Abstract
Background/Objective
Adiponectin exerts beneficial effects by reducing inflammation, and improving lipid metabolism and insulin-sensitivity. Although adiponectin is lower in obese individuals, whether weight gain reduces adiponectin expression in humans is controversial. We sought to investigate the role of weight gain, and consequent changes in leptin, on altering adiponectin expression in humans.
Methods/Results
Forty four normal-weight healthy subjects were recruited (mean age 29 years; 14 women) and randomized to either gain 5% of body weight by 8-weeks of overfeeding (n=34) or maintain weight (n=10). Modest weight gain of 3.8 ± 1.2 kg resulted in increased adiponectin (p=0.03) while weight maintenance resulted in no changes in adiponectin. Further, changes in adiponectin correlated positively with changes in leptin (p=0.0085). In-vitro experiments using differentiated human white preadipocytes showed that leptin increased adiponectin mRNA and protein expression, while a leptin-antagonist had opposite effects. To understand the role of leptin in established obesity, we compared adipose tissue samples obtained from normal weight versus obese subjects. We noted, first, that leptin activated cellular signaling pathways and increased adiponectin mRNA in adipose tissue from normal-weight participants, but did not do so in adipose tissue from obese participants; and second, that obese subjects had increased caveolin-1 expression, which attenuates leptin-dependent increases in adiponectin.
Conclusions
Modest weight gain in healthy individuals is associated with increases in adiponectin, which correlate positively with changes in leptin. In-vitro, leptin induces adiponectin expression which is attenuated by increased caveolin-1 expression. Additionally, adipose tissue from obese subjects shows increased caveolin-1 expression, and impaired leptin signaling. This leptin signal impairment may prevent concordant increases in adiponectin in obese subjects despite their high levels of leptin. Therefore, impaired leptin signaling may contribute to low adiponectin expression in obesity and may provide a target for increasing adiponectin expression, hence improving insulin sensitivity and cardio-metabolic profile in obesity.
Introduction
Adiponectin is an adipokine with profound anti-atherogenic, anti-inflammatory, and insulin-sensitizing properties.1–3 Paradoxically, it is the only adipokine which is decreased in obesity.4–6 This negative association between obesity and adiponectin raises the possibility that weight gain decreases adiponectin expression. However, the effect of weight gain on adiponectin expression in healthy humans is controversial and the molecular mechanisms underlying decreased adiponectin expression in human obesity remain largely unknown.7–11 On the other hand, cross-sectional population based studies have shown the presence of higher adiponectin levels in the metabolically healthy obese population, which provides support for the potential role of adiponectin in disassociating obesity per se from cardiometabolic dysfunction.12 Indeed, increasing adiponectin expression is being targeted as a mechanism to improve insulin sensitivity and decrease cardiovascular risk in the obese population.13
Several lines of evidence suggest that leptin, an adipokine increased in obesity, may regulate adiponectin expression. Absence of leptin, as seen in leptin deficient ob/ob and leptin receptor deficient db/db mice, is characterized by low adiponectin expression.4, 14 Additionally, supplementation of leptin in ob/ob mice results in increased adiponectin expression.15 Importantly, increases in adiponectin expression upon leptin administration were observed before weight loss.16 Furthermore, adipocyte-selective reduction of leptin receptor expression diminishes adiponectin expression, suggesting that leptin may be directly signaling adipocytes to induce adiponectin expression.17 Similarly, in leptin deficient conditions in humans, such as lipodystrophy, decreased expression of both leptin and adiponectin is seen,18 and leptin treatment in leptin-deficient adults increases adiponectin expression.19 However, the role of leptin in regulation of adiponectin in humans is unclear, since cross-sectional population studies show a negative correlation between leptin and adiponectin.20, 21 Therefore, we designed a study to first, examine the effect of weight gain on adiponectin expression in normal weight healthy subjects, and second, to investigate the role of leptin in regulation of adiponectin. We measured adiponectin expression in normal weight healthy humans, at baseline and after overfeeding-induced weight gain versus weight maintenance (controls). We also explored the role of leptin in regulating adiponectin expression in-vitro, and extended our studies to include cross-sectional ex-vivo studies in normal weight versus obese subjects, so as to determine novel molecular mechanisms which may play a role in decreasing adiponectin expression in established obesity. We hypothesized that leptin up-regulates adiponectin expression, and that the decreased adiponectin expression in established obesity is secondary to an impairment of leptin signaling.
Materials and methods
Longitudinal weight gain study
We used a longitudinal overfeeding study in humans to determine the effects of weight gain on adiponectin expression.22, 23 Forty four healthy adults (30 men and 14 women) aged 29 ± 6 years who were sedentary, and free of any chronic diseases such as diabetes, hypertension and dyslipidemia, were recruited to participate in the overfeeding protocol. Tobacco users and shift workers were excluded. The study was conducted at the Mayo Clinic Center for Translational Science Activities (CTSA) Clinical research Unit (CRU) and the protocol was approved by the Institutional Review Board. Informed written consent was obtained from all participants. Findings from this study relating to endothelial dysfunction, heart rate-variability, and adipose tissue changes have been published elsewhere.22, 24–26 All the subjects in whom leptin and adiponectin data was available at both baseline and after weight gain were included to test the hypothesis.
The details of the longitudinal weight gain model have been described previously.22, 23 Briefly, after a three day period during which calories required to maintain weight were estimated, subjects were randomized 4:1 to either gain weight or maintain weight. Weight gain was induced by increasing calorie consumption using 1–3 supplements/day (400–1200 extra kcal) in addition to their usual caloric intake and did not differ in macronutrient composition (40% carbohydrates, 40% fat, and 20% protein). The goal of the controlled weight gain intervention was to gain 5% body weight over 8-weeks. The weight maintainer group was advised to maintain their body weight for 8-weeks. For both groups, subjects were weighed > 5 days/week which allowed the dietitians to monitor and adjust the calorie intake on a regular and frequent basis. We measured body weight, total body composition (data were obtained in duplicate with DXA; DPX-IQ; Lunar Radiation), fasting lipid profile (standard turbidimetry method, Hitachi 912 chemistry analyzer), insulin (2 site-immunoenzymatic assay; Beckman Instruments Inc.), glucose (hexokinase reagent; Hitachi 912 chemistry analyzer), leptin (radioimmunoassay; Linco research) and adiponectin (ELISA; Mediagnost GmbH) at baseline (TP1) and after 8-weeks follow-up (TP2). For body composition, the average values from the two scans were used to assess total body fat mass, and total body fat free mass (not including the bone mass).
