
| Pulmonary | 1,489 (19.2%) | 22 (14.6%) | 1,467 (19.3%) | 0.176 |
| Infection | 1,938 (25%) | 47 (31.1%) | 1,891 (24.9%) | 0.096 |
| Infection—hepatitis | 4 (0.1%) | 0 (0%) | 4 (0.1%) | 0.999 |
| Liver failure | 53 (0.7%) | 1 (0.7%) | 52 (0.7%) | 0.999 |
| Cardio- or cerebrovascular | 733 (9.5%) | 13 (8.6%) | 720 (9.5%) | 0.829 |
| Malignancy | 626 (8.1%) | 18 (11.9%) | 608 (8%) | 0.109 |
| Multiple-organ failure | 455 (5.9%) | 7 (4.6%) | 448 (5.9%) | 0.635 |
| Other | 868 (11.2%) | 18 (11.9%) | 850 (11.2%) | 0.875 |
p-values represent results of Pearson’s chi-square test or Fisher’s exact test as appropriate, with the exception of median survival, which was compared using the log-rank test. Results in parentheses for long-term survival represent 95% confidence interval (CI).
p-values for cause of death represent the overall chi-square test, while the individual p-values compare each cause as a binary variable. The type of infectious hepatitis was not defined.
Figure 2.
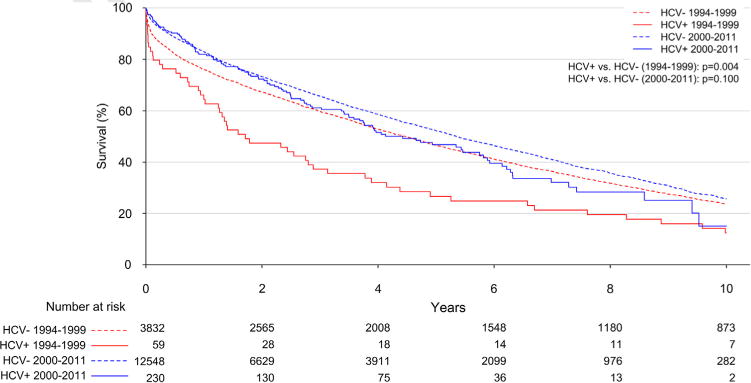
Unadjusted Kaplan–Meier survival curve after lung transplantation by recipient HCV status. Overall survival was similar in HCV+ vs HCV− recipients after 2000 by log-rank test (p = 0.100); however, HCV+ recipients had significantly decreased overall survival compared with HCV− recipients before 2000 according to log-rank test (p = 0.004).
Cases of HCV+ recipients with HCV+ donor lungs were extremely rare, representing <0.1% of cases (n = 7). Six of these were performed before 2003. After eliminating cases after 2003 to generate a less biased comparison, cases of HCV+ R (with HCV D; n = 103), HCV+ D (with HCV− R; n = 25) and HCV+ R/D (n = 6) were relatively similar in donor and recipient characteristics. Median waitlist days were considerably shorter for both HCV+ D (141 days) and HCV+ R/D (176 days) compared with HCV+ R with HCV− D (310 days; p < 0.001). One- and 5-year survival were not statistically different among cases of HCV+ R (71% and 34%), HCV+ D (60% and 23%) and HCV+ R/D (67% and 17%; p = 0.41), although the small sample size made significance difficult to achieve.
When examining institutional use of HCV+ D lungs, we found that 21% (n = 19) of lung transplant centers had used HCV+ D, compared with 71% of centers (n = 65) who had transplanted into HCV+ R. Since 2005, only 6% of centers (n = 4) have used HCV+ D.
Discussion
Lung transplant specialists are in the difficult position of weighing the needs of a challenging patient population and availability of a valuable but limited resource. The use of lung transplantation in HCV infection is rare, and this study we have described the trends in utilization, the patient population involved, and outcomes that can be expected. Use of HCV+ D in lung transplantation has decreased significantly, with only 3 cases recorded since 2005, whereas cases in HCV+ R have remained stable, near 1.7%. The dramatically worse outcomes after lung transplantation from HCV+ D, with median survival of 1.3 vs 5.1 years, could not be accounted for after adjusting for confounders. Alternatively, use of lung transplantation in HCV+ vs HCV− recipients did not demonstrate significant differences in overall survival since 2000, an improvement from earlier study periods. Given improvements in diagnostic and therapeutic options for HCV, this issue may need to be re-addressed when the population-level benefits of newer drugs, such as sofosbuvir and simeprevir, have been realized.12
Studies of renal transplantation have indicated higher rates of graft failure and mortality in HCV+ R, due potentially to increased complications of the HCV infection and immunosuppression.15,16 More limited studies in cardiac transplantation have identified evidence of worse outcomes in HCV+ candidates, although the largest adjusted analysis did not reach statistical significance.8,17 Nonetheless, experts have been hesitant to recommend HCV+ candidates for transplantation.18 Data on transplantation with HCV+ D are even more limited. Although the use of HCV+ kidneys has been restricted to HCV+ R, the use of HCV+ organs for other types of non-liver transplantation is less clear.18
Current guidelines allow for consideration of lung transplantation in candidates with HCV infection where the infection is stable on therapy and no evidence of cirrhosis and portal hypertension exist.1 A small case series demonstrated promising results in lung transplant candidates treated with aggressive pre-transplant anti-viral therapy.19 With the recent advances in anti-viral HCV therapy,9 it is not surprising that HCV seropositivity was not associated with worse long-term outcomes after lung transplant in multiple post-2000 cohorts.11,20
Our results in the HCV+ vs HCV-recipient comparison confirms previous findings from the UNOS database during the period 2000 to 2007.11 That report indicated a stable rate for HCV+ R in lung transplantation. Our current study adds 4 years of more recent data and examines temporal trends going back to 1994, demonstrating stable use throughout this period. We have also provided an analysis of the interaction between HCV status and study period that showed significant improvement in outcomes for HCV+ recipients from the pre-2000 to post-2000 period. Survey data indicated that two thirds of transplant centers are willing to transplant a lung into a HCV-seropositive recipient; however, <20% indicated a willingness to transplant in HCV viremic patients.11 These data also show that HCV RNA testing became routine practice 10 to 15 years ago. The advent of reliable HCV RNA testing to identify and exclude potential viremic recipients may explain the improved outcomes in HCV+ recipients since 2000. With no significant differences in cause of death among HCV+ recipients from the pre- to post-2000 periods, this theory remains speculative based solely on a temporal association.
