Abstract
Anxious youth are at heightened risk for subsequent development of depression; however, little is known regarding which anxious youth are at the highest prospective risk. Biased attentional patterns (e.g., vigilance and avoidance of negative cues) are implicated as key mechanisms in both anxiety and depression. Aberrant attentional patterns may disrupt opportunities to effectively engage with, and learn from, threatening aspects of the environment during development and/or treatment, compounding risk over time. Sixty-seven anxious youth (age 9–14; 36 female) completed a dot-probe task to assess baseline attentional patterns provoked by fearful-neutral face pairs. The time course of attentional patterns both during and after threat was assessed via eyetracking and pupilometry. Self-reported depressive and anxiety symptoms were assessed two years after the conclusion of a larger psychotherapy treatment trial. Eyetracking patterns indicative of threat avoidance predicted greater 2-year depression scores, over and above baseline and post-treatment symptoms. Sustained, post-threat pupillary avoidance (reflecting preferential neural engagement with the neutral relative to the previously threatening location) predicted additional variance in depression scores, suggesting sustained avoidance in the wake of threat further exacerbated risk. Identical eyetracking and pupil indices were not predictive of anxiety at 2 years. These biobehavioral markers imply that avoidant attentional processing in the context of anxiety may be a gateway to depression across a key maturational window. Excessive avoidance of threat could interfere with acquisition of adaptive emotion regulation skills during development, culminating in the broad behavioral deactivation that typifies depression. Prevention efforts explicitly targeting avoidant attentional patterns may be warranted.
Keywords: attentional bias, anxiety, depression, adolescence
Depression rates increase markedly during the transition to adolescence (Angold, Costello, & Worthman, 1998). Both biological and psychosocial changes characterize the transition from late childhood to early adolescence (occurring at approximately 9–13 years of age) and contribute to the post-pubertal increase in depression rates (Cyranowski, Frank, Young, & Shear, 2000). Hormonal changes occurring in adolescence may sensitize the brain to the harmful effects of stress and increase vulnerability to depression (Angold & Costello, 2006; Crone & Dahl, 2012; Hyde, Mezulis, & Abramson, 2008), particularly for females (Green, McGinnity, Meltzer, Ford, & Goodman, 2005). While such peri-pubertal changes are normative, specific individuals respond to them with cascading detrimental effects. Identifying specific youth at highest risk, and ideally, intervening before adverse developmental trajectories set in, is an unrealized healthcare goal with substantial public health ramifications (Weissman et al, 1999).
Pediatric anxiety is a key risk factor for subsequent development of depression, with the majority of depressed youth having a history of anxiety (Kessler, Avenevoli, & Merikangas, 2001; Pine, Cohen, Gurley, Brook, & Ma, 1998). However, only a minority of anxious youth go on to develop depression. Identifying biobehavioral markers of prospective depression risk within this high-risk population is therefore critical to prevention efforts, promoting the ability to design mechanistic interventions that target modifiