Abstract
Objective
To determine the role of cell swelling in severe hemorrhagic shock and resuscitation injury.
Summary Background Data
Circulatory shock induces the loss of energy dependent volume control mechanisms. As water enters ischemic cells, they swell, die, and compress nearby vascular structures, which further aggravates ischemia by reducing local microcirculatory flow and oxygenation. Loading the interstitial space with cell impermeant molecules prevents water movement into the cell by passive biophysical osmotic effects, which prevents swelling injury and no-reflow.
Methods
Adult rats were hemorrhaged to a pressure of 30–35 mm Hg, held there until the plasma lactate reached 10 mM, and given a low volume resuscitation (LVR) (10–20% blood volume) with saline or various cell impermeants (sorbitol, raffinose, trehalose, gluconate, and Polyethylene glycol-20k (PEG-20k). When lactate again reached 10 mM following LVR, full resuscitation was started with crystalloid and red cells. One hour after full resuscitation, the rats were euthanized. Capillary blood flow was measured by the colored microsphere technique.
Results
Impermeants prevented ischemia-induced cell swelling in liver tissue and dramatically improved LVR outcomes in shocked rats. Small cell impermeants and PEG-20k in LVR solutions increased tolerance to the low flow state by 2 and 5 fold, respectively, normalized arterial pressure during LVR, and lowered plasma lactate after full resuscitation, relative to saline. This was accompanied by higher capillary blood flow with cell impermeants.
Conclusions
Ischemia-induced lethal cell swelling during hemorrhagic shock is a key mediator of resuscitation injury, which can be prevented by cell impermeants in low volume resuscitation solutions.
Introduction
Deaths due to injury in the US reached over 171,000 and costs over $400 billion a year in health care costs and lost productivity in 2010 1. Deaths from trauma are the number 1 cause of death for people under 44 years of age in the US and the third leading cause of death overall for all age groups. Trauma accounts for about 30% of all life years lost in the US, compared to cancer (16%), heart disease (12%), and HIV (2%) 2. For all traumatic injuries, hemorrhagic shock is responsible for over 35% of pre-hospital deaths and over 40% of all deaths within the first 24 hours. This is second only to trauma deaths induced by severe CNS injury 3. Finally, hemorrhagic hypotension exposes the patient to immediate complications of life threatening infections, coagulopathies, and multiple organ failure 4,5.
Early resuscitation strategies include the use of low volumes of intravenous blood products to increase oxygen delivery and to replace lost coagulation and clotting factors (coagulation proteins and platelets). While this approach is fine for hospital emergency departments, it is not currently practical in pre-hospital settings where early intervention may be the key to preventing future complications following more definitive resuscitation. Crystalloids are available for pre-hospital use because the can be safely transported and stored but they are generally limited in their effectiveness. Attempts to modify basic intravenous crystalloids for pre-hospital resuscitation by adding hypertonic NaCl or starch (Hextend) as a volume expander have had disappointing results 6,7. The future use of effective spray dried blood products will be a valuable tool in pre-hospital settings since they replace chemical coagulation precursors and factors. The use of fresh frozen plasma in the field, which is currently being tested at many centers, will also be useful but it too is limited by the need for refrigeration. There remains a need for a better crystalloid to resuscitate patients with severe hemorrhagic shock, especially in a pre-hospital setting. The successful design of such a solution is highly dependent on understanding the pathophysiological mechanisms that lead to injury during hemorrhagic hypotension and subsequent resuscitation. The optimal solution will likely be an effective new stable crystalloid that targets these mechanisms used together with reconstituted dried plasma products for the replacement and reconstitution of coagulation potential.
The predominant root mechanism of injury in hemorrhagic shock is energy failure. While global ischemia and reperfusion injury are causally based at many levels, they all arise from changes that occur when the cell energetics drops because of a loss of adequate microvascular oxygen transport and subsequent loss of aerobically produced high energy adenine nucleotides 8–10. One mechanism of cell, tissue, and organ injury is cell swelling that occurs from the loss of ATP-dependent cell volume regulatory control mechanisms. In most cells, the single highest energy consuming process is the running of the Na/K ATPase pumps in the cell membrane. These pumps actively transport sodium ions out of the cell to maintain membrane potentials and to run numerous Na+-dependent facilitated membrane transport processes such as calcium, glucose, amino acids, and organic cation transporters. In the absence of ATP to run those pumps, as occurs in ischemia following hemorrhagic shock, the Na/K ATPase turns off and sodium enters the cell as it runs back down its electrochemical gradient. The elevated intracellular sodium futilely stimulates the sodium pump that can’t run because of loss of ATP11. Chloride then enters the cell down an electrical gradient and water follows the sodium chloride down a developing osmotic gradient, which causes the cell to swell. Hydropic degeneration from energy failure damages membrane and mitochondrial structures12, which may lead to cell death.
This basic mechanism of cell ischemic injury has been well described in organ preservation associated with transplantation13–15. Effective modern organ preservation solutions were developed around this concept and contain high concentrations of cell impermeants16. These are classes of non-toxic molecules, usually saccharides and small organic cations and anions, which are small enough to freely egress the capillary space in the microcirculation but are too large or too charged to cross the cell membrane. As such, they preferentially load into the interstitial space where they create an osmotic force that prevents the movement of water into the cell as the sodium concentrations rise during ischemia. They prevent lethal cell swelling. Cell impermeant, as a class of agents, are one of the most effective components of organ preservation solutions used today17. The University of Wisconsin solution contains high amounts of raffinose, lactobionic acid, sulfate, and phosphate, which all act as cell impermeants to prevent water movement. The Belzer-UW MPS solution uses gluconate and HTK solution uses both high concentrations of histidine and mannitol as impermeants. Histidine at physiological pH is charged and is an impermeant. Water movement in organ preservation is slower than ischemia at normal mammalian temperatures because hypothermia is used to preserve organs, which slows down the process. Since cell swelling during ischemia induced by hemorrhagic hypotension also occurs18 and at a much faster rate than in organ preservation because of the warmer temperatures, it was hypothesized that loading the interstitial space with nontoxic cell impermeants during the low volume period would prevent lethal cell swelling and increase the tolerance of the patient to the low volume state and improve outcomes at resuscitation. Testing this was the objective of the study.
Methods
All animal work was conducted under a protocol approved by the VCU Institutional Animal Care and Use Committee, which is governed by the rules and regulations set forth in the NIH guide and the USDA.