In contrast to the highly controlled and deliberate setting of recipient work-up for lung transplantation, the urgent and time-sensitive nature of lung transplant donor evaluation makes HCV RNA testing more problematic. Although selective testing for donor HCV RNA levels is performed,21 there are few data to guide clinicians on the use of HCV+ lungs,18 with the limited data reflecting poor outcomes for recipients of these organs.22 Lack of data in this area and the lack of routine assessment for active donor HCV infection may explain 2 of the primary findings of our HCV+ D analysis, namely: (1) the significant decrease in the use of HCV-seropositive donor lungs; and (2) the worse outcomes among patients receiving HCV+ lungs. With only 3 reported cases of transplantation using HCV+ lungs since 2005, it appears that transplant teams have all but stopped using this source of donation.
The outcomes among patients receiving HCV+ lungs supports this decreased use, with median survival of 1.3 years compared with 5.1 years after HCV− lungs. Our analysis indicates that recipients of HCV+ lungs were sicker at the time of transplant. However, even after adjustment for these factors through 2 methods, differences in survival between these groups remain striking. The increased time to transplant in patients receiving HCV− lungs, a difference that was exaggerated in the propensity-matched analysis, may represent the increased wait time associated with waiting for a HCV− lung. Despite longer wait times, recipients of HCV− lungs had improved overall survival compared with those receiving HCV+ lungs. Although the cohort was limited by sample size and pre-2003 data, making strong conclusions impossible, unadjusted comparison of HCV+ R with HCV− D to HCV+ R with HCV+ D demonstrated similar overall survival. With survey data suggesting that >60% of centers accepting HCV+ D restrict their use to HCV+ R,10 this strategy may deserve further examination as a method to expand limited donor availability.
Due to the severely limited cohort of lung transplant cases with HCV+ D, we were unable to analyze changes in outcomes over time. The improvements seen in the HCV+ R of lung transplantation may also be present in recipients of HCV+ D; however, significant decreases in use of HCV+ D over time did not permit this analysis. Results indicating increased infectious hepatitis and possibly increased liver failure as the cause of death suggest that HCV+ D status may have played a role in these worse outcomes, although the type of hepatitis infection leading to death could not be evaluated in our analysis. These results may justify decreased use of HCV+ D over time. Although survey data from 1999 suggest that 55% of centers were willing to consider the use of HCV+ D,10 the actual use of this resource has been much more limited, with only 6% of centers using HCV+ D since 2005.
With improved treatment for HCV, which may lead to cure rates of >90%, a significant improvement from current interferon-β cure rate of 50%,9,12 HCV seropositivity may be less indicative of viremia in both HCV+ R and D populations, allowing for increased use of lung transplantation in these cases. With significant cost barriers to widespread use of these improved therapies, we must await the population-level impact of these new regimens before reassessing use of HCV+ lung donation.
Our study has several limitations to consider when interpreting the results, due to its observational nature and limited sample of HCV+ D cases. Unmeasured confounders could not be adjusted for and may have biased our results, although the UNOS database provides an extensive set of variables. Cases in which HCV+ lungs were not used and the transplant candidate subsequently died before surgery would have been excluded, potentially favoring HCV− D; however, by excluding patients from centers where no HCV+ lungs were transplanted, we assured that centers were willing to transplant HCV+ lungs. Data on the number of patients denied access to the waitlist due to HCV status could not be addressed, and the number of HCV+ D lungs rejected due to HCV status is unavailable.