In-vitro studies
Commercially available human white preadipocytes (HWP’s; PromoCell, Heidelberg, Germany) were used for the in-vitro experiments after differentiation for 12 days as per manufacturer’s instructions. The cells were kept overnight in serum free minimal media, and treated with increasing concentrations of leptin (10 ng/ml −150 ng/ml; Sigma, St. Louis, MO, USA) for 6 hours and 24 hours respectively. The effect of leptin on adiponectin mRNA and protein was determined semi-quantitatively by RT-PCR using commercially available TaqMan probes (Applied Biosystems, Foster City, CA) and Western blot analysis (anti-adiponectin antibody AB22554, anti-GAPDH antibody, AB9485 AbCam, Cambridge, MA). The effect of leptin treatment on adiponectin secretion was determined in conditioned media from 24 h leptin-treated cells using total and high molecular weight adiponectin ELISA kits as per manufacturer’s instruction (RnD systems, Minneapolis, MN). Similarly, differentiated HWPs were treated with increasing concentrations of leptin antagonist (0.5 – 2 µg.ml; SHLA; Protein Laboratories Rehovot Ltd, Rehovot, Israel) to determine the effect of endogenous leptin expression on basal adiponectin mRNA and protein expression. The leptin antagonist (SHLA) is an inactive leptin-mutant (L39A/D40A/F41A) with high affinity to the leptin-receptor and acts via competitively binding to the leptin receptor. Further, the ability of LA to antagonize central leptin-dependent changes in appetite, weight, and hypertension has been previously demonstrated.27–29
To determine the cell signaling pathway through which leptin modulates adiponectin mRNA, we treated differentiated HWP with ERK inhibitor (PD98059, 30µM, Sigma, St. Louis, MO), AMPK inhibitor (Insolution AMPK inhibitor Compund C, 20µM, EMD Chemicals Inc, Gibbstown, USA), AKT inhibitor IV (1µM, EMD Chemicals Inc), and STAT3 inhibitor VII (3µM, EMD Chemicals Inc) for 30 minutes prior to and during 6 hours of leptin (100ng/ml) treatment. The effect of pathway inhibition on adiponectin mRNA was determined by RT-PCR analysis. We also examined the ability of leptin-antagonist (1.5 µg/ml) to prevent leptin-dependent (100ng/ml) activation of ERK and STAT3 pathways in differentiated HWP.
To determine the effect of caveolin-1 overexpression on leptin-dependent increases in adiponectin mRNA, differentiated HWPs were infected with adenovirus encoding caveolin-1 gene or control null adenovirus at an MOI of 100 (Vector BioLabs, Philadelphia, PA). These cells were than treated with leptin (10 ng/ml; 100 ng/ml) for 6 hours prior to determination of adiponectin mRNA transcripts by RT-PCR.
Ex-vivo adipose tissue studies
To understand the dynamics of direct leptin action in established obesity, we examined the ability of leptin to activate cellular signaling pathways and increase adiponectin mRNA expression in human adipose tissue from a cross-sectional population comprising of normal weight, and obese individuals. Seventeen adults (8 men and 9 women) aged 20–67 years were recruited to participate in these studies. The protocol was approved by the Mayo Clinic Institutional Review Board and written informed consent was obtained from all participants. Subcutaneous abdominal adipose tissue biopsies were collected either from subjects undergoing elective abdominal surgery, or from subjects participating in our ongoing research studies at the CRU.
Fresh adipose tissue was collected in basal adipocyte nutrition media, cut into approx. 0.5 cm3 pieces, and incubated for 30 min at 37°C for conditioning. The tissue was then aliquoted and incubated with either 0 ng/ml (control) or 100 ng/ml leptin for 15 minutes (leptin signaling), or 6 hours (adiponectin mRNA). Since adipose tissue is the main source of leptin, cells of adipose tissue would be exposed to higher leptin levels than those present in the circulation. Furthermore, these experiments sought to measure the maximal response to leptin which would be relevant to both lean as well obese individuals. Therefore, a concentration of 100 ng/ml leptin was used for these ex-vivo experiments. Additionally, the 15 min treatment to examine the leptin-dependent activation of cell signaling pathways and 6 hour treatment to examine the effects on mRNA transcripts were based on our previous experiments during which maximal responses would likely be observed.22 The effect of leptin on activation of cellular signaling pathways was determined by Western blot analysis (anti-p/t STAT3, and anti-p/tERK1/2, 9134, 9139, 9101, and 9102, Cell Signaling, Danvers, MA). Further, a small aliquot of the adipose tissue sample was immediately flash frozen in liquid nitrogen and used to investigate the expression of proteins involved in leptin signaling by Western blot analysis (anti Ob-R, sc80255,Santa Cruz Biotech, Dallas, TX; anti-SOCS3, AB16030, AbCam, Cambridge, MA; anti-cav-1, 610407, BD Transduction lab, San Diego, CA).
Statistical analysis
Data were analyzed using JMP 9.0.1 software (SAS Institute Inc.) Continuous variables from study participants were recorded as means ± SDs. For the longitudinal weight gain study, each measurement during baseline and after weight gain was compared using paired Wilcoxon’s signed-rank test. Changes in adiponectin and leptin during weight gain were compared using Pearson correlation. For the cross-sectional study, variables across the groups were compared using Wilcoxon rank sum test. It was tested whether variance in the groups that were being statistically compared was similar. All in-vitro experiments were performed at least four times. Data from the in-vitro and ex-vivo experiments are presented as mean±SEM. Statistical significance and pairwise analysis were determined using Wilcoxon rank sum test.
Results
Effects of modest weight gain in healthy normal weight participants
Increased calorie intake over a period of 8 weeks caused an average weight gain of 3.8 ± 1.2 kg. The increase in total body weight resulted mainly from increased body fat mass (3.6 ± 1.5 kg); fat free mass did not change (Table 1). Among the variables measured, expected increases in insulin, and leptin were observed. Importantly, an increase in circulating adiponectin was observed with weight gain. On the other hand, the weight maintainer group did not show any changes in weight, total body fat mass, leptin or adiponectin during the 8 week follow-up. There was also inter-individual variability in response to weight gain in the variables measured, including adiponectin and leptin. The changes in adiponectin ranged from 8.9 to −5.5 µg/ml and 13 subjects actually showed reduced adiponectin even though they gained similar amounts of weight and fat mass as the subjects manifesting increased adiponectin with weight gain. Similarly, changes in leptin in the weight gain group ranged from 17.9 to −0.1 ng/ml. The individual changes in these measures are depicted in Figure 1A.
Table 1.
Characteristics of the study population
| Weight gainers (n=34, 23 men) | Weight maintainers (n=10, 7 men) | |||
|---|---|---|---|---|
| TP1 | TP2 | TP1 | TP2 | |
| Weight (kg) | 71.23 ± 14.54 | 75.00 ± 15.00* | 73.72 ± 14.59 | 73.54 ± 14.37 |
| Total body fat mass (kg) | 17.81 ± 7.61 | 21.43 ± 8.04* | 18.62 ± 5.95 | 18.87 ± 5.99 |
| Total body fat-free mass (kg) | 51.88 ± 12.61 | 51.96 ± 12.72 | 51.91 ± 10.51 | 51.49 ± 10.73 |
| BMI (kg/m2) | 23.25 ± 3.58 | 24.50 ± 3.71* | 23.95 ± 2.68 | 23.89 ± 2.52 |
| Cholesterol (mmol/l) | 4.05 ± 0.63 | 4.16 ± 0.85 | 4.32 ± 0.63 | 4.22 ± 0.52 |
| HDL (mmol/l) | 0.97 ± 0.29 | 0.96 ± 0.26 | 1.13 ± 0.19 | 1.12 ± 0.16 |
| Triglycerides (mmol/l) | 0.92 ± 0.34 | 1.06 ± 0.60 | 0.95 ± 0.25 | 0.81 ± 0.19 |
| Leptin (ng/ml) | 6.93 ± 5.54 | 11.11 ± 8.72* | 5.27 ± 3.12 | 6.41 ± 4.18 |
| Adiponectin (µg/ml) | 8.76 ± 3.8 | 9.53 ± 4.30* | 7.21 ± 2.89 | 7.75 ± 3.01 |
| Leptin/Adiponectin ratio | 0.91 ± 0.83 | 1.33 ± 1.10* | 0.72 ± 0.29 | 0.82 ± 0.41 |
| Glucose (mmol/l) | 5.11 ± 0.34 | 5.31 ± 0.61 | 4.92 ± 0.35 | 4.96 ± 0.26 |
| Insulin (pmol/l) | 30.9 ± 19.44 | 38.58 ± 20.76* | 31.20 ± 18.36 | 29.10 ± 13.86 |
Data are presented as mean ± SD, TP1: baseline measure; TP2: 8-week follow-up,
is p < 0.05 determined by paired Wilcoxon Signed-Rank test,
BMI: Body mass index, HDL: high density lipoprotein.