In-Vitro Model
Warm ischemia-induced cell swelling and the effects of cell impermeants on this response was first characterized in mouse liver slices. Liver slices have been used before to characterize cell impermeants 19 since they are easy to prepare and there is abundant mass for many groups per liver. Adult mice (C57BL/6) were anesthetized with isoflurane and the liver was isolated, quickly removed, and immersed in cold saline on ice. Liver slices (3–4 per condition) were prepared with a Staddie-Riggs microtome to give a uniform thickness of < 0.5 mm. About 150 mg of liver slices were incubated in 25-ml Erlenmeyer flasks in 1.5 ml Krebs buffer in a Dubanoff style metabolic shaking water bath under an atmosphere of oxygen or nitrogen always containing 5% CO2. Tissue slices underwent ischemia by incubation under an atmosphere of 95% nitrogen and 5% CO2 for 1 hour followed by reperfusion under an atmosphere of 95% oxygen and 5% CO2 for an additional hour. Some tissue slice conditions contained impermeants in the Krebs buffer during ischemia and some did not (controls). Impermeants were used at 0, 25, 50, 100, and150 mM final concentration. These impermeants consisted of sorbitol, gluconate, trehalose, raffinose, and an equal molar mixture of raffinose and trehalose. Tissue slices were sampled after preparation (Fresh), after ischemia, and after reperfusion in untreated and impermeant treated groups for analysis of total tissue water (TTW) content by calculating [wet-dry]/dry weight ratios. Dry weights were determined after drying the tissue slices in a 65° C oven for 48 hours.
Rodent Shock Model
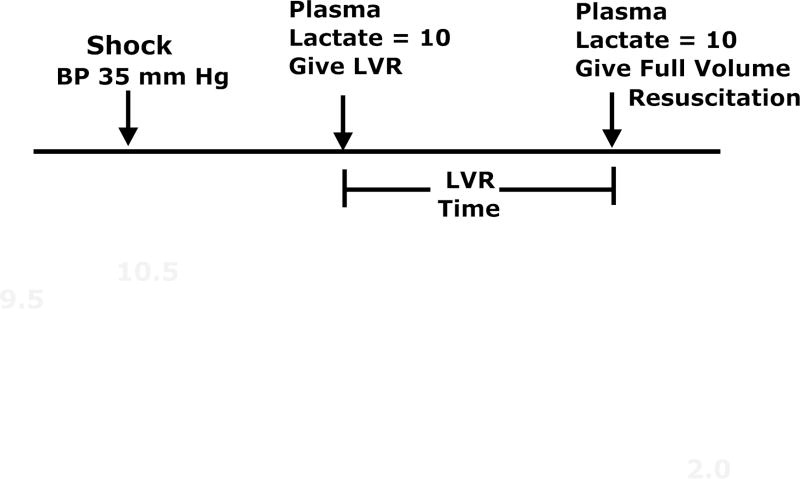
A low volume resuscitation (LVR) model was used in adult rats to test both the cell swelling hypothesis and to develop the impermeant based LVR solution used for pre-hospital resuscitation of patients with severe hemorrhagic shock. Adult Sprague Dawley rats were anesthetized with isofluorane and maintained in a light surgical plane of anesthesia during the study. Polyethylene catheters were placed in both femoral arteries for blood pressure monitoring and blood sampling and a catheter was placed in one femoral vein for administration of fluids. The animals were allowed to ventilate on their own to establish normal arterial blood gas (ABG) values. A 1-cm midline incision was created to induce soft tissue injury and for the placement of a temperature probe in the abdomen. The animals were kept at 38 C using a heating pad and an incandescent light source above them. Arterial blood pressure, heart rate, and temperature were continuously recorded using a PowerLab (ADInstruments, Boston, MA). After a 30 min stabilization period, heparin was given (500 U/kg) and arterial blood was slowly removed at 1 ml/min into a syringe to maintain blood pressure at 30–35 mm Hg. This hypotension was maintained until the plasma lactate reached a value between 9–10 mM, as measured with both a hand held lactate analyzer (Lactate Plus, Nova Biomedical, Waltham, MA) and a blood gas analyzer (Radiometer 800). Once the target lactate was reached, a low volume resuscitation equal to 10–20% of the calculated blood volume20 of saline was administered I.V. over a 10 min period using a syringe infusion pump. When the blood lactate again reached 9–10 mM, full resuscitation was started, which consisted of a volume of saline equal to the volume of the blood loss (about 55–60% of total blood volume) plus 30% of the removed red blood cells (washed) infused I.V. over 10 minutes (while this full resuscitation protocol using saline is now outdated, the study was started when it was acceptable to use saline so the authors finished the project using the same protocol). After 1 hour of full resuscitation, the animals were euthanized by an anesthetic overdose and terminal blood was removed for analysis. The time from the start of the LVR period until the start of full resuscitation is called the LVR time and it represents the tolerance of the animal to the low volume state or the maximum amount of time that a shocked subject can safely remain in the low volume state until more definitive resuscitation is required. This was a major outcome used in the study. The protocol is illustrated in Figure 1.
Figure 1.
The Low Volume Resuscitation (LVR) protocol that was used in these studies. Arterial hemorrhage is used to maintain a low volume state (30–35 mm hg) until the plasma lactate reaches 9–10 mM. At that time, a low volume resuscitation solution is given, which temporarily reduces plasma lactate (due to dilution and increased perfusion) and increases arterial blood pressure. When lactate again reaches 10 mM, full resuscitation is started with 1 volume of saline containing 30% of the washed red cells that were hemorrhaged. The time from the start of the LVR solution until the start of the full resuscitation solution is called the LVR time and it represents the tolerance of the patient to the low volume state or the maximum amount of time that a patient can safely remain in the low volume state until more definitive medical care and full resuscitation can be started. The LVR time was a key outcome variable for these studies.
Regional Blood Flow
In another series of studies (n=6 per group), the effects of hemorrhagic shock, low volume resuscitation, and LVR with impermeants on local capillary blood flow were studied using the colored microsphere technique 21,22. Animals were prepared as previously described but a catheter was also placed into the left ventricle by advancing the catheter through the right carotid artery using real time pressure and pressure waveforms as indicators of the catheter location. Once all catheters were in place, 0.2 ml colored microspheres (Triton Technologies, San Diego, CA) were rapidly injected into the left ventricle. A calibrated arterial reference blood sample was simultaneously removed from the femoral artery catheter by a withdrawal pump to calibrate the microsphere measurement. Three different microsphere colors were used at baseline, during LVR (immediately before full resuscitation), and 60 min after full resuscitation. After the study, tissue samples were removed from major organs and the microspheres in the tissues and in the reference arterial blood samples were recovered by alkaline digestion and repeated centrifugations. Dye coating the purified microspheres was extracted with dimethylformamide and quantitiated using a UV-VIS spectrophotometer (Shimadzu). Individual colors were resolved using a matrix inversion algorithm from the composite spectra. Blood flow was calculated by the tissue dye content using the reference blood draw as a standard.