The present results demonstrate that lung transplantations from HCV+ D have significantly worse outcomes compared to those with HCV− D, suggesting that decreased use of HCV+ lungs may be the appropriate response. On the other hand, outcomes among HCV+ R since 2000 have approximated those of their HCV− counterparts after lung transplantation, an improvement over HCV+ R before 2000. Although these results support the use of lung transplantation in HCV+ R and call for extreme caution when using transplant lungs from HCV+ D, these questions will need to be revisited after the widespread implementation of new HCV therapies that hold potential to dramatically decrease the prevalence of active HCV infection.
Acknowledgments
B.R.E. is partially supported by the National Institutes of Health (Grant U01 HL088942).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose
This study was presented at the 34th annual meeting and scientific sessions of the International Society for Heart and Lung Transplantation, April 2014, San Diego, California.
References
- 1.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Maurer JR, Frost AE, Estenne M, et al. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Transplantation. 1998;66:951–6. doi: 10.1097/00007890-199810150-00033. [DOI] [PubMed] [Google Scholar]
- 3.van Trigt P, Davis RD, Shaeffer GS, et al. Survival benefits of heart and lung transplantation. Ann Surg. 1996;223:576–84. doi: 10.1097/00000658-199605000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geertsma A, ten Vergert EM, Bonsel GJ, et al. Does lung trans-plantation prolong life? A comparison of survival with and without transplantation. J Heart Lung Transplant. 1998;17:511–6. [PubMed] [Google Scholar]
- 5.Chaparro C, Scavuzzo M, Winton T, et al. Status of lung transplant recipients surviving beyond five years. J Heart Lung Transplant. 1997;16:511–6. [PubMed] [Google Scholar]
- 6.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 annual data report: Lung: Department of Health and Human Services, Health Resources and Services Administration Healthcare Systems Bureau, Division of Transplantation. 2012 http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/06_lung_12.pdf/. Accessed March 26,2014.
- 7.Fong TL, Valinluck B, Govindarajan S, et al. Short-term prednisone therapy affects aminotransferase activity and hepatitis C virus RNA levels in chronic hepatitis C. Gastroenterology. 1994;107:196–9. doi: 10.1016/0016-5085(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 8.Delgado J, Munoz de Bustillo E, Ibarrola C, et al. Hepatitis C virus −related fibrosing cholestatic hepatitis after cardiac transplantation: is azathioprine a contributory factor? J Heart Lung Transplant. 1999;18:607–10. doi: 10.1016/s1053-2498(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotler SJ, Jensen DM, Kesten S. Hepatitis C virus infection and lung transplantation: a survey of practices. J Heart Lung Transplant. 1999;18:456–9. doi: 10.1016/s1053-2498(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 11.Fong TL, Cho YW, Hou L, et al. Outcomes after lung transplantation and practices of lung transplant programs in the United States regarding hepatitis C seropositive recipients. Transplantation. 2011;91:1293–6. doi: 10.1097/TP.0b013e3182193cd3. [DOI] [PubMed] [Google Scholar]
- 12.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–87. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 13.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 annual data report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [Google Scholar]
- 14.Daily OP, Kauffman HM. Quality control of the OPTN/UNOS Transplant Registry. Transplantation. 2004;77:1309. doi: 10.1097/01.tp.0000120943.94789.e4. [DOI] [PubMed] [Google Scholar]
- 15.Fabrizi F, Martin P, Dixit V, et al. Hepatitis C virus antibody status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5:1452–61. doi: 10.1111/j.1600-6143.2005.00864.x. [DOI] [PubMed] [Google Scholar]
- 16.Contreras AM, Monteon FJ, Flores MR, et al. Drug-related hepatotoxicity in a renal transplant recipient with long-term survival and hepatitis C. Ann Hepatol. 2007;6:70–3. [PubMed] [Google Scholar]
- 17.Fong TL, Hou L, Hutchinson IV, et al. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88:1137–41. doi: 10.1097/TP.0b013e3181bd3e59. [DOI] [PubMed] [Google Scholar]
- 18.Carbone M, Mutimer D, Neuberger J. Hepatitis C virus and nonliver solid organ transplantation. Transplantation. 2013;95:779–86. doi: 10.1097/TP.0b013e318273fec4. [DOI] [PubMed] [Google Scholar]
- 19.Doucette KE, Weinkauf J, Sumner S, et al. Treatment of hepatitis C in potential lung transplant candidates. Transplantation. 2007;83:1652–5. doi: 10.1097/01.tp.0000264561.18380.22. [DOI] [PubMed] [Google Scholar]
- 20.Sahi H, Zein NN, Mehta AC, et al. Outcomes after lung transplantation in patients with chronic hepatitis C virus infection. J Heart Lung Transplant. 2007;26:466–71. doi: 10.1016/j.healun.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Viral hepatitis guidelines in hemodialysis and transplantation. Am J Transplant. 2004;4(suppl 10):72–82. doi: 10.1111/j.1600-6135.2004.00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartwig MG, Patel V, Palmer SM, et al. Hepatitis B core antibody positive donors as a safe and effective therapeutic option to increase available organs for lung transplantation. Transplantation. 2005;80:320–5. doi: 10.1097/01.tp.0000165858.86067.a2. [DOI] [PubMed] [Google Scholar]