Figure 1. Effects of overfeeding-induced weight gain versus weight maintenance in normal weight subjects.
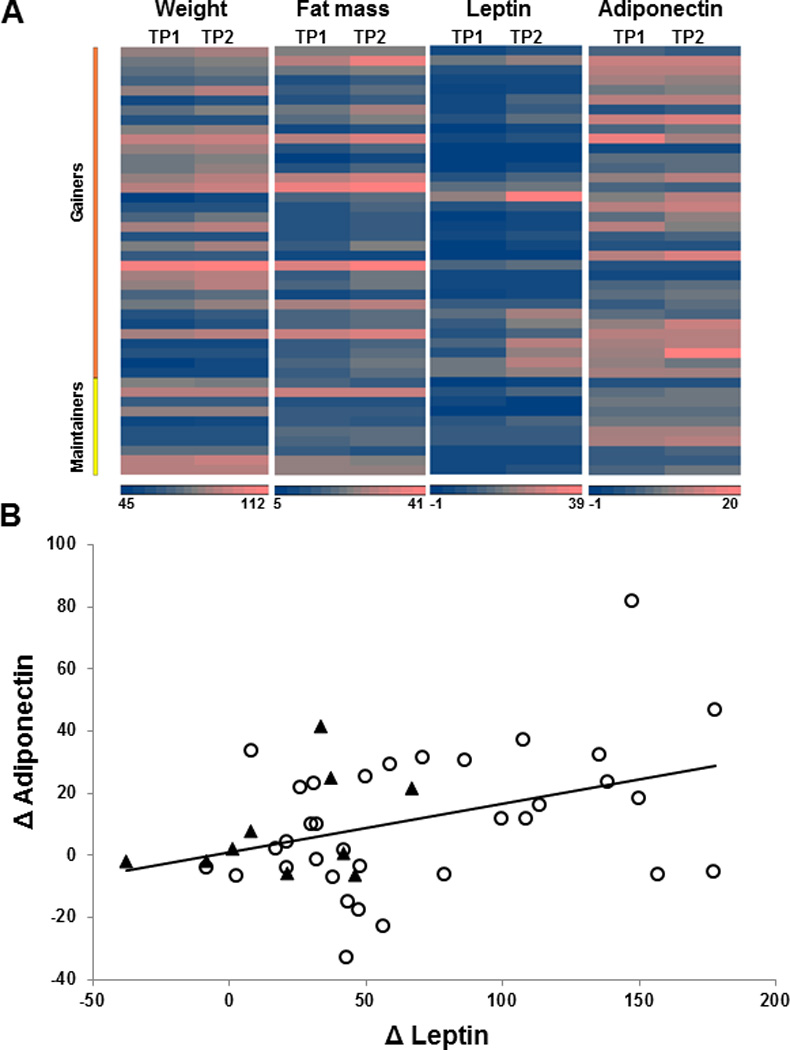
Heat map depicting individual responses of the study subjects at baseline (TP1) and after 8 week of weight gain or maintenance (TP2) (A). The heat map highlights the inter-individual variations in response to overfeeding-induced changes in weight, fat mass, leptin and adiponectin in weight gainers along with variations observed in the subjects assigned to the weight maintainer group. Relation between relative changes in adiponectin and leptin with modest weight gain in study participants (B). Circles represent data from weight gainers (n=34), and triangles represent data from weight maintainers (n=10). Changes in adiponectin correlated positively only with changes in leptin (Pearson coefficient, r = 0.39, P = 0.008; n = 44), but not with any other variable.
To examine the role of leptin in regulating adiponectin expression, a statistical approach was undertaken and the relationship between adiponectin and leptin was examined. A positive correlation between leptin and adiponectin was evident at baseline and after weight gain, and changes in adiponectin expression correlated positively with changes in leptin (r=0.3920, P=0.0085) (Figure 1B). Importantly, there was no correlation evident between changes in adiponectin with changes in any other measured variable (supplementary table 1).
Leptin up-regulates adiponectin expression
We examined the effect of leptin on adiponectin expression using differentiated HWPs in culture. Leptin increased adiponectin mRNA (P<0.0001) (Figure 2A) and protein (P=0.006) (Figure 2B) expression in a dose-dependent manner. However, leptin treatment did not change secretion of total and high-molecular weight adiponectin in the conditioned media (Figure 2C). Since differentiated HWPs also express leptin, we determined the effect of increasing concentrations of leptin antagonist on adiponectin expression as well. Treatment of differentiated HWPs with leptin antagonist decreased adiponectin mRNA (P=0.002) (Figure 2D), intracellular-protein (P=0.05) (Figure 2E), extracellular secreted total adiponectin (P=0.007) and high molecular weight adiponectin (P=0.02) (Figure 2F).
Figure 2. Leptin regulates adiponectin expression in differentiated human white preadipocytes (HWP).
