Abstract
Background
High level physical activity is associated with lower colorectal cancer mortality, likely through insulin sensitization. IRS1 (insulin receptor substrate 1) is a mediator of insulin and insulin-like growth factor (IGF) signaling pathways, and its down-regulation is associated with insulin resistance. Therefore, we hypothesized that tumor IRS1 expression status might modify cellular sensitivity to insulin and IGF, and the prognostic association of physical activity.
Methods
We assessed IRS1 expression level in 371 stage I–III rectal and colon cancers in the Nurses’ Health Study and the Health Professionals Follow-up Study by immunohistochemistry. In survival analysis, Cox proportional hazards model was used to assess an interaction between post-diagnosis physical activity (ordinal scale of sex-specific quartiles Q1 to Q4) and IRS1 expression (ordinal scale of negative, low, and high), controlling for potential confounders including microsatellite instability, CpG island methylator phenotype, LINE-1 methylation level, and KRAS, BRAF and PIK3CA mutation status.
Results
There was a statistically significant interaction between post-diagnosis physical activity and tumor IRS1 expression in colorectal cancer-specific mortality analysis (Pinteraction=0.005). Multivariable hazard ratio (95% confidence interval) for higher post-diagnosis physical activity (Q3–Q4 vs. Q1–Q2) was 0.15 (0.02–1.38) in IRS1-negative group, 0.45 (0.19–1.03) in IRS1-low group, and 1.32 (0.50–3.53) in IRS1-high group.
Conclusions
The association of post-diagnosis physical activity with colorectal carcinoma patient survival may differ by tumor IRS1 expression level. If validated, tumor IRS1 expression status may serve as a predictive marker to identify subgroups of patients who might gain greater survival benefit from increased level of exercise.
Keywords: Carcinoma, Colon Cancer, Energy Metabolism, Metabolism, Public Health
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of cancer death worldwide, and its complex, heterogeneous etiology has not been fully elucidated. Insulin resistance may be causally linked to CRC incidence1–8 and cancer survival,9,10 whereas, physical activity may reduce CRC risk11–17 and mortality.18–23 Although the underlying mechanism remains uncertain, accumulating evidence suggests that physical activity may influence CRC patient survival by increasing insulin sensitivity.24,25
IRS1 (insulin receptor substrate 1; the HUGO-approved official symbol; HGNC ID: 6215) is cytoplasmic substrate of the insulin receptor (INSR) and insulin-like growth factor 1 receptor (IGF1R) signaling pathways.26–28 IRS1 mediates glucose homeostasis29,30 as well as proliferative and anti-apoptotic function of insulin and IGF1 by transmitting signals from the activated receptors to downstream effectors.31 IRS1 also plays prominent roles in human malignancy and is activated in various human cancers, including CRC.26,27,32–37
Considering the possible effect of physical activity on insulin sensitization, and the roles of IRS1 in insulin resistance, we hypothesized that the tumor IRS1 expression level might influence the prognostic association of post-diagnosis physical activity with patient survival in CRC. To test this hypothesis, we designed a molecular pathological epidemiology (MPE) study to assess statistical interaction between post-diagnosis physical activity and tumor IRS1 expression levels in analysis of CRC-specific survival, controlling for major tumor molecular features, including microsatellite instability (MSI), CpG island methylator phenotype (CIMP), and KRAS, BRAF, and PIK3CA mutations.
MATERIALS AND METHODS
Study Population and Ascertainment of Cases
We used two U.S. nationwide prospective cohort studies, the Nurses’ Health Study (NHS, N=121,701 women observed since 1976) and the Health Professionals Follow-Up Study (HPFS, N=51,529 men observed since 1986).38,39 Collection of clinical information and tumor tissue is described in Supplementary Materials. Written informed consent was obtained from all study participants. Tissue collection and analyses were approved by the Human Subjects Committees at Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital.
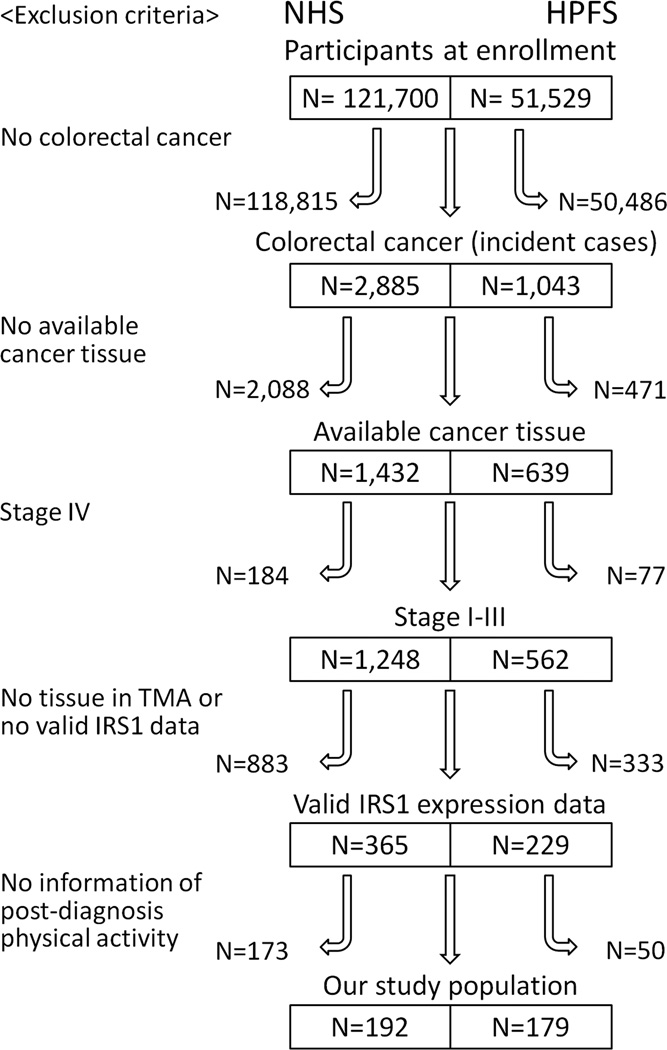
A total of 371 stage I-III CRC cases diagnosed by 2008 were included in this study based on the availability of tissue, IRS1 expression data, and post-diagnosis physical activity data (Figure 1). Cases of stage IV CRCs were excluded from the analyses to minimize bias related to differential reporting of physical activity data according to severity of disease.40 Patients were observed until death, or January 2012, whichever came first. Death was ascertained by use of the National Death Index. Study physicians, unaware of exposure information, reviewed medical and pathological records to retrieve information on tumor location and disease stage.