Experimental Design
Shocked animals were treated according to the following groups:
Saline Controls: Received saline as the LVR solution (n=12)
Gluconate: Received a LVR solution of 15% gluconate in saline, a prototypical cell impermeant (n=11).
Gluconate + PEG-20k: Received a LVR solution of 15% gluconate and 10% polyethylene glycol with a molecular weight of 20 kDa (PEG-20k). PEG-20k acts as an oncotic agent (n=8)
PEG-20k: Received a LVR solution of 10% PEG-20k (n=6)
BSA: Received a LVR solution of 10% Bovine Serum Albumin (BSA, a prototypical oncotic agent, (n=6)
The outcome variables for the study included LVR time, plasma lactate, mean arterial blood pressure, and regional tissue blood flow rates.
Statistical Analysis
Most data are expressed as the group mean +/− the standard deviation. Each group consisted of 6–12 subjects per group, which was derived from power analysis and the known variance of the data in the studies. Data were analyzed by ANOVA and Bonferroni’s multiple comparison test. All data were first analyzed for normality of distribution. A P value < 0.05 was considered statistically significant.
Results
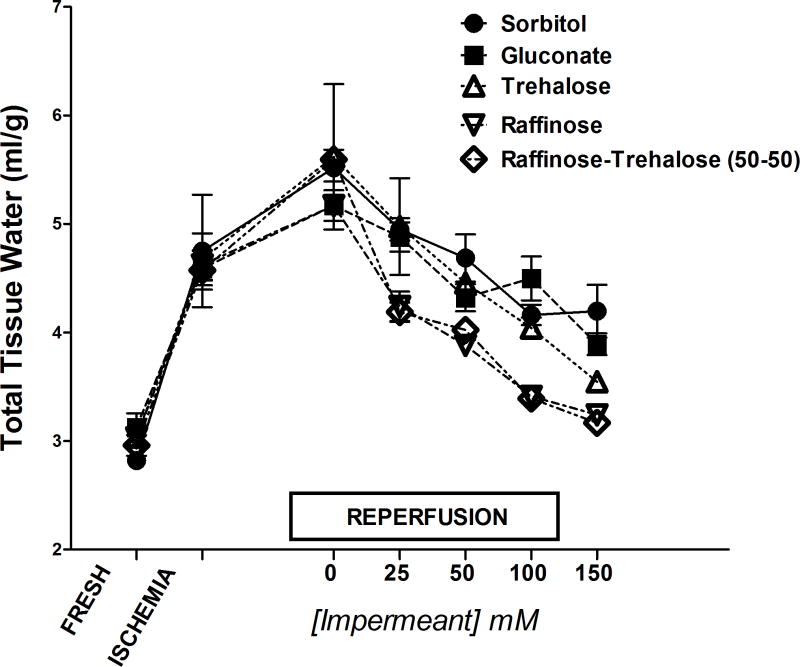
The impermeant effects of a variety of common cell impermeants in the in-vitro tissue slice model is shown in Figure 2. Total tissue water measurements indicate that 60 minutes of hypoxic ischemia to murine liver slices caused tissue water accumulation to increase almost 2 fold after ischemia alone and after 1 hour of normoxic reperfusion. The addition of all of the molecular species of cell impermeants to the incubation media during ischemia prevented the ischemia-induced water accumulation after reperfusion. The magnitude of the response was generally directly proportional to both the molecular weight of the impermeant and the molar concentration in the media (25–150 mM). The optimal responses were observed with raffinose and mixtures of raffinose and trehalose used at about 60–100 mM.
Figure 2.
Cell swelling of liver tissue slices in-vitro in response to hypoxic ischemia and the effects of various concentrations of cell impermeants on the cell swelling response. Cell swelling was indexed by measuring total tissue water (TTW) of the liver slices an hour after ischemia and an hour after normoxic reperfusion or in fresh controls. Cell impermeants were in the Krebs buffer suffusing the slices during ischemia. In general, the impermeant effect is proportional to the molecular weight of the impermeant and its concentration in the extracellular space. n=6 liver samples per group, values are mean ± SD. Each impermeant group also has a zero concentration control, which sees ischemia and reperfusion without any impermeants.
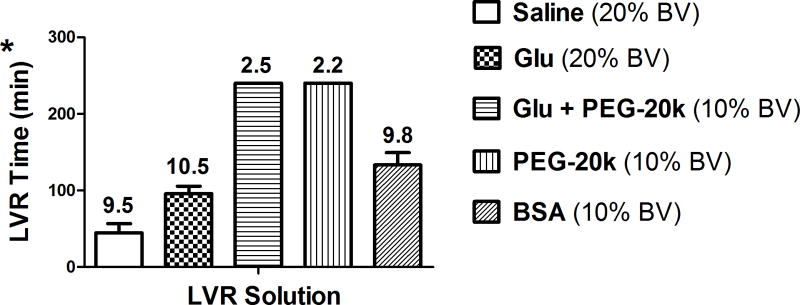
The amount of time that a shocked subject can safely remain in the low volume state is indexed by the LVR time in this experiment. These times are shown in Figure 3 for the various treated groups of shocked rats. The trigger to end the LVR period after the LVR solutions were given was the lactate climbing back up to 9–10 mM. Gluconate (15%) added to the saline control LVR solution increased the LVR time by 100% from about 45 minutes for the saline control to over 96 minutes for the gluconate solution. The addition of 10% PEG-20k to the gluconate LVR solution further increased the LVR time 5.3 fold over the saline control to 240 minutes. This LVR time was arbitrarily stopped because of anesthesia effects but it likely could have gone much longer since the target lactate of 10 mM was never reached even after 240 minutes after the start of the LVR solution. The lactate in the gluconate + PEG-20k group after 240 min was only 2.5 mM. Similarly, the LVR time in the group with only PEG-20k was also ended after 240 minutes with a lactate of only 2.2 mM. Since the time limits of these two groups was never met because the target lactates were never met, we do not know if there is in fact a difference in the two groups with respect to LVR time. Finally, LVR solutions containing 10% BSA were also effective at increasing the LVR time (133 min) but not nearly as effective as LVR solutions containing PEG-20k. It is also important to note that the volume of LVR solution used that contained PEG-20k was half the volume (10%) used in the other groups (20%: saline control, gluconate, and BSA). Thus, PEG-20k based LVR solutions were over 5 times more effective at expanding the LVR time compared to saline, at half the dose.