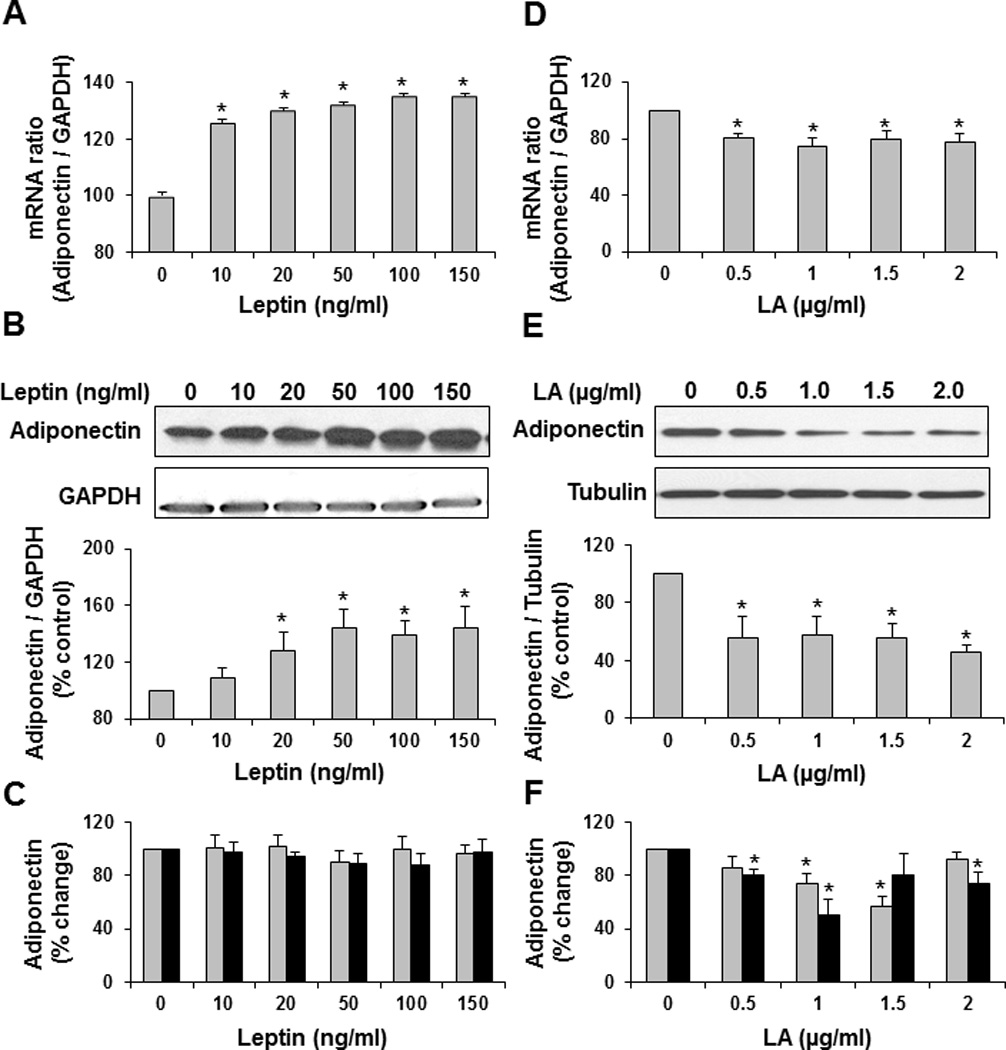
In-vitro treatment of differentiated HWP with increasing concentrations of leptin increases adiponectin mRNA (A) and protein (B) in a dose-dependent manner. However, leptin does not increase adiponectin secretion (C). Treatment of differentiated HWP with leptin antagonist decreases adiponectin mRNA (D), protein (E), and secretion (F). Data presented as mean ± SEM (n=4). *P ≤ 0.05 compared with control as determined by Wilcoxon rank-sum test. Total adiponectin: grey bars; high molecular weight adiponectin: black bars.
Furthermore, the leptin-dependent increase in adiponectin mRNA was attenuated in the presence of ERK1/2 and STAT3 inhibitor (Figure 3A). Further, treatment with leptin in the presence of leptin-antagonist prevented leptin-dependent increases in pERK1/2 (Figure 3B) while leptin-dependent activation of STAT3 was not altered by the presence of leptin-antagonist (Figure 3C), suggesting only partial antagonism to leptin actions.
Figure 3. Activation of ERK pathway is required for leptin-dependent increases in adiponectin.
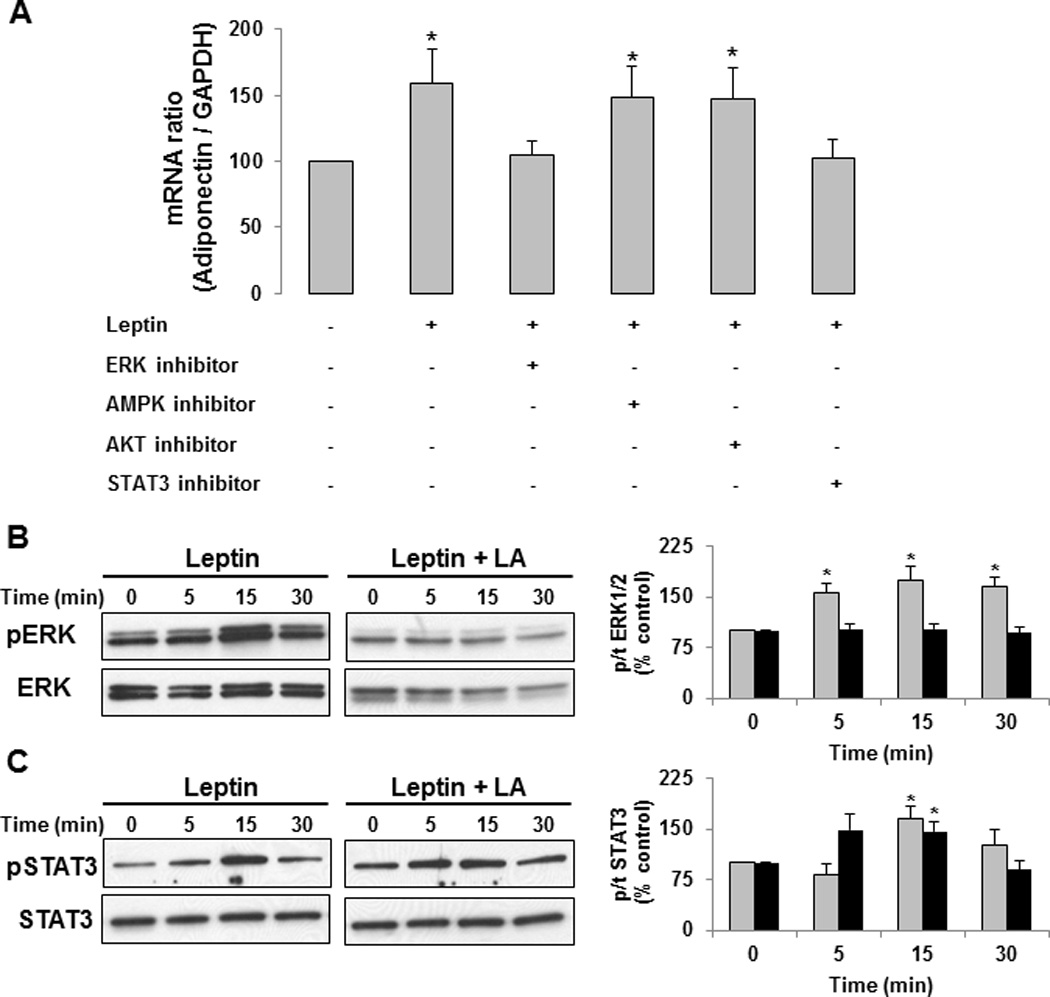
In-vitro treatment of differentiated HWP with leptin in presence of ERK and STAT3 pathway inhibitor prevented leptin-dependent increases in adiponectin (A). However, treatment with leptin-antagonist attenuated leptin-dependent activation of ERK1/2 (B). Leptin-antagonist does not prevent leptin-dependent activation of STAT3 pathways (C). Data presented as mean ± SEM (n=4). *P ≤ 0.05 compared with control as determined by Wilcoxon rank-sum test. Grey bars present data from leptin treatment. Black bars present data from leptin-antagonist and leptin treatment.
Leptin cellular signaling in adipose tissue of normal weight versus obese subjects
Leptin plays an important role in energy homeostasis; therefore the presence of increased leptin levels in obesity suggests impaired leptin signaling at the central level. Hence, we sought to determine if adipose tissue from obese participants was non-responsive to external leptin stimuli.