Figure 1. Flow chart of case selection.
Among the incident cases of colorectal cancer, those with one or more of the followings were excluded from this study: no available cancer tissue, stage IV, no tissue in tissue microarray or valid IRS1 data, or no information of post-diagnosis physical activity. (HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study, TMA, tissue microarray)
Assessment of Physical Activity
Leisure time physical activity was evaluated every two years, and validated against physical activity diaries.41 Participants reported the duration of physical activity (ranging from 0–11 or more hours/week) engaged in walking at usual pace, jogging, running, bicycling, swimming laps, racket sports, other aerobic exercises, lower intensity exercise (yoga, toning, stretching), or other vigorous activities.41 Each activity on the questionnaire was assigned a metabolic equivalent task score (METS). METS is defined as the ratio of the metabolic rate of specific activities to the resting metabolic rate; one METS is the energy expenditure for sitting quietly.42,45 The METS from the individual activities were summed to yield a total METS hours/week. To avoid the period of active anticancer treatment, the first assessment of post-diagnosis physical activity was conducted between 1 year and 4 years after the diagnosis of CRC (median, 17 months).41 To minimize bias due to declining physical activity in the period around cancer recurrence or death, physical activity was assessed at a single point of time after the diagnosis of CRC and not updated thereafter.20,40 To minimize bias associated with occult cancer recurrence, we excluded deaths within 6 months of the activity assessment.
We classified post-diagnosis physical activity level (METS/week) into sex-specific quartiles (Q1, the lowest, to Q4, the highest), considering that the distribution of physical activity level considerably differed between men and women.42,44 We primarily used a combined cohort of men and women to maximize statistical power. As a secondary analysis, we examined the relation between post-diagnosis physical activity and patient survival in strata of tumor IRS1 expression level (negative, low, or high) in each cohort, and confirmed consistency in results between men and women.
Analyses of BRAF, KRAS, PIK3CA Mutations, Microsatellite Instability (MSI), CpG Island Methylator Phenotype (CIMP), and LINE-1 Methylation
Tumor molecular features of CRCs were analyzed as previously described for KRAS,43,44 BRAF,45 PIK3CA,46,47 CIMP (and MSI),48–50 and LINE-1 methylation51,52 (see Supplementary Materials).
Immunohistochemistry
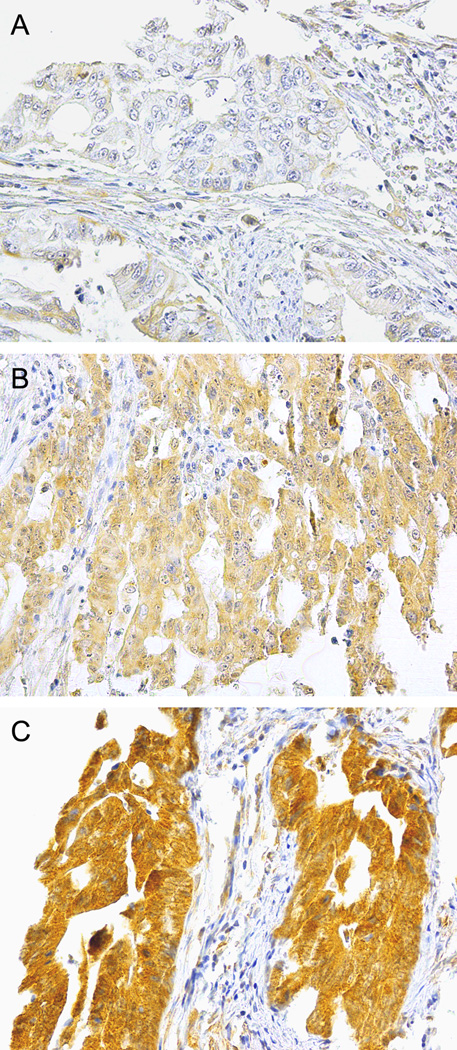
Tissue microarray was constructed as described.38 Immunostaining methods for CTNNB1 (β-catenin),53 TP53,54 and PTGS2 (COX-2)55 were previously described. IRS1 and IRS2 immunostaining procedures are described in Supplementary Materials. Cytoplasmic IRS1 and IRS2 expression status were classified as negative, low, or high (Figure 2).
Figure 2. IRS1 immunohistochemical analysis in colorectal cancer cells.
(A) Colorectal cancer cells with no/little staining for IRS1 are classified to be IRS1-negative. (B) Weak staining for IRS1 in cytoplasm of colorectal cancer cells indicates low-level IRS1 expression. (C) Strong staining for IRS1 in cytoplasm of colorectal cancer cells indicates high-level IRS1 expression.
IRS1 and IRS2 expression levels in all cases were interpreted by a pathologist (T.M.). A random group of 122 cases was independently reviewed by a second pathologist (S.A.K.). Both pathologists were unaware of other data. Concordance between the two pathologists indicated substantial agreement for both IRS1 status (three levels) (weighted κ=0.69; P<0.001) and IRS2 status (three levels) (weighted κ=0.77; P<0.001).