Figure 3.
The Low Volume Resuscitation (LVR) time measured in 5 groups of shocked rats. The LVR time is the time from the start of the low volume resuscitation until the time of full resuscitation, based on the response of the plasma lactate levels. The values are mean ± SD with 6–12 animals per group. The numbers above the bars are the average plasma lactate value at the end of LVR. Since 10 was the cut off to end LVR by definition, most of the lactate values are very close to 10. However, the two groups with PEG-20k in the LVR solution were arbitrarily ended at a LVR time of 240 min and the ending lactate values were still far below the target of 10 mM. The standard LVR volume was chosen to be 20% of the calculated blood volume but this had to be cut in half for the PEG-20k groups because the response and diuresis were too intense. * P< 0.05 between all groups except between the two groups with PEG-20k, GLU = sodium gluconate.
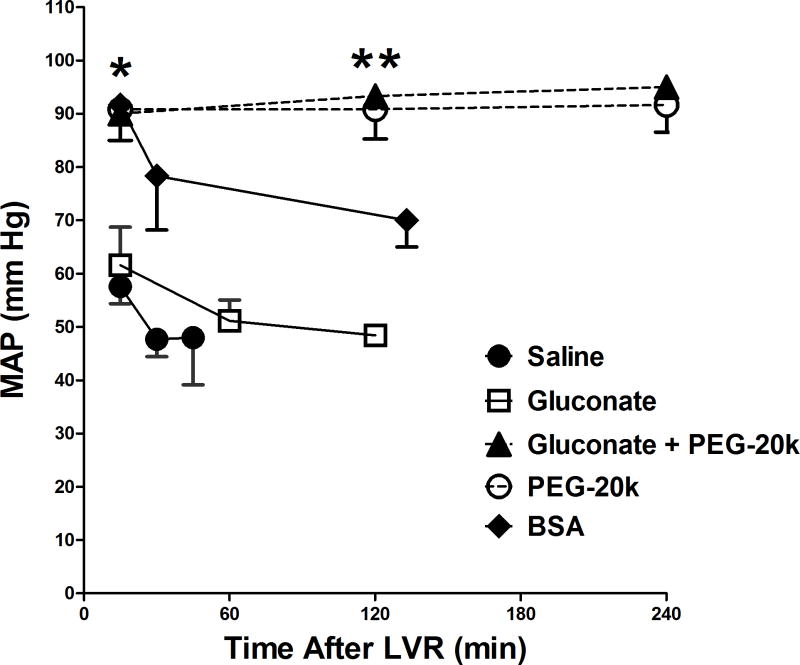
The mean arterial blood pressure in rats after shock and after administration of the LVR solution (for as long as the LVR period lasted) is shown in Figure 4. In the saline controls, the blood pressure after the shock period was 30–35 mm Hg, by definition of the model. After 10 min of saline LVR administration, the MAP rose initially to about 55 mm Hg but then rapidly fell back below 50 mm Hg as the LVR period ended after 45 min, due to the lactate reaching 10 mM. The gluconate group showed a similar pattern. Although the MAP did not get higher than the control group, it did last longer since gluconate doubled the LVR time. Groups resuscitated with PEG-20k in the LVR solution, however, had normal MAP throughout the 240 min LVR period and this was accomplished with only 50% of the LVR resuscitation volume of the controls. The BSA treated oncotic controls started with a normal blood pressure immediately after LVR solution administration, which fell off to about 70 mm Hg at the end of the LVR period. This was significantly higher than the control MAP but significantly lower than the MAP for the groups resuscitated with LVR solutions containing PEG-20k.
Figure 4.
Mean arterial pressure (MAP) in animals during the LVR time after hemorrhagic shock in the 5 groups of shocked rats. Values are mean ± SD, n=6–12 per group, * P<0.05 relative to the gluconate and saline groups,** P< 0.05, relative to the BSA and gluconate groups (at the same approximate time point).
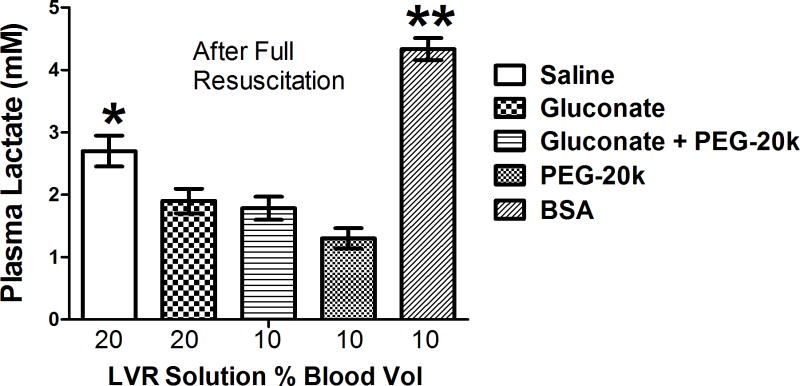
Figure 5 shows the final plasma lactate levels in shocked rats after LVR and one hour after full resuscitation. The lactate levels were all significantly lower in animals given a LVR solution with an impermeant (gluconate, PEG-20k, or both) relative to the saline control group. Lactate in the BSA group after full resuscitation was significantly higher than all of the groups, including the saline controls.
Figure 5.
Plasma lactate values measured after one hour of full resuscitation in the 5 shocked rat groups. Values are mean ± SD, n=6–12 animals per group, * ** P< 0.05 relative to every other group.