To test whether leptin signaling is impaired in adipose tissue from obese humans, we examined the effect of leptin on activation of cellular signaling pathways and adiponectin expression ex-vivo. The characteristics of the study population from which adipose tissue was obtained are presented in Supplemental table 2. As designed, the BMI differed significantly between the two groups. However, compared to the normal weight group, the obese group was also somewhat older (p=0.03). Compared to normal weight participants, leptin was unable to phosphorylate STAT3 (Figure 4A) and ERK (Figure 4B) pathways in adipose tissue from obese participants. Furthermore, adiponectin protein expression was significantly reduced in adipose tissue of obese subjects (Figure 4C) and leptin-dependent increases in adiponectin mRNA were also attenuated (Figure 4D). Leptin treatment increased adiponectin mRNA transcripts significantly only in adipose tissue obtained from normal weight study participants (P=0.027), but did not significantly change adiponectin mRNA in adipose tissue from obese participants (P=0.19).
Figure 4. Leptin-dependent activation of cellular signaling pathways is evident in normal weight subjects (N, white bars) but impaired in adipose tissue from obese (Ob, black bars) participants.
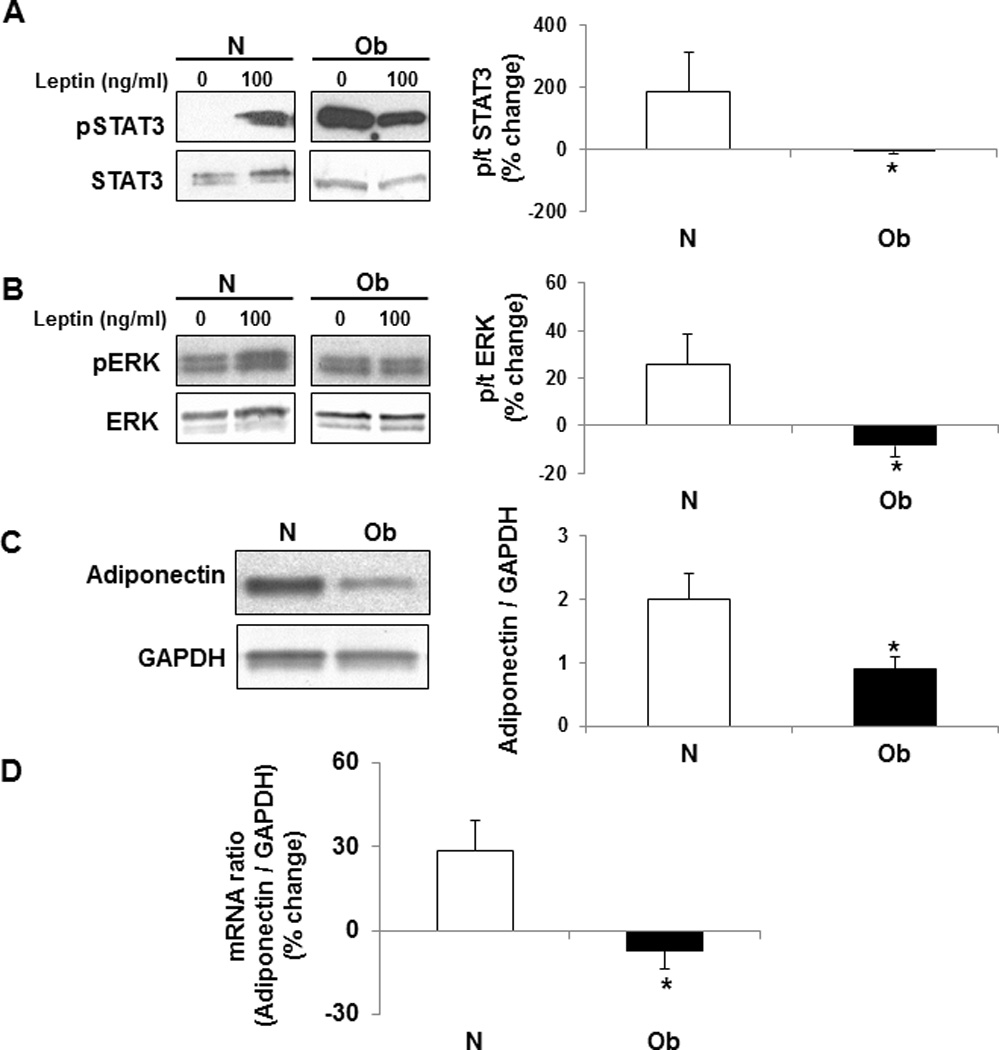
Ex-vivo treatment of adipose tissue with leptin (100 ng/ml) for 15 min activated STAT3 (A), and ERK (B) pathways in samples obtained from normal weight participants. Activation of these pathways was impaired in adipose tissue samples obtained from obese subjects. Furthermore, adiponectin protein expression was elevated in adipose tissue from normal weight subjects (C). The impaired cellular signaling prevented leptin-dependent increases in adiponectin mRNA in adipose tissue from obese participants (D). Data presented as mean ± SEM. *P ≤ 0.05 compared with percent activation in normal weight participants as determined by Wilcoxon rank-sum test.
To investigate the molecular mechanisms underlying leptin cellular signal impairment, we examined the expression of proteins that are known to play a critical role in leptin signaling (Figure 5). The expression of leptin-receptor (Ob-R) (Figure 5A) and suppressor of cytokine signaling 3 (SOCS3) (Figure 5B) did not differ significantly in our study participants, even though the expression of SOCS3 tended to be higher in adipose tissue from obese subjects. However, caveolin-1 protein expression was significantly elevated in adipose tissue from obese individuals (Figure 5C).
Figure 5. Increased caveolin-1 expression contributes to impaired leptin-dependent activation of cellular signaling pathways in adipose tissue from obese (Ob, black bars) subjects.
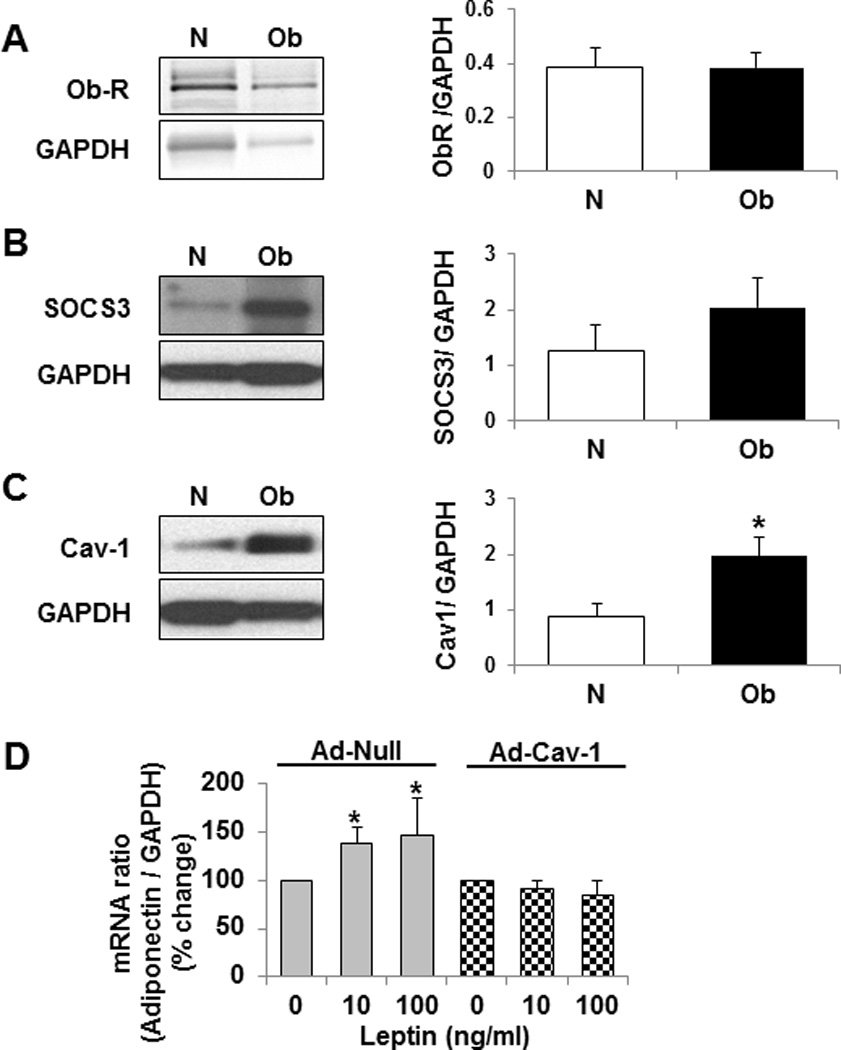
The expression of leptin receptor (Ob-R) (A), and SOCS-3 (B) was not different in adipose tissue from obese participants, but caveolin-1 protein expression (C) was increased significantly. Furthermore, caveolin-1 overexpression impaired leptin-dependent increases in adiponectin mRNA in cultured differentiated HWP (D, checkered bars). Data presented as mean ± SEM. *P ≤ 0.05 compared to normal weight subjects (N) / control (Leptin: 0 ng/ml) as determined by Wilcoxon rank-sum test.
We further sought to determine if increased caveolin-1 expression as seen in adipose tissue from obese participants could impair leptin-dependent increases in adiponectin mRNA. We infected the differentiated HWPs with an adenovirus encoding caveolin-1 gene to increase caveolin-1 expression. Leptin treatment did not increase adiponectin mRNA transcription in those cells overexpressing caveolin-1 (Figure 5D).
Discussion
The important and novel findings of our study are first, that weight gain increases adiponectin expression in healthy humans; second, that leptin up-regulates adiponectin expression; and third, that the decreased adiponectin expression in established obesity may be secondary to impairment of leptin signaling in adipose tissue, perhaps related to increased caveolin-1 expression in these adipocytes.
Using a unique longitudinal weight gain model in healthy humans, we show that modest weight gain increases adiponectin expression. These findings are consistent with the recently published short-term overfeeding-induced weight gain studies.7, 8, 11 However, previous studies examining the effect of substantial weight gain (approximately 10–15% increase in body weight) showed either no change or a decrease in adiponectin expression with weight gain.9, 10 Taken together, these studies suggest that decreases in adiponectin with weight gain may be related to a threshold effect. Modest weight gain is associated with increases in adiponectin, but more substantial weight gain, especially to the point of obesity, induces decreases in adiponectin. This threshold effect would therefore contribute to reduced adiponectin in the obese population.4–6 Furthermore, changes in the adipose tissue microenvironment, including increases in inflammation along with molecular and cellular changes during excessive weight gain and establishment of obesity, may together play a key role in decreasing adiponectin expression.30–33 Notably, similar studies examining the effect of modest weight gain in humans did not show any changes in adipose tissue inflammation.34, 35
The longitudinal weight gain study was conducted in free-living conditions; as a result not all subjects gained weight or maintained weight as designed (figure 1A). Additionally, inter-individual variability in response to weight gain in the variables measured, including adiponectin and leptin was observed. The broad range of changes in leptin and adiponectin in response to similar amounts of weight gain highlights the multifactorial nature of leptin and adiponectin regulation, including genetic and epigenetic differences.36 In spite of this variability, we observed a positive correlation between changes in adiponectin and changes in leptin in our study population. This was intriguing, especially since clinical studies have demonstrated an inverse relation between the two adipokines.20, 21 However, these prior clinical studies were limited by the cross-sectional study design and may thus reflect an outcome of established obesity. Also, changes in adiponectin did not correlate with any other measures that were obtained in our study population including insulin.
We have confirmed leptin-dependent up-regulation of adiponectin expression using cultured differentiated HWPs. Complementary to these data, we also found that treatment of differentiated HWPs with a leptin-antagonist decreased adiponectin expression, suggesting that leptin may contribute importantly to tonic adiponectin production in basal physiologic conditions. However, in the in-vitro studies, leptin-dependent increases in intracellular-adiponectin did not mirror increases in total and high molecular weight adiponectin secretion. The lack of increase in adiponectin secretion may be secondary to increases in caveolin-1 expression.37 Interestingly, caveolin-1 knock-out mice have decreased total and high molecular weight adiponectin despite normal intracellular levels of adiponectin.38, 39 Nevertheless, the physiological relevance of leptin-dependent up-regulation of adiponectin in healthy humans is highlighted by positive associations observed between leptin and adiponectin at baseline and after 8 weeks follow-up, as well as the changes in each during modest weight gain in our study.
We also undertook studies to examine the specific cellular signaling pathways through which leptin mediates increases in adiponectin mRNA and establish that 1) SHLA attenuates leptin-mediated activation of these pathways; and 2) leptin-dependent activation of these pathways is impaired in adipose tissue from obese individuals. We observed that in the presence of inhibitors of ERK1/2 and STAT3 pathways, leptin did not increase adiponectin mRNA (fig 3A) which suggested that activation of both these pathways may be important for leptin-dependent increases in adiponectin expression. Importantly, ERK-dependent or ERK-independent pathways may contribute to STAT3 mediated increase in adiponectin mRNA.40–42 Indeed, several studies have shown that leptin may activate STAT3 via both ERK-dependent and ERK-independent pathways.43–46 Furthermore, we also show that in the presence of SHLA, leptin-dependent activation of ERK1/2 was impaired (fig 3b) but SHLA did not alter leptin-dependent activation of the STAT3 (fig 3c). This observation was surprising as previous animal studies have shown that LA prevents leptin-dependent activation of STAT3 in the hypothalamus.27, 28 Taken together, our results suggest that leptin may regulate adiponectin mRNA via ERK-dependent activation of STAT3. However, further detailed studies examining the signaling cascade through which leptin regulates adiponectin mRNA transcripts are warranted. Furthermore, we also demonstrate that leptin does not activate ERK and STAT3 pathways or increases adiponectin expression in adipose tissue from obese individuals.
Leptin plays a vital role in energy homeostasis by modulating food intake and energy expenditure. Therefore, increased leptin expression in obesity suggests impaired leptin signaling at the central level. However, in obesity, the role of hyperleptinemia versus impaired leptin signaling in peripheral tissues is not completely understood.47–49 We previously reported that increases in leptin induced by modest weight gain result in up-regulation of adipose tissue caveolin-1 expression, which in-turn may impair leptin-dependent cellular signaling in adipose tissue.22 This study suggested the presence of a “threshold effect” of leptin action, whereby leptin-dependent increases in caveolin-1 cause impairment of leptin signaling after a certain amount of body fat has been accumulated. Therefore, modest fat gain in normal-weight healthy individuals cause increases in leptin, and hence leptin-mediated increases in adiponectin. However further substantial increases in weight to the point of obesity are likely to trigger impairment of leptin-cellular actions and blunt concordant increases in adiponectin. Although our prior study22 suggested that adipose tissue in an obese individual is likely leptin resistant, to our knowledge, whether adipose tissue remains leptin sensitive or becomes leptin resistant in obesity is unknown. In the present study, we demonstrate that leptin-dependent activation of cellular signaling pathways and adiponectin mRNA transcription is impaired in adipose tissue from obese individuals. Moreover, we explored the mechanisms that may underlie impaired adipose tissue leptin signaling in obese individuals. Of the several mechanisms that have been proposed to attenuate leptin cellular signaling, we observed a markedly increased caveolin-1 expression in our obese study population. We demonstrate that increased caveolin-1 expression in differentiated HWPs, to levels seen in adipose tissue from obese subjects, prevents leptin-dependent increases in adiponectin expression. These studies suggest that in obesity, the discordant decrease in adiponectin with increases in leptin may be a result of impaired leptin signaling in the adipose tissue of obese individuals. Thus, targeting therapeutics aimed at improving leptin sensitivity via modulation of caveolin-1 expression may be an important strategy to increase adiponectin in obesity.