Statistical Analysis
Detailed statistical methodologies are described in Supplementary Materials. All statistical analyses were conducted using SAS software (version 9.3, SAS Institute, Cary, NC, USA). All P-values were two-sided. Our primary hypothesis testing was assessment of the interaction between post-diagnosis physical activity and tumor IRS1 expression level in CRC-specific survival analysis in the combined cohort. All other analyses and hypothesis testing in this study were secondary analyses, and therefore, results were interpreted cautiously. In particular, we were aware of multiple testing inherent in the subgroup analyses of prognostic associations of physical activity in strata of IRS1 status. To test differences in the frequency distribution of categorical data, the chi-square test was performed. One-way analysis of variance (ANOVA) was used to compare mean age and mean LINE-1 methylation level.
For the primary endpoint CRC-specific survival, participants were censored at the time of death if death was not due to CRC. Multivariable Cox proportional hazards regression models were used to control for potential confounders, and were stratified by stage and sex to limit the number of variables in multivariable models. A statistical interaction was assessed by a likelihood ratio test, using the cross-product of post-diagnosis physical activity (ordinal variable of four categories: Q1, Q2, Q3 and Q4) and IRS1 status (three ordinal categories of negative, low, and high level) as the interaction term. Pinteraction value was calculated by comparing the model with the interaction term to the model without the interaction term. We used an initial model including the interaction term, post-diagnosis physical activity, tumor IRS1 status, and other possible covariates, and conducted a backward elimination procedure.
In addition to regression models for the interaction term, in our secondary analysis, we calculated survival hazard ratio (HR) for high post-diagnosis physical activity (vs. low activity) in each stratum of IRS1 expression level (negative, low, or high). We divided post-diagnosis physical activity into two categories (Q1–Q2 as low level and Q3–Q4 as high level).
Combined categories of disease stage (I, II, III, missing) and sex were used as a stratifying variable using the “strata” option in the SAS “proc phreg” command to minimize residual confounding and overfitting. The proportionality of hazards assumption was evaluated using a time-dependent variable, which was cross-product of IRS1 variable and survival time (all P-values >0.20).
RESULTS
Frequency of clinical, pathologic, and molecular features of 371 stage I-III colorectal cancers (CRCs) included in this study are summarized in Table 1 (the features in each cohort in Tables S1 and S2) according to post-diagnosis physical activity quartiles. There was no significant association between tumor IRS1 expression status and post-diagnosis physical activity in both cohorts.
Table 1.
Clinical, pathologic, and molecular characteristics in colorectal cancer cases according to post-diagnosis physical activity
| Clinical, pathologic, or molecular characteristics |
Total No. | Post-diagnosis physical activity quartile* | P† | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| All cases | 371 | 91 | 91 | 95 | 94 | |
| Sex | 0.95 | |||||
| Male (HPFS) | 179 (48%) | 43 (47%) | 46 (51%) | 45 (47%) | 45 (48%) | |
| Female (NHS) | 192 (52%) | 48 (53%) | 45 (49%) | 50 (53%) | 49 (52%) | |
| Age, years (mean ± SD) | 67.6 ± 8.1 | 68.9 ± 8.8 | 67.7 ± 8.9 | 67.0 ± 7.7 | 66.7 ± 6.9 | 0.24‡ |
| Year of diagnosis | 0.13 | |||||
| Prior to 1996 | 168 (45%) | 33 (36%) | 45 (49%) | 44 (46%) | 46 (49%) | |
| 1996 to 2008 | 203 (55%) | 58 (64%) | 46 (51%) | 51 (54%) | 48 (51%) | |
| Body mass index, kg/m2 | 0.061 | |||||
| <30 | 309 (83%) | 71 (78%) | 75 (82%) | 80 (84%) | 83 (88%) | |
| ≥30 | 62 (17%) | 20 (22%) | 16 (18%) | 15 (16%) | 11 (12%) | |
| Family history of colorectal cancer in first degree relatives | 0.66 | |||||
| Absent | 288 (78%) | 70 (77%) | 66 (73%) | 81 (85%) | 71 (76%) | |
| Present | 83 (22%) | 21 (23%) | 25 (28%) | 14 (15%) | 23 (25%) | |
| Tumor location | 0.18 | |||||
| Caecum | 70 (19%) | 17 (19%) | 14 (15%) | 20 (22%) | 19 (20%) | |
| Ascending and transverse colon | 102 (28%) | 23 (25%) | 24 (26%) | 31 (33%) | 24 (26%) | |
| Splenic flexure to sigmoid colon | 117 (32%) | 25 (28%) | 33 (36%) | 25 (27%) | 34 (36%) | |
| Rectum | 80 (22%) | 26 (29%) | 20 (22%) | 17 (18%) | 17 (18%) | |
| Cancer stage | 0.11 | |||||
| I | 96 (28%) | 25 (30%) | 18 (21%) | 26 (29%) | 27 (31%) | |