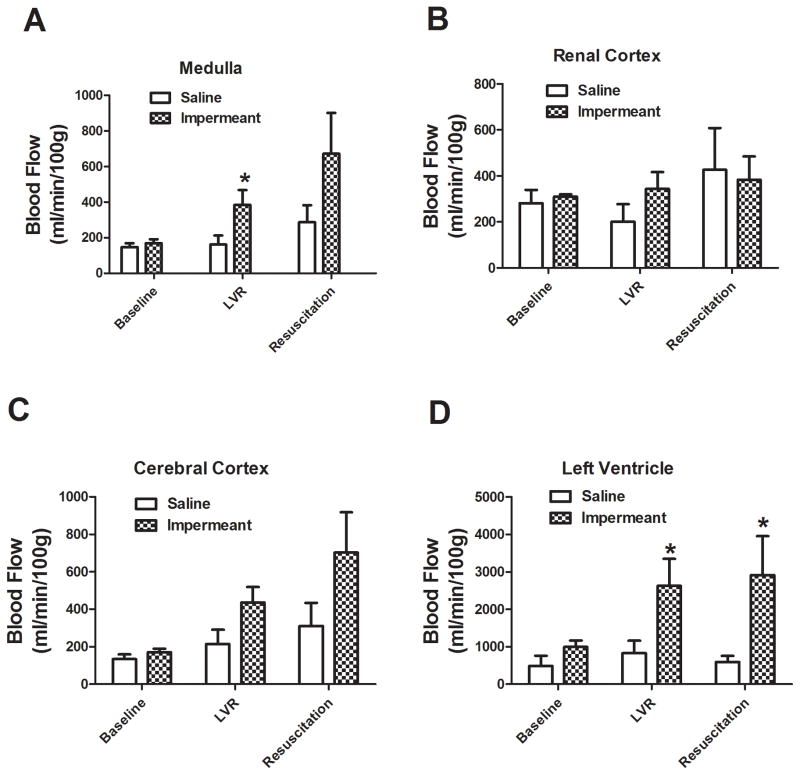
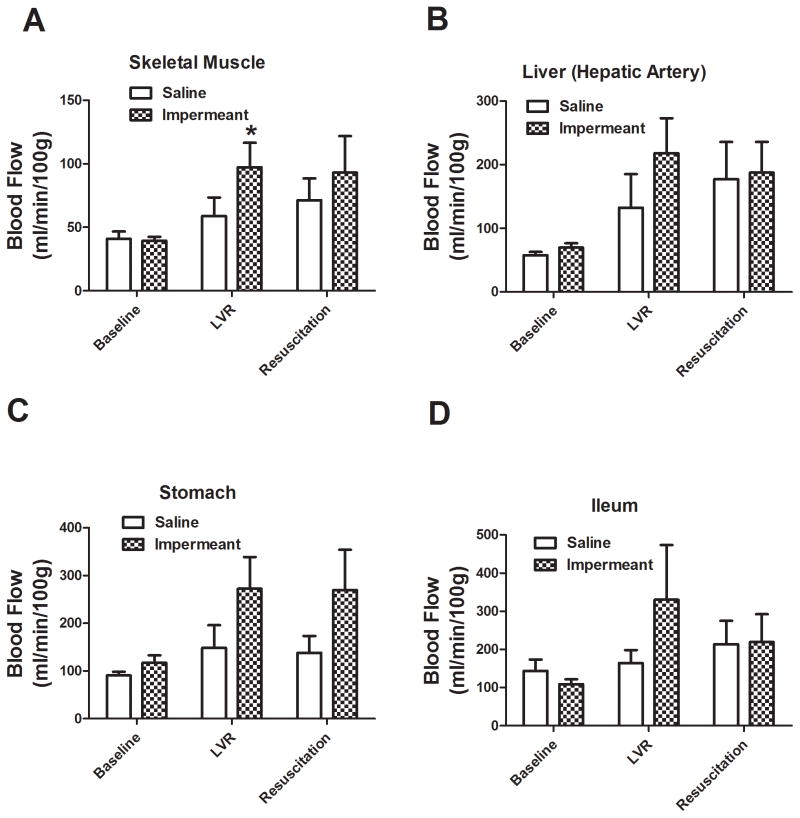
Regional capillary blood flow in major organs and tissues in shocked rats treated with gluconate or with saline is shown in Figures 6 and 7. Local blood flow in the skeletal muscle, left ventricle, and brain (medulla) was significantly higher during the LVR period when an impermeant based LVR solution was used, compared to saline. There were higher trends in other tissue beds too. After full resuscitation, regional blood flow was significantly higher in the left ventricle after impermeant based resuscitation compared to saline. Again, there were strong trends in other beds.
Figure 6.
Capillary blood flow in medulla, cerebral cortex, renal cortex, and left ventricle at baseline, after the LVR period, and after the full resuscitation period in 6 rats per group. The two groups compared were saline LVR solution (Saline) and a saline LVR solution with an impermeant mixture. * P< 0.05 relative to the corresponding value for saline. Capillary blood flow was measured using the colored microsphere technique.
Figure 7.
Capillary blood flow in skeletal muscle, stomach, liver (hepatic artery flow), and terminal ileum at baseline, after the LVR period, and after the full resuscitation period in 6 rats per group. The two groups compared were saline LVR solution (Saline) and a saline LVR solution with an impermeant mixture. * P< 0.05 relative to the corresponding value for saline. Capillary blood flow was measured using the colored microsphere technique.
Arterial Blood Gas (ABG) data are shown in the table for rats given saline, saline with gluconate, or saline with gluconate + PEG20k during the low volume resuscitation period. ABG parameters are reported for each group after the baseline period before shock, after the hemorrhagic shock period, and after the low volume resuscitation period (immediately before full resuscitation). In all groups, the changes in the ABG data from baseline to shock are predictable and not different between groups. Specifically, lactate rose to 10 mM in each group because the amount of shock that was induced was titrated and controlled to that level of oxygen debt (lactate). Additionally, HCO3- and pCO2 values fell as the pH remained unchanged. After LVR, however, some differences in the ABG data were apparent between the group receiving PEG20k in the LVR solution and the other groups. Specifically, PEG20k LVR prevented lactate levels from significantly rising above baseline (1.2 ± 0.4 mM during baseline Vs. 2.6 ± 1.1 mM after a 240 min LVR period). A significant metabolic alkalosis with higher HCO3- and higher pH was also observed with PEG-20k resuscitation, relative to the other LVR groups. The pH of all of the LVR solutions was 7.2.
Discussion
Severe hemorrhagic shock in the field can be life threatening because the blood pressure drops and the microcirculatory exchange capacity deteriorates, which cause the delivery of oxygen to tissues (DO2) to fall. First responders are severely limited in what they can do to stabilize the DO2. Recognizing now that high volume crystalloid resuscitation that was once used to raise perfusion pressure is harmful, pre-hospital care now amounts to delivering low volume resuscitation solutions. Given those constraints, low volume resuscitation (< 500 ml) should be looked upon as a vehicle to deliver agents that increase tolerance to the low volume state rather than as a temporary volume expander to raise blood pressure per se. This is best accomplished by targeting significantly important causal mechanism and pathways of global ischemia and resuscitation injury. This study targeted cell swelling, which is a very specific and highly underestimated mechanism that contributes to the phenotypic changes associated with hemorrhagic shock and global ischemia.