Some studies including that of Labruna et al have shown that high leptin/adiponectin ratio may be useful as a marker to identify populations at-risk of developing metabolic disorders including insulin-resistance.50 Therefore, we examined the changes in leptin/adiponectin (L/A) ratio with weight gain. We show that modest weight gain is associated with increases in L/A ratio (P<0.001) but the baseline L/A ratio did not correlate with changes in any variables measured including adiponectin and insulin. However, the relative changes in L/A ratio correlate with increases in insulin (P=0.0165, r=0.3638). In view of our findings that leptin regulates adiponectin expression, increases in leptin without concordant increases in adiponectin may suggest the presence of impaired leptin signaling. In other words, increases in leptin/adiponectin ratio may suggest decreased leptin sensitivity. Interestingly, several studies have suggested use of the leptin/adiponectin ratio as a surrogate marker for the presence of insulin-resistance as well.51, 52 The similarities between leptin resistance and insulin resistance are concordant with studies showing that the molecules underlie impaired leptin and impaired insulin signaling and that there is cross-talk between the cellular signaling pathways for leptin and insulin.48, 53, 54 Therefore, the leptin/adiponectin ratio could be a marker for both altered leptin and altered insulin sensitivity.
The strengths of our study include the comprehensive, translational approach ranging from a longitudinal weight gain model in well characterized normal weight healthy humans, and in-vitro studies demonstrating for the first time that leptin increases adiponectin expression in human adipocytes. These are complemented by ex-vivo studies using adipose tissue explants from a cross-sectional study population of varying levels of BMI showing differential effects of leptin in weight gain versus obesity. Our study also has some limitations. While we addressed the physiologic relationships between leptin and adiponectin during weight gain, and its implications in obesity, adiponectin regulation is multifactorial. Our study was not designed to comprehensively address factors other than leptin which would contribute to the low adiponectin expression in obesity. Furthermore, some subjects included in the longitudinal weight gain study had a high baseline total body fat mass; this may have contributed to the variability in response to weight gain. However, baseline fat mass did not show any relationship with either changes in leptin or in adiponectin. Also, subjects included in the cross-sectional population were not well characterized as we did not have data on body composition as well as systemic measures of leptin and adiponectin, and the obese group was significantly older than the normal weight subjects. Age difference may affect caveolin-1, adiponectin, and leptin sensitivity.
The increasing prevalence of obesity, along with associated diabetes mellitus and cardiovascular disease, highlight the compelling need for novel therapeutic strategies aimed at either reducing obesity, or attenuating its association with cardiovascular and metabolic disease. Increasing adiponectin expression is being targeted as a mechanism to decrease cardiometabolic risk.13 Extrinsic adiponectin administration has significant limitations and would require administration of adiponectin at very high concentrations. Therefore, activating endogenous adiponectin expression would be an important potential approach, and requires an understanding of the molecular mechanisms that lead to low adiponectin expression in human obesity. Our study suggests a strategy of targeting leptin resistance so as to reduce obesity-associated metabolic and cardiac disorders, by increasing adiponectin expression. This is especially important as leptin is being explored as a therapeutic agent in treatment of other metabolic disorders including lipodystrophy and diabetes.55
Supplementary Material
Acknowledgments
This work was supported by American Heart Association Scientist Development Grant 11SDG7260046 (to PS); a grant from the European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123) (to PS, FLJ, TK and VKS), NIH grant [DK81014; HL73211; HL65176] (to VKS) and DK45343 (to MDJ)]; the National Center for Research Resources (grant 1UL1 TR000135), which is a component of the NIH; and the NIH Roadmap for Medical Research.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Fruhbeck G. The Sir David Cuthbertson Medal Lecture. Hunting for new pieces to the complex puzzle of obesity. The Proceedings of the Nutrition Society. 2006;65(4):329–347. doi: 10.1017/s0029665106005106. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. The Journal of clinical investigation. 2006;116(7):1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. The Journal of biological chemistry. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 7.Heilbronn LK, Campbell LV, Xu A, Samocha-Bonet D. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS One. 2013;8(10):e78864. doi: 10.1371/journal.pone.0078864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brons C, Jensen CB, Storgaard H, Hiscock NJ, White A, Appel JS, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. The Journal of physiology. 2009;587(Pt 10):2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ukkola O, Teran-Garcia M, Tremblay A, Despres JP, Bouchard C. Adiponectin concentration and insulin indicators following overfeeding in identical twins. Journal of endocrinological investigation. 2008;31(2):132–137. doi: 10.1007/BF03345579. [DOI] [PubMed] [Google Scholar]
- 10.Astrand O, Carlsson M, Nilsson I, Lindstrom T, Borga M, Nystrom FH. Weight gain by hyperalimentation elevates C-reactive protein levels but does not affect circulating levels of adiponectin or resistin in healthy subjects. European journal of endocrinology / European Federation of Endocrine Societies. 2010;163(6):879–885. doi: 10.1530/EJE-10-0763. [DOI] [PubMed] [Google Scholar]
- 11.Cahill F, Amini P, Wadden D, Khalili S, Randell E, Vasdev S, et al. Short-term overfeeding increases circulating adiponectin independent of obesity status. PLoS One. 2013;8(8):e74215. doi: 10.1371/journal.pone.0074215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguilar-Salinas CA, Garcia EG, Robles L, Riano D, Ruiz-Gomez DG, Garcia-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. The Journal of clinical endocrinology and metabolism. 2008;93(10):4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 13.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30(5):234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Makimura H, Mizuno TM, Bergen H, Mobbs CV. Adiponectin is stimulated by adrenalectomy in ob/ob mice and is highly correlated with resistin mRNA. American journal of physiology. 2002;283(6):E1266–E1271. doi: 10.1152/ajpendo.00227.2002. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Della-Fera MA, Hartzell DL, Hausman D, Baile CA. Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life sciences. 2008;83(1–2):35–42. doi: 10.1016/j.lfs.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Delporte ML, El Mkadem SA, Quisquater M, Brichard SM. Leptin treatment markedly increased plasma adiponectin but barely decreased plasma resistin of ob/ob mice. American journal of physiology. 2004;287(3):E446–E453. doi: 10.1152/ajpendo.00488.2003. [DOI] [PubMed] [Google Scholar]
- 17.Huan JN, Li J, Han Y, Chen K, Wu N, Zhao AZ. Adipocyte-selective reduction of the leptin receptors induced by antisense RNA leads to increased adiposity, dyslipidemia, and insulin resistance. J Biol Chem. 2003;278(46):45638–45650. doi: 10.1074/jbc.M304165200. [DOI] [PubMed] [Google Scholar]