| II | 136 (40%) | 27 (33%) | 35 (42%) | 36 (40%) | 38 (43%) | |
| III | 112 (33%) | 30 (37%) | 31 (37%) | 28 (31%) | 23 (26%) | |
| Tumor grade | 0.36 | |||||
| Low | 348 (94%) | 88 (97%) | 86 (95%) | 85 (90%) | 89 (95%) | |
| High | 22 (5.9%) | 3 (3.3%) | 5 (5.5%) | 9 (9.6%) | 5 (5.3%) | |
| MSI status | 0.56 | |||||
| MSI-low/MSS | 303 (82%) | 74 (81%) | 74 (83%) | 74 (79%) | 81 (86%) | |
| MSI-high | 65 (18%) | 17 (19%) | 15 (17%) | 20 (21%) | 13 (14%) | |
| MLH1 promoter hypermethylation | 0.33 | |||||
| Negative | 317 (86%) | 76 (85%) | 77 (86%) | 79 (83%) | 85 (91%) | |
| Positive | 50 (14%) | 13 (15%) | 13 (14%) | 16 (17%) | 8 (8.6%) | |
| CIMP status | 0.60 | |||||
| CIMP-negative | 165 (45%) | 39 (44%) | 42 (47%) | 40 (42%) | 44 (47%) | |
| CIMP-low | 148 (40%) | 34 (38%) | 38 (42%) | 38 (40%) | 38 (41%) | |
| CIMP-high | 54 (15%) | 16 (18%) | 10 (11%) | 17 (18%) | 11 (12%) | |
| LINE-1 methylation, % (mean ± SD) | 60.9 ± 9.7 | 60.0 ± 10.6 | 59.1 ± 9.4 | 61.8 ± 9.7 | 62.6 ± 8.7 | 0.052‡ |
| BRAF mutation | 0.31 | |||||
| Negative | 330 (90%) | 83 (92%) | 83 (92%) | 81 (87%) | 83 (89%) | |
| Positive | 36 (9.8%) | 7 (7.8%) | 7 (7.8%) | 12 (13%) | 10 (11%) | |
| KRAS mutation | 0.45 | |||||
| Negative | 212 (57%) | 54 (59%) | 51 (57%) | 58 (61%) | 49 (52%) | |
| Positive | 158 (43%) | 37 (41%) | 39 (43%) | 37 (39%) | 45 (48%) | |
| PIK3CA mutation | 0.13 | |||||
| Negative | 285 (84%) | 71 (87%) | 72 (89%) | 73 (81%) | 69 (80%) | |
| Positive | 54 (16%) | 11 (13%) | 9 (11%) | 17 (19%) | 17 (20%) | |
| TP53 expression | 0.30 | |||||
| Negative | 209 (57%) | 53 (58%) | 44 (48%) | 54 (58%) | 58 (63%) | |
| Positive | 158 (43%) | 38 (42%) | 47 (52%) | 39 (42%) | 34 (37%) | |
| CTNNB1 (β-catenin) expression (nuclear) | 0.12 | |||||
| Negative | 178 (50%) | 49 (58%) | 43 (48%) | 47 (53%) | 39 (43%) | |
| Positive | 176 (50%) | 36 (42%) | 47 (52%) | 42 (47%) | 51 (57%) | |
| PTGS2 (COX-2) expression | 0.48 | |||||
| Negative | 141 (38%) | 37 (41%) | 34 (37%) | 38 (40%) | 32 (34%) | |
| Positive | 229 (62%) | 54 (59%) | 57 (63%) | 57 (60%) | 61 (66%) | |
| IRS1 expression | 0.61 | |||||
| Negative | 34 (9.2%) | 6 (6.6%) | 8 (8.8%) | 11 (12%) | 9 (9.6%) | |
| Low | 221 (60%) | 57 (63%) | 64 (70%) | 46 (48%) | 54 (58%) | |
| High | 116 (31%) | 28 (31%) | 19 (21%) | 38 (40%) | 31 (33%) | |
| IRS2 expression | 0.22 | |||||
| Negative | 28 (7.9%) | 6 (7.1%) | 10 (11%) | 9 (9.7%) | 3 (3.4%) | |
| Low | 207 (58%) | 50 (59%) | 55 (62%) | 51 (55%) | 51 (58%) | |
| High | 120 (34%) | 29 (34%) | 24 (27%) | 33 (35%) | 34 (39%) | |
CIMP, CpG island methylator phenotype; HPFS, Health Professionals Follow-up Study; LINE-1, long interspersed nucleotide element 1; METS, metabolic equivalent task score; MSI, microsatellite instability; MSS, microsatellite stable; NHS, Nurses’ Health Study; SD, standard deviation
Q1, Q2, Q3, and Q4 represent sex-specific quartile of post-diagnosis physical activity. Male patients were grouped as follows: Q1, 0≤METS hour/week<6.1; Q2, 6.1≤METS hour/week<18.3; Q3, 18.3≤METS hour/week<46.3; Q4, ≥46.3 METS hour/week. Female patients were grouped as follows: Q1, 0≤METS hour/week<2.5; Q2, 2.5≤METS hour/week<7.7; Q3, 7.7≤METS hour/week<18.4; Q4, ≥18.4 METS hour/week.
P-values were calculated by Chi-square test. These P-values do not relate to the primary hypothesis testing, hence, Bonferroni-correction for multiple hypothesis testing was not performed.
ANOVA was used to compare the means of age and LINE-1 methylation.
During follow-up of CRC patients (with a median of 15.1 years), there were 168 deaths, including 52 CRC-specific deaths. Tumor IRS1 expression status was not significantly associated with either CRC-specific or overall survival in univariable or multivariable analysis (Table 2).
Table 2.
Colorectal cancer mortality by IRS1 expression level
| Colorectal cancer-specific mortality | Overall mortality | ||||||
|---|---|---|---|---|---|---|---|
| IRS1 expression |
No. of cases |
No. of events |
Univariable HR (95% CI) |
Multivariable HR* (95% CI) |
No. of events |
Univariable HR (95% CI) |
Multivariable HR* (95% CI) |
| Negative | 34 | 5 | 1 (reference) | 1 (reference) | 17 | 1 (reference) | 1 (reference) |
| Low | 221 | 28 | 0.91 (0.35–2.36) | 0.89 (0.34–2.32) | 91 | 0.90 (0.53–1.51) | 1.02 (0.60–1.75) |
| High | 116 | 19 | 1.19 (0.44–3.18) | 1.18 (0.44–3.18) | 58 | 1.14 (0.66–1.96) | 1.36 (0.76–2.26) |
| Ptrend† | 0.50 | 0.49 | 0.31 | 0.21 | |||
CI, confidence interval; HR, hazard ratio
Combined categories of disease stage (I, II, III, missing) and sex were used as a stratifying variable. Selection of covariates included in the final model is described in Materials and Methods.