Hemorrhagic shock is characterized by changes secondary to the loss or reduction in cellular energetics. As the cell ATP levels fall because of low oxygen delivery, the cell begins to lose ATP dependent processes, including the active volume control mechanisms driven by the Na/K ATPase pump. Hydropic cellular degeneration then leads to cell and organelle membrane dysfunction, which can cause cell homeostasis abnormalities, lysis, and death. Furthermore, swollen parenchymal cells compress capillary exchange vessels to reduce capillary blood flow, which causes more ischemia and swelling in a vicious cycle. Although this mechanism is well appreciated in preservation injury of organs stored for transplantation, it is mysteriously unappreciated in global warm ischemia associated with shock, stroke, or infarction injury. The main objective of this study was to test this mechanism of shock by attempting to reverse it with cell impermeants that are known to prevent cell swelling but have few other biological effects. The results are clear, dramatic, and may represent a significant step forward in treating severe hemorrhagic shock with low volume crystalloid based resuscitation, especially in an austere pre-hospital environment.
Cell swelling plays a major role in organ preservation injury and may do the same in circulatory shock after trauma. Organ Preservation causes cell swelling because depletion of ATP during cold ischemia and cold per se cause disruption of the normal ATP-dependent cell volume control mechanisms. Cell swelling is a major contributor to preservation injury in recovered donor organs since it can be largely mitigated by using cell impermeants in organ preservation solutions. In fact, cell impermeants are one of the most important and effective components of modern day organ preservation solutions 17. The concept is simple, load the interstitial space with molecules that escape the capillary but are impermeant to the cell membrane. This preferentially increases the extracellular osmolarity and prevents water from moving into the cell, which is its natural propensity when the intracellular sodium concentration increases after ischemia-induced pump failure. A similar mechanism is proposed to exist in ischemic shock and a similar solution was tried since most cell impermeants are relatively nontoxic and can be administered in high enough concentrations to be theoretically effective at preventing ischemic cell swelling. The evidence supporting this parallel mechanism is seen in the impermeant effects on liver cell swelling after warm ischemia and the effects of impermeants on LVR times, blood lactate levels after shock, and capillary blood flow to major organs during shock. As cell swelling is prevented with gluconate in the LVR solutions, microcirculatory exchange improves, which lowers plasma lactate values in treated subjects. This is manifest as both increasing LVR times and higher capillary blood flow to vital organs with impermeant treatment during the low volume period. This is consistent with reduced swelling compression on microcirculatory exchange vessels and reduced obstructive swelling of endothelial cells forming the capillary lumen 23–25, which allows for better capillary perfusion and more efficient cellular metabolism (lower lactates). All these data are consistent with the hypothesis that circulatory shock states promote cell swelling, which is an important cause of tissue, organ, and systems injury, acting in part, through a microvascular mechanism.
In an attempt to further test the cell swelling hypothesis and to make impermeant treatment more effective, a model of a microcirculatory osmotic gradient was developed and tested. In the osmotic gradient model, three microvascular compartments are identified in a shocked patient as intracellular, interstitial (I.S.), and the capillary compartment. An osmotic gradient could be established both between the intracellular and extracellular space by the use of conventional cell impermeants (like gluconate), which occupy both capillary and interstitial spaces, and a gradient could be established between the interstitial and capillary spaces by the addition of an oncotic agent to the circulation, which only occupies the capillary space. The combination of impermeant and oncotic agent would create this double gradient, which may not only prevent cell swelling but also keep water flowing out of the interstitial space and into the capillary space where it belongs. There, the water that would otherwise have entered the ischemic cells in the tissue will expand the circulatory volume and promote capillary blood flow and oxygen exchange, which mitigates the shock state by increasing the efficiency of oxygen delivery during low flow. When this was tried by combining both gluconate (an impermeant) with PEG-20k, an oncotic agent, a huge potentiation effect was seen on LVR times. Furthermore, blood pressure during the low volume resuscitation period was completely normalized with PEG20k LVR solutions. While gluconate doubled the LVR time, relative to the saline control, gluconate and PEG-20k increased the LVR time 5–6 fold. It is not even known what the upper limits are to this effect since the PEG-Gluconate studies were cut off by the experimenter 5 hours after the start of the LVR period. At that time, the lactate levels were still only at about 2.5 mM, far below the threshold of 10 mM needed to trigger full resuscitation in our model. Furthermore, the volume of PEG-20k or gluconate needed for this effect was half of the volume used for the saline control LVR group. This supports the concept that the addition of PEG-20k served to move water into the capillary space where it supports intravascular volume, blood pressure, and microcirculatory flow. The latter is supported by the low plasma lactate levels throughout the LVR period, which suggest good microcirculatory flow secondary to high capillary driving pressures (fluid expansion). What this means clinically is that a severely shocked patient (MAP in the 40’s with 50% blood loss) can receive half of the volume of an impermeant based low volume resuscitation solution (I.V) and safely remain in the low volume state for at least 6 times longer than if conventional saline resuscitation were used, before definitive full resuscitation is needed.
LVR solutions containing PEG20k largely prevent the accumulation of lactate in the blood even after 240 minutes after the initial 55% blood volume hemorrhage. Accompanying the low lactate was a slightly higher pH and a significantly higher bicarbonate concentration (double that of the other LVR groups). This metabolic alkalosis served to correct the lactacidosis of the low volume state and is likely of renal origin since the pCO2 remained normal and the pH of the LVR solutions were held at 7.2. After administration of the LVR solution in these studies, we always observed a temporary diuresis, which was attributable to the osmotic retention of water in the renal tubules secondary to PEG20k filtration across Bowman’s space and trapping in the tubular lumen. This diuresis (and maybe a concomitant naturesis) may result in the significant excretion of hydrogen ions into the urine resulting in a normalization of pH and even a slight alkalosis. Metabolic studies are needed to define this possible mechanism. In any case, preserving proper pH during shock and low volume resuscitation may help maintain the normal blood pressure observed in the PEG20k LVR group.
To test the oncotic-impermeant model further, we conducted studies using albumin and PEG-20k alone in the LVR solution. Since high molecular weight PEG molecules are known to have other biological properties besides their oncotic ones, we used the physiological prototype oncotic agent albumin as an oncotic control. Albumin used alone to control for oncotic effects was not at all as effective as PEG-20k alone but it was better than saline. This suggests that there is something different about PEG-20k. The effects of PEG-20k in LVR shock models are attributable to more than just purely oncotic properties. There are two reasonable possibilities to consider; 1.) The involvement of non-oncotic PEG effects such as PEG’s known effects on cell membranes, protein binding and hydration properties, or immunocamouflage effects, or 2.) Oncotic-impermeant hybrid effects, where, because of the unique molecular weight and attributes of PEG-20k, it is able to act both as a cell impermeant and as an oncotic agent.