- 18.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. The Journal of clinical endocrinology and metabolism. 2002;87(5):2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 19.Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4531–4536. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26(8):2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. European journal of endocrinology / European Federation of Endocrine Societies. 2002;147(2):173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, Peterson TE, Sert-Kuniyoshi FH, Glenn JA, Davison DE, Romero-Corral A, et al. Leptin signaling in adipose tissue: role in lipid accumulation and weight gain. Circ Res. 2012;111(5):599–603. doi: 10.1161/CIRCRESAHA.112.273656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. Journal of the American College of Cardiology. 2010;56(8):662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi T, Sert-Kuniyoshi FH, Calvin AD, Singh P, Romero-Corral A, van der Walt C, et al. Effect of Weight Gain on Cardiac Autonomic Control During Wakefulness and Sleep. Hypertension. 2011;57(4):723–730. doi: 10.1161/HYPERTENSIONAHA.110.163147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero-Corral A, Sert-Kuniyoshi FH, Sierra-Johnson J, Orban M, Gami A, Davison D, et al. Modest Visceral Fat Gain Causes Endothelial Dysfunction in Healthy Humans. Journal of the American College of Cardiology. 2010;56(8):662–666. doi: 10.1016/j.jacc.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh P, Somers VK, Romero-Corral A, Sert-Kuniyoshi FH, Pusalavidyasagar S, Davison DE, et al. Effects of weight gain and weight loss on regional fat distribution. American Journal of Clinical Nutrition. 2012;96(2):229–233. doi: 10.3945/ajcn.111.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Matheny MK, Tumer N, Mitchell MK, Scarpace PJ. Leptin antagonist reveals that the normalization of caloric intake and the thermic effect of food after high-fat feeding are leptin dependent. American journal of physiology. Regulatory, integrative and comparative physiology. 2007;292(2):R868–R874. doi: 10.1152/ajpregu.00213.2006. [DOI] [PubMed] [Google Scholar]
- 28.Tumer N, Erdos B, Matheny M, Cudykier I, Scarpace PJ. Leptin antagonist reverses hypertension caused by leptin overexpression, but fails to normalize obesity-related hypertension. J Hypertens. 2007;25(12):2471–2478. doi: 10.1097/HJH.0b013e3282e9a9fd. [DOI] [PubMed] [Google Scholar]
- 29.Shpilman M, Niv-Spector L, Katz M, Varol C, Solomon G, Ayalon-Soffer M, et al. Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem. 2011;286(6):4429–4442. doi: 10.1074/jbc.M110.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284(46):31936–31944. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simons PJ, van den Pangaart PS, Aerts JM, Boon L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. The Journal of endocrinology. 2007;192(2):289–299. doi: 10.1677/JOE-06-0047. [DOI] [PubMed] [Google Scholar]
- 32.Hajri T, Tao H, Wattacheril J, Marks-Shulman P, Abumrad NN. Regulation of adiponectin production by insulin: Interactions with tumor necrosis factor-alpha and interleukin-6. American journal of physiology. doi: 10.1152/ajpendo.00307.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tam CS, Covington JD, Bajpeyi S, Tchoukalova Y, Burk D, Johannsen DL, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab. 2014;99(5):1749–1757. doi: 10.1210/jc.2013-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alligier M, Meugnier E, Debard C, Lambert-Porcheron S, Chanseaume E, Sothier M, et al. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97(2):E183–E192. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- 35.Tam CS, Viardot A, Clement K, Tordjman J, Tonks K, Greenfield JR, et al. Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes. 2010;59(9):2164–2170. doi: 10.2337/db10-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. The Biochemical journal. 2010;425(1):41–52. doi: 10.1042/BJ20091045. [DOI] [PubMed] [Google Scholar]
- 37.Khan T, Hamilton MP, Mundy DI, Chua SC, Scherer PE. Impact of simvastatin on adipose tissue: pleiotropic effects in vivo. Endocrinology. 2009;150(12):5262–5272. doi: 10.1210/en.2009-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razani B, Combs TP, Wang XB, Frank PG, Park DS, Russell RG, et al. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J Biol Chem. 2002;277(10):8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- 39.Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15(2):171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuroki M, O'Flaherty JT. Extracellular signal-regulated protein kinase (ERK)-dependent and ERK-independent pathways target STAT3 on serine-727 in human neutrophils stimulated by chemotactic factors and cytokines. The Biochemical journal. 1999;341(Pt 3):691–696. [PMC free article] [PubMed] [Google Scholar]
- 41.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Molecular and cellular biology. 1997;17(11):6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gough DJ, Koetz L, Levy DE. The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and Ras-mediated transformation. PLoS One. 2013;8(11):e83395. doi: 10.1371/journal.pone.0083395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Rourke L, Shepherd PR. Biphasic regulation of extracellular-signal-regulated protein kinase by leptin in macrophages: role in regulating STAT3 Ser727 phosphorylation and DNA binding. The Biochemical journal. 2002;364(Pt 3):875–879. doi: 10.1042/BJ20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, Neupane M, Zhou HR, Wu D, Chang CC, Moustaid-Moussa N, et al. Leptin differentially regulate STAT3 activation in ob/ob mouse adipose mesenchymal stem cells. Nutr Metab (Lond) 2012;9(1):109. doi: 10.1186/1743-7075-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bravard A, Vial G, Chauvin MA, Rouille Y, Bailleul B, Vidal H, et al. FTO contributes to hepatic metabolism regulation through regulation of leptin action and STAT3 signalling in liver. Cell Commun Signal. 2014;12:4. doi: 10.1186/1478-811X-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Wu X, He Y, Kastin AJ, Hsuchou H, Rosenblum CI, et al. Melanocortin potentiates leptin-induced STAT3 signaling via MAPK pathway. J Neurochem. 2009;110(1):390–399. doi: 10.1111/j.1471-4159.2009.06144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mark AL. Selective leptin resistance revisited. American journal of physiology. Regulatory, integrative and comparative physiology. 2013;305(6):R566–R581. doi: 10.1152/ajpregu.00180.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16(2):144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. Journal of the American College of Cardiology. 2008;52(15):1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labruna G, Pasanisi F, Nardelli C, Caso R, Vitale DF, Contaldo F, et al. High leptin/adiponectin ratio and serum triglycerides are associated with an "at-risk" phenotype in young severely obese patients. Obesity. 2011;19(7):1492–1496. doi: 10.1038/oby.2010.309. [DOI] [PubMed] [Google Scholar]
- 51.Finucane FM, Luan J, Wareham NJ, Sharp SJ, O'Rahilly S, Balkau B, et al. Correlation of the leptin:adiponectin ratio with measures of insulin resistance in non-diabetic individuals. Diabetologia. 2009;52(11):2345–2349. doi: 10.1007/s00125-009-1508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue M, Yano M, Yamakado M, Maehata E, Suzuki S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism. 2006;55(9):1248–1254. doi: 10.1016/j.metabol.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24(1):1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 54.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106(4):473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. American journal of physiology. Endocrinology and metabolism. 2011;301(4):E567–E584. doi: 10.1152/ajpendo.00315.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