Ptrend values were calculated using IRS1 expression levels as an ordinal categorical variable in a proportional hazards model.
Our primary aim was to examine an interaction between post-diagnosis physical activity level and tumor IRS1 expression in CRC-specific survival analysis. There was a significant interaction between post-diagnosis physical activity and IRS1 expression status in univariable and multivariable CRC-specific survival analyses (Pinteraction=0.005 in both analyses, Table 3). In each stratum of IRS1 expression level, we calculated survival hazard ratio (HR) for high post-diagnosis physical activity (Q3–Q4 vs. Q1–Q2). In multivariable analyses, CRC-specific survival HR (95% confidence interval [CI]) for high post-diagnosis physical activity (vs. low activity) was 0.15 (0.02–1.38) in IRS1-negative CRC, 0.45 (0.19–1.03) in IRS1-low CRC, and 1.32 (0.50–3.53) in IRS1-high CRC. In a combined group of the IRS1-negative and IRS1-low strata, CRC-specific survival HR (95% CI) for high post-diagnosis physical activity was 0.39 (0.17–0.82) (Table 3). As an exploratory survival analysis, we examined an interaction between post-diagnosis physical activity level and tumor IRS1 expression among colon cancer cases (Table S3). Similar to the results using all CRC cases, we demonstrated a statistically significant interaction between post-diagnosis physical activity and IRS1 expression level in colon cancer-specific survival analysis.
Table 3.
Post-diagnosis physical activity and colorectal cancer mortality in strata of tumor IRS1 expression level
| Colorectal cancer-specific mortality HR for high level of physical activity (Q3–Q4 vs. Q1–Q2)* |
Overall mortality HR for high level of physical activity (Q3–Q4 vs. Q1–Q2)* |
|||||||
|---|---|---|---|---|---|---|---|---|
| IRS1 expression | No. of cases |
No. of events |
Univariable HR (95% CI) |
Multivariable HR† (95% CI) |
No. of events |
Univariable HR (95% CI) |
Multivariable HR† (95% CI) |
|
| Negative | 34 | 5 | 0.16 (0.02–1.46) | 0.15 (0.02–1.38) | 17 | 0.62 (0.24–1.61) | 0.53 (0.20–1.39) | |
| Low | 221 | 28 | 0.44 (0.19–1.00) | 0.45 (0.19–1.03) | 94 | 0.58 (0.38–0.84) | 0.71 (0.46–1.11) | |
| High | 116 | 19 | 1.38 (0.52–3.62) | 1.32 (0.50–3.53) | 58 | 0.64 (0.38–1.07) | 0.77 (0.45–1.32) | |
| Pinteraction‡ | 0.004 | 0.005 | 0.29 | 0.14 | ||||
| Secondary analysis (combined IRS1-negative and IRS-low strata) | ||||||||
| Negative/low | 255 | 33 | 0.38 (0.18–0.82) | 0.39 (0.17–0.82) | 111 | 0.59 (0.40–0.88) | 0.68 (0.46–1.02) | |
CI, confidence interval; HR, hazard ratio; METS, metabolic equivalent task score
Q1, Q2, Q3, and Q4 represent sex-specific quartiles of post-diagnosis physical activity. Male patients were grouped as follows: Q1, 0≤METS hour/week<6.1; Q2, 6.1≤METS hour/week<18.3; Q3, 18.3≤METS hour/week<46.3; Q4, ≥46.3 METS hour/week. Female patients were grouped as follows: Q1, 0≤METS hour/week<2.5; Q2, 2.5≤METS hour/week<7.7; Q3, 7.7≤METS hour/week<18.4; Q4, ≥18.4 METS hour/week.
The multivariable, stage and sex-stratified Cox regression model initially included covariates described in Supplementary materials. With a backward stepwise elimination with a threshold of P=0.05, selected covariates in the final model were "year of CRC diagnosis (continuous)" for CRC-specific survival analysis, and "age at diagnosis (continuous)" and "tumor location (caecum vs. ascending to transverse vs. splenic flexure to sigmoid vs. rectum)" for overall survival analysis.
Pinteraction value (two-sided) was calculated using likelihood ratio test which compared the model with interaction term to the model without interaction term. The interaction term represents the cross-product of post-diagnosis physical activity (4 ordinal categories from Q1 to Q4) and IRS1 expression level (3 ordinal categories from negative to high expression).
In each cohort, we examined CRC-specific survival HR for high post-diagnosis physical activity (Q3-Q4 vs. Q1-Q2) in strata of IRS1 expression level, and confirmed that results were generally consistent between men and women, although statistical power was limited (Table S4).
Table S5 shows survival HR for one-category increase in physical activity quartiles, in strata of IRS1 expression level, in men, women, and a combined cohort. CRC-specific survival HR for one-quartile increase in post-diagnosis physical activity became consistently higher for a higher category of tumor IRS1 expression status.
Because our previous studies showed that the association of post-diagnosis physical activity with survival of CRC patients differed by status of tumor CTNNB156 or PTGS2 (cyclooxygenase-2)42 expression, we performed secondary analyses stratified by combined IRS1 and CTNNB1 (Table S6), and by combined IRS1 and PTGS2 status (Table S7). Although statistical power was limited, we observed a trend toward lower CRC-specific survival HR (for high physical activity level vs. low activity) in combined IRS1-negative and IRS1-low CRC group, compared to IRS1-high CRC, and this trend did not appear to be appreciably altered by CTNNB1 and PTGS2 status.