There is evidence to support the idea that PEG-20k acts as both a cell impermeant by escaping the capillary space while remaining impermeable to the cell and as an oncotic agent whereby a large amount of the material remains trapped in the capillary space. This property may be caused by a slow equilibration time to cross the capillary barrier into the interstitial space, based on its size and other attributes. In fact, PEG-20k has been shown to effectively expand the vascular space and move water out of the interstitial space to stimulate thirst in rats and pigeons26, which demonstrates its oncotic effects. It has also been detected immunohisochemically in renal tubule epithelium and in monocytes in the liver and lung after I.V. administration, suggesting that it leaves glomerular capillaries and hepatic sinusoids27, which demonstrates its partial impermeant effects. Our own studies and observations indicate that it leaves Bowman’s space since a significant but temporary diuresis is seen in rats after severe shock after receiving PEG-20k in the LVR solution. This diuresis may have been due to PEG-20k induced restoration of the arterial pressure during the LVR period or it may have been due to an osmotic diuresis from PEG being filtered and trapped in the renal tubules, similar to mannitol. The latter is more plausible since we do not see a diuresis in shocked rats when their blood pressure is normalized using conventional resuscitation solutions but we do with PEG-20k solutions. Normalization of renal perfusion pressure and the institution of a mild filtration may be desirable during a shock state, as long as the diuresis does not jeopardize the newly normalized blood pressure. There is no evidence in our studies that this happens. The renal effects would tend to prevent the development of ATN after resuscitation. The diuresis observed in this study with PEG-20k is temporary and dose dependent since a 10% blood volume LVR dose of PEG-20k produces much milder diuresis compared to a 20% blood volume LVR dose. That’s why we decreased the dose of the LVR solutions containing PEG-20k from 20% to 10%. The molecular weight of PEG-20k appear to be right on the size limit for partial capillary permeability since higher molecular weights approaching 30 kDa do not cross capillary spaces including Bowman’s space27. A proposed hybrid oncotic-impermeant property of PEG-20k is also consistent with the observation from our study that PEG-20k was as effective alone as it was in combination with gluconate. In essence, partial capillary permeability characteristics of PEG-20k may allow enough osmotically active material to escape into the interstitial space to mimic the impermeant effect of gluconate while the majority of material stays behind in the capillary to act oncotically. Therefore, gluconate or other impermeants may not be necessary in LVR solutions using just PEG-20k alone, but further testing in survival studies is needed.
There are limitations to this study and to its projected clinical use. Our study used a controlled hemorrhagic shock model, which is highly relevant in limb or extremity injuries in the field or other compressible hemorrhagic injuries. In both cases, good hemorrhage control can be achieved with tourniquets and compression techniques. This stops the bleeding and allows the impermeant based LVR solutions to expand the circulatory volume, drive up arterial pressure, and improve flow and oxygen exchange (in addition to protecting tissues from lethal cell swelling). In trauma cases where bleeding remains uncontrolled or hard to control, then the model is over simplistic and overestimates its clinical utility because bleeding will continue. Bleeding will continue because; 1.) compression or hemostasis in the field is limited, 2.) the increased arterial pressure (in the absence of hemostasis) from the osmotic volume shifts will exacerbate the pressure gradient for further hemorrhaging, and 3.) the crystalloid solution does not provide any replacement of clotting factors and precursors, which could limit bleeding by active coagulation and platelet activation. These factors all limit the use of an impermeant based LVR solution in many clinical settings. However, combining impermeant based LVR solutions with plasma product replacements such as fresh frozen plasma or spray dried plasma products may prove the most useful in many pre-hospital trauma settings involving uncontrolled hemorrhaging.
In conclusion, cell swelling due to global ischemia from severe hemorrhagic shock plays a significant role in the sensitivity of the victim to the low volume state. This is attributable to effects on the microcirculation with improved efficiency of microvascular oxygenation and perfusion during low volume states. The use of cell impermeants with oncotic agents or PEG-20k alone in low volume resuscitation solutions dramatically increases the time that a patient can safely remain in the low volume state until definitive medical care and full resuscitation are needed. The new LVR solution may be important in civilian pre-hospital resuscitation and for combat casualty care and resuscitation on the battlefield, especially when combined with plasma component replacement.
Table.
Blood gas data during the shock and LVR protocol in rats receiving saline, Gluconate (Glu), or Gluconte + PEG20k LVR solutions
| Group | ABG Parameter | Baseline | Hemorrhagic Shock | LVR |
|---|---|---|---|---|
|
| ||||
| Saline (20% BW) | Lactate (mM) | 1.35 (0.23) | 9.72 (0.83) | 9.49 (1.95) |
| HCO3− (mM) | 24.1 (2.6) | 14.5 (3.17) | 14.2 (1.63) | |
| pO2 (mm Hg) | 441 (32) | 381 (63.8) | 369 (36.3) | |
| pH | 7.37 (0.04) | 7.37 (0.04) | 7.36 (0.05) | |
| pCO2 (mm Hg) | 39.8 (3.10) | 26.4 (3.75) | 22.9 (3.32) | |
|
| ||||
| Glu (20% BW) | Lactate (mM) | 1.15 (0.33) | 9.34 (0.37) | 10.5 (1.67) |
| HCO3− (mM) | 26.2 (1.47) | 16.3 (1.55) | 13.0 (2.92) | |
| pO2 (mm Hg) | 406 (49.1) | 398 (38.1) | 379 (62.6) | |
| pH | 7.39 (0.05) | 7.39 (0.05) | 7.30 (0.15) | |
| pCO2 (mm Hg) | 41.6 (3.11) | 26.1 (2.59) | 22.1 (2.76) | |
|
| ||||
| Glu + PEG20k (10% BW) | Lactate (mM) | 1.20 (0.37) | 9.42 (0.21) | 2.62 (1.06) * |
| HCO3− (mM) | 25.8 (1.28) | 16.3 (0.99) | 31.2 (2.54) * | |
| pO2 (mm Hg) | 448 (39.7) | 417 (19.9) | 419 (31.4) | |
| pH | 7.40 (0.03) | 7.34 (0.03) | 7.48 (0.02) * | |
| pCO2 (mm Hg) | 36.1 (2.95) | 25.2 (1.94) | 39.9 (5.67) * | |
Values are mean ± (SD), n=6 per group,
P< 0.05 relative to the corresponding value in the other LVR groups, BW = volume based on calculated body weight
Hemorrhagic shock causes lethal cell swelling, which was mitigated by administration of cell impermeant based low volume resuscitation solutions. Impermeants increased the clinically safe time that a patient can remain in the low volume state (golden hour) by 5 fold over conventional saline solutions, in part, by improving microcirculatory perfusion.