As secondary analyses, we examined interaction between post-diagnosis physical activity and tumor expression of IRS2 (another cytoplasmic substrate of the INSR and IGF1R signaling pathways). There was no significant interaction between post-diagnosis physical activity and IRS2 expression status in CRC-specific survival analysis (Pinteraction=0.15) and overall survival analysis (Pinteraction=0.94). In addition, tumor IRS2 expression status was not significantly associated with CRC-specific survival in univariable or multivariable analysis (Ptrend>0.20) (Table S8).
DISCUSSION
We tested the hypothesis that the prognostic association of post-diagnosis physical activity with CRC survival might differ by IRS1 expression level. To our knowledge, this is the first study to evaluate the interactive role of IRS1 expression and physical activity on survival in patients with CRC. We found a statistically significant interaction between post-diagnosis physical activity and IRS1 expression level in CRC-specific survival analysis. Our data suggest that tumor IRS1 expression status can identify individuals who may particularly benefit from exercise.
Given the generally harmless nature of most exercise activities, colorectal cancer patients may be recommended to engage in physical activity regardless of tumor biomarker data.18–23 Nonetheless, our current analysis represents a valuable hypothesis-generating study, which can inform future studies to elucidate the mechanism and develop colorectal cancer prevention and treatment strategies targeting the insulin and IGF1 signaling pathway.
Insulin-resistance states contribute to higher CRC incidence and mortality.8–10,57 One possible reason may arise from that exercise improves systemic insulin sensitivity and decreases blood insulin level, leading to prevention of CRC incidence and death.24,26,57 Our human population-based data, along with these lines of experimental evidence, support the hypothesis that the prognostic association of post-diagnosis physical activity may differ by tumor IRS1 expression level, although further studies are needed to clarify the exact mechanism.
Integrative analysis of lifestyle factors and tumor characteristics is increasingly important,58–64 because those factors contribute to heterogeneity of tumor.65,66 As previously shown, the association between post-diagnosis physical activity and CRC-specific survival might differ by tumor CTNNB156 or PTGS242 expression status. In our secondary analysis, the association between physical activity and survival appeared to be stronger in patients with IRS1-negative/low CRCs than in those with IRS1-high CRC, irrespective of CTNNB1 and PTGS2 status, although statistical power was limited. Further large-scale studies should assess tumor IRS1 status together with other markers as potential predictive biomarkers for benefit from exercise.
We observed no interaction between post-diagnosis physical activity and tumor IRS2 expression. Although both IRS1 and IRS2 are involved in insulin-related metabolism, they have different tissue-specific function.67–69 IRS1 is mainly associated with insulin resistance in skeletal muscle, while IRS2 is mainly involved in insulin resistance and lipid metabolism in liver.67,69 Similarly, our results may be consistent with differential functions of IRS1 and IRS2 in CRC cells in relation to exercise-induced change of the tumor microenvironment.
There are strengths in this study. Extensive epidemiologic and molecular characterization of our cohorts enabled us to examine interactive effects of a specific lifestyle factor in relation to tumor characteristics. This MPE approach can link exposures to specific molecular pathologic signatures, give clues to mechanisms, enhance causal inference, and identify potential biomarkers for clinical use.70–75 Study participants were distributed throughout the U.S. and in general represent CRC cases in the U.S. population. Data on lifestyle and tumors were collected prospectively by investigators blinded to patient outcomes.
Limitations of our study include the lack of treatment information after the diagnosis of CRC. However, due to the unaware of molecular data on physicians, it was unlikely that chemotherapy use substantially differed according to IRS1 status. Second, information on cancer recurrence was not available, but with long follow-up on censored cases, CRC-specific mortality is a reasonable proxy for CRC-specific outcomes. Third, the possibility of reverse causation cannot be excluded. Nonetheless, reverse causation may not be the sole explanation to the observed interaction between tumor IRS1 expression status and post-diagnosis physical activity. Fourth, the likelihood of return of the physical activity assessment may be higher among patients who were relatively healthier. Thus, we limited our study to stage I-III cases in order to minimize effect of this selection bias in an attempt to retain statistical power. Lastly, our overall sample size and statistical power were such that the results require validation in independent studies.
In conclusion, the positive association of post-diagnosis physical activity with CRC patient survival may be stronger for tumors with IRS1-negative/low expression than for tumors with IRS1-high expression. These findings need to be validated in additional populations. Upon validation, tumor IRS1 expression status may serve as a potential biomarker to identify subgroups of CRC patients who might gain greater survival benefit from increased level of exercise.
Supplementary Material
Acknowledgments
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data. All study participants provided written informed consents. This study was approved by the Institutional Review Boards at Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital. The results of the present study do not constitute endorsement by American College of Sports Medicine.
Funding: This work was supported by U.S. National Institutes of Health (NIH) grants [K07 CA190673 to R.N.; R01 CA137178 and K24 DK098311 to A.T.C.; P50 CA127003 to C.S.F.; R01 CA151993 and R35 CA197735 to S.O.; UM1 CA186107 and P01 CA87969 to Meir J. Stampfer, Nurse’s Health Study; P01 CA55075 and UM1 CA167552 to Walter C. Willett, Health Professional’s Follow-up Study], and by grants from the Paula and Russell Agrusa Fund for Colorectal Cancer Research, the Friends of the Dana-Farber Cancer Institute, the Bennett Family Fund and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. A.H. was supported by the Japan-United States Educational Exchange Promotion Foundation (Fulbright Foundation), Japan and the U.S. S.A.K. was supported by an early exchange postdoctoral fellowship grant from Asan Medical Center, Seoul, Korea. A.M.-F. was supported by a fellowship from Fundacion Caja Madrid, Spain. K.I. was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad and by Takashi Tsuruo Memorial Fund, Japan. K.M. was supported by a fellowship grant from the Uehara Memorial Foundation, Japan. A.T.C. is a Damon Runyon Clinical Investigator.
A.T.C. previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc, and Pfizer Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc, or Pfizer Inc.
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- CI
confidence interval
- CIMP
CpG island methylator phenotype
- CRC
colorectal cancer
- HPFS
Health Professionals Follow-up Study
- HR
hazard ratio
- LINE-1
long interspersed nucleotide element-1
- METS
metabolic equivalent task score
- MSI
microsatellite instability
- MSS
microsatellite stable
- NHS
Nurses’ Health Study
- SD
standard deviation.
Footnotes
Conflict of Interest: All remaining authors have declared no conflicts of interest.
Use of Standardized Official Symbols: We use HUGO (Human Genome Organisation)-approved official symbols for genes and gene products (proteins), including BRAF, CTNNB1 (catenin [cadherin-associated protein], beta 1, 88kDa; so-called β-catenin); IGF1, IGF1R (insulin-like growth factor 1 receptor); INSR (insulin receptor); IRS1 (insulin receptor substrate 1); IRS2 (insulin receptor substrate 2); KRAS, PIK3CA, PTGS2, and TP53; all of which are described at www.genenames.org. Gene names are italicized and gene product (protein) names are non-italicized.
References
- 1.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 3.Chang CK, Ulrich CM. Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia. 2003;46(5):595–607. doi: 10.1007/s00125-003-1109-5. [DOI] [PubMed] [Google Scholar]
- 4.Komninou D, Ayonote A, Richie JP, Jr, Rigas B. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003;228(4):396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 5.Limburg PJ, Stolzenberg-Solomon RZ, Vierkant RA, et al. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin Gastroenterol Hepatol. 2006;4(12):1514–1521. doi: 10.1016/j.cgh.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86(3):s836–s842. doi: 10.1093/ajcn/86.3.836S. c syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007; 86(3):s836–s842. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasuga M, Ueki K, Tajima N, et al. Report of the Japan Diabetes Society/Japanese Cancer Association Joint Committee on Diabetes and Cancer. Cancer Sci. 2013;104(7):965–976. doi: 10.1111/cas.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19(5):F27–F45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- 10.Sciacca L, Vigneri R, Tumminia A, et al. Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23(9):808–815. doi: 10.1016/j.numecd.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11(7):579–588. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 12.IARC. Weight control and physical activity. Int Agency Res Cancer Press. 2002;6 [Google Scholar]
- 13.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Color Dis. 2005;7(3):204–213. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 14.WCRF. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Gloval Perspective. Am Inst Cancer Res. 2007;2 [Google Scholar]
- 15.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67(3):253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 16.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125(1):171–180. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 17.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100(4):611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Giovannucci EL, Ogino S, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–2108. doi: 10.1001/archinternmed.2009.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–1913. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 23.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 24.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle T, Fritschi L, Platell C, Heyworth J. Lifestyle factors associated with survival after colorectal cancer diagnosis. Br J Cancer. 2013;109(3):814–822. doi: 10.1038/bjc.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bommer GT, Feng Y, Iura A, et al. IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype. J Biol Chem. 2010;285(3):1928–1938. doi: 10.1074/jbc.M109.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito DL, Aru F, Lattanzio R, et al. The insulin receptor substrate 1 (IRS1) in intestinal epithelial differentiation and in colorectal cancer. PLoS One. 2012;7(4):e36190. doi: 10.1371/journal.pone.0036190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slattery ML, Samowitz W, Curtin K, et al. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1206–1214. [PubMed] [Google Scholar]
- 29.Wang Y, Nishina PM, Naggert JK. Degradation of IRS1 leads to impaired glucose uptake in adipose tissue of the type 2 diabetes mouse model TALLYHO/Jng. J Endocrinol. 2009;203(1):65–74. doi: 10.1677/JOE-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wainszelbaum MJ, Liu J, Kong C, et al. TBC1D3, a hominoid-specific gene, delays IRS-1 degradation and promotes insulin signaling by modulating p70 S6 kinase activity. PLoS One. 2012;7(2):e31225. doi: 10.1371/journal.pone.0031225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dearth RK, Cui X, Kim HJ, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6(6):705–713. doi: 10.4161/cc.6.6.4035. http://www.ncbi.nlm.nih.gov/pubmed/17374994. [DOI] [PubMed] [Google Scholar]
- 32.Guo YS, Narayan S, Yallampalli C, Singh P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992;102(4 Pt 1):1101–1108. [PubMed] [Google Scholar]
- 33.Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. C1 Chang Q, Li Y, White MF, Fletcher JA, Xiao S Const Act Insul Recept substrate 1 is a Freq event Hum tumors Ther Implic Cancer Res 2002;62(21)6035-6038.ancer Res. 2002;62(21):6035–6038. [PubMed] [Google Scholar]
- 34.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94(13):972–980. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 35.Durai R, Yang W, Gupta S, Seifalian AM, Winslet MC. The role of the insulin-like growth factor system in colorectal cancer: review of current knowledge. Int J Colorectal Dis. 2005;20(3):203–220. doi: 10.1007/s00384-004-0675-4. [DOI] [PubMed] [Google Scholar]