Acknowledgments
This work was financially supported by grants from the National Institutes of Health (R01 DK 087737) and the Department of Defense (W81XWH-12-1-0599) to Dr. Mangino.
Footnotes
Conflicts of Interest
There were no conflicts of interest to declare.
References
- 1.National Center for Injury Prevention and Control. Web–based Injury Statistics Query and Reporting System (WISQARS) 2013. Ref Type: Online Source. [Google Scholar]
- 2.Finkelstein EA, Corso PS, Miller TR. The Incidence and Economic Burden of Injuries in the United States. USA. Oxford: University Press; 2006. [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 4.Heckbert SR, Vedder NB, Hoffman W, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45:545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Franklin GA, Boaz PW, Spain DA, et al. Prehospital hypotension as a valid indicator of trauma team activation. J Trauma. 2000;48:1034–1037. doi: 10.1097/00005373-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Riha GM, Kunio NR, Van PY, et al. Hextend and 7. 5% hypertonic saline with Dextran are equivalent to Lactated Ringer’s in a swine model of initial resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2011;71:1755–1760. doi: 10.1097/TA.0b013e3182367b1c. [DOI] [PubMed] [Google Scholar]
- 7.Riha GM, Kunio NR, Van PY, et al. Uncontrolled hemorrhagic shock results in a hypercoagulable state modulated by initial fluid resuscitation regimens. J Trauma Acute Care Surg. 2013;75:129–134. doi: 10.1097/ta.0b013e3182984a9b. [DOI] [PubMed] [Google Scholar]
- 8.Chaudry IH, Sayeed MM, Baue AE. Depletion and restoration of tissue ATP in hemorrhagic shock. Arch Surg. 1974;108:208–211. doi: 10.1001/archsurg.1974.01350260062014. [DOI] [PubMed] [Google Scholar]
- 9.Gomez H, Mesquida J, Hermus L, et al. Physiologic responses to severe hemorrhagic shock and the genesis of cardiovascular collapse: can irreversibility be anticipated? J Surg Res. 2012;178:358–369. doi: 10.1016/j.jss.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudry IH. Use of ATP following shock and ischemia. Ann N Y Acad Sci. 1990;603:130–140. doi: 10.1111/j.1749-6632.1990.tb37667.x. [DOI] [PubMed] [Google Scholar]
- 11.Barlet-Bas C, Khadouri C, Marsy S, et al. Enhanced intracellular sodium concentration in kidney cells recruits a latent pool of Na-K-ATPase whose size is modulated by corticosteroids. J Biol Chem. 1990;265:7799–7803. [PubMed] [Google Scholar]
- 12.Petit PX, Goubern M, Diolez P, et al. Disruption of the outer mitochondrial membrane as a result of large amplitude swelling: the impact of irreversible permeability transition. FEBS Lett. 1998;426:111–116. doi: 10.1016/s0014-5793(98)00318-4. [DOI] [PubMed] [Google Scholar]
- 13.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–247. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 14.Southard JH, Beltzer FO. Principles of Organ Preservation Part I. Surgical Rounds. 1993:353–360. [Google Scholar]
- 15.Southard JH, Beltzer FO. Principles of Organ Preservation Part II. Surgical Rounds. 1993:443–448. [Google Scholar]
- 16.Southard JH, Belzer FO. Control of canine kidney cortex slice volume and ion distribution at hypothermia by impermeable anions. Cryobiology. 1980;17:540–548. doi: 10.1016/0011-2240(80)90068-1. [DOI] [PubMed] [Google Scholar]
- 17.Southard JH, van Gulik TM, Ametani MS, et al. Important components of the UW solution. Transplantation. 1990;49:251–257. doi: 10.1097/00007890-199002000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Mees N, Southard JH, Belzer FO. Inhibition of ischemic induced cellular swelling in kidney cortex tissue by lactobionate anions. J Trauma. 1982;22:118–120. doi: 10.1097/00005373-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lindell S, Ametani M, Belzer FO, et al. Hypothermic perfusion of rabbit livers: effect of perfusate composition (Ca and lactobionate) on enzyme release and tissue swelling. Cryobiology. 1989;26:407–412. doi: 10.1016/0011-2240(89)90065-5. [DOI] [PubMed] [Google Scholar]
- 20.Arora TK, Malhotra AK, Ivatury R, et al. L-arginine infusion during resuscitation for hemorrhagic shock: impact and mechanism. J Trauma Acute Care Surg. 2012;72:397–402. doi: 10.1097/ta.0b013e3181d039fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams JA, Mangino MJ, Bassuk J, et al. Novel CPR with periodic Gz acceleration. Resuscitation. 2001;51:55–62. doi: 10.1016/s0300-9572(01)00373-2. [DOI] [PubMed] [Google Scholar]
- 22.Adams JA, Mangino MJ, Bassuk J, et al. Regional blood flow during periodic acceleration. Crit Care Med. 2001;29:1983–1988. doi: 10.1097/00003246-200110000-00022. [DOI] [PubMed] [Google Scholar]
- 23.Zakaria eR, Li N, Matheson PJ, et al. Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation. Surgery. 2007;142:487–496. doi: 10.1016/j.surg.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretschmar K, Engelhardt T. Swelling of capillary endothelial cells contributes to traumatic hemorrhagic shock-induced microvascular injury: a morphologic and morphometric analysis. Int J Microcirc Clin Exp. 1994;14:45–49. doi: 10.1159/000178205. [DOI] [PubMed] [Google Scholar]
- 25.Behmanesh S, Kempski O. Mechanisms of endothelial cell swelling from lactacidosis studied in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H1512–H1517. doi: 10.1152/ajpheart.2000.279.4.H1512. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman S, Kaesermann HP, Peters G. The mechanism of drinking induced by parenteral hyperonocotic solutions in the pigeon and in the rat. J Physiol. 1980;301:91–99. doi: 10.1113/jphysiol.1980.sp013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudmann DG, Alston JT, Hanson JC, et al. High molecular weight polyethylene glycol cellular distribution and PEG-associated cytoplasmic vacuolation is molecular weight dependent and does not require conjugation to proteins. Toxicol Pathol. 2013;41:970–983. doi: 10.1177/0192623312474726. [DOI] [PubMed] [Google Scholar]