- 36.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8(12):915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 37.Porter HA, Perry A, Kingsley C, Tran NL, Keegan AD. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett. 2013;338(2):239–248. doi: 10.1016/j.canlet.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 39.Liao X, Lochhead P, Nishihara R, et al. Aspirin use tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyerhardt JA, Ogino S, Kirkner GJ, et al. Interaction of molecular markers and physical activity on mortality in patients with colon cancer. Clin Cancer Res. 2009;15(18):5931–5936. doi: 10.1158/1078-0432.CCR-09-0496. doi:1078-0432.CCR-09-0496 [pii] 10.1158/1078-0432.CCR-09-0496 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morikawa T, Kuchiba A, Lochhead P, et al. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with beta-catenin (CTNNB1) status. Cancer Res. 2013;73(5):1600–1610. doi: 10.1158/0008-5472.CAN-12-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamauchi M, Lochhead P, Imamura Y, et al. Physical Activity, Tumor PTGS2 Expression, and Survival in Patients with Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1142–1152. doi: 10.1158/1055-9965.EPI-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by Pyrosequencing. J Mol Diagnostics. 2005;7(3):413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imamura Y, Morikawa T, Liao X, et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res. 2012;18(17):4753–4763. doi: 10.1158/1078-0432.CCR-11-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogino S, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. CpG island methylator phenotype-low (CIMP-low) in colorectal cancer: possible associations with male sex and KRAS mutations. J Mol Diagnostics. 2006;8(5):582–588. doi: 10.2353/jmoldx.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nosho K, Kawasaki T, Ohnishi M, et al. PIK3CA mutation in colorectal cancer: relationship with genetic and epigenetic alterations. Neoplasia. 2008;10(6):534–541. doi: 10.1593/neo.08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao X, Morikawa T, Lochhead P, et al. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18(8):2257–2268. doi: 10.1158/1078-0432.CCR-11-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogino S, Kawasaki T, Brahmandam M, et al. Precision and performance characteristics of bisulfite conversion and real-time PCR (MethyLight) for quantitative DNA methylation analysis. J Mol Diagnostics. 2006;8(2):209–217. doi: 10.2353/jmoldx.2006.050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3(11):e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58(1):90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogino S, Kawasaki T, Nosho K, et al. LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer. 2008;122(12):2767–2773. doi: 10.1002/ijc.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Irahara N, Nosho K, Baba Y, et al. Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagnostics. 2010;12(2):177–183. doi: 10.2353/jmoldx.2010.090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawasaki T, Nosho K, Ohnishi M, et al. Correlation of beta-catenin localization with cyclooxygenase-2 expression and CpG island methylator phenotype (CIMP) in colorectal cancer. Neoplasia. 2007;9(7):569–577. doi: 10.1593/neo.07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morikawa T, Kuchiba A, Liao X, et al. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131(5):1169–1178. doi: 10.1002/ijc.26495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogino S, Brahmandam M, Kawasaki T, Kirkner GJ, Loda M, Fuchs CS. Combined analysis of COX-2 and p53 expressions reveals synergistic inverse correlations with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Neoplasia. 2006;8(6):458–464. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. JAMA. 2011;305(16):1685–1694. doi: 10.1001/jama.2011.513. doi:305/16/1685 [pii] 10.1001/jama.2011.513 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee DH, Kim JY, Lee MK, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013;21(9):2537–2545. doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- 58.Chia WK, Ali R, Toh HC. Aspirin as adjuvant therapy for colorectal cancer--reinterpreting paradigms. Nat Rev Clin Oncol. 2012;9(10):561–570. doi: 10.1038/nrclinonc.2012.137. [DOI] [PubMed] [Google Scholar]
- 59.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers (Basel) 2013;5(2):676–713. doi: 10.3390/cancers5020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14(8):16365–16385. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bishehsari F, Mahdavinia M, Vacca M, Malekzadeh R, Mariani-Costantini R. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20(20):6055–6072. doi: 10.3748/wjg.v20.i20.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okugawa Y, Grady WM, Goel A. Epigenetic Alterations in Colorectal Cancer: Emerging Biomarkers. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weisenberger DJ, Levine AJ, Long TI, et al. Association of the colorectal CpG island methylator phenotype with molecular features, risk factors, and family history. Cancer Epidemiol Biomarkers Prev. 2015;24(3):512–519. doi: 10.1158/1055-9965.EPI-14-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogino S, Fuchs CS, Giovannucci E. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn. 2012;12(6):621–628. doi: 10.1586/erm.12.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogino S, Lochhead P, Chan AT, et al. Molecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and disease. Mod Pathol. 2013;26(4):465–484. doi: 10.1038/modpathol.2012.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kido Y, Burks DJ, Withers D, et al. Tissue-specific insulin resistance in mice with mutations in the insulin receptor, IRS-1, and IRS-2. J Clin Invest. 2000;105(2):199–205. doi: 10.1172/JCI7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C, Thirone AC, Huang X, Klip A. Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J Biol Chem. 2005;280(19):19426–19435. doi: 10.1074/jbc.M412317200. [DOI] [PubMed] [Google Scholar]
- 69.Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys. 2007;48(2–3):103–113. doi: 10.1007/s12013-007-0030-9. http://www.ncbi.nlm.nih.gov/pubmed/17709880. [DOI] [PubMed] [Google Scholar]
- 70.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102(6):365–367. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60(3):397–411. doi: 10.1136/gut.2010.217182. doi:gut.2010.217182 [pii] 10.1136/gut.2010.217182 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8(12):711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ogino S, King EE, Beck AH, Sherman ME, Milner DA, Giovannucci E. Interdisciplinary education to integrate pathology and epidemiology: towards molecular and population-level health science. Am J Epidemiol. 2012;176(8):659–667. doi: 10.1093/aje/kws226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogino S, Lochhead P, Giovannucci E, Meyerhardt JA, Fuchs CS, Chan AT. Discovery of colorectal cancer PIK3CA mutation as potential predictive biomarker: power and promise of molecular pathological epidemiology. Oncogene. 2014;33(23):2949–2955. doi: 10.1038/onc.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogino S, Campbell PT, Nishihara R, et al. Proceedings of the second international molecular pathological epidemiology (MPE) meeting. Cancer Causes Control. 2015;26(7):959–972. doi: 10.1007/s10552-015-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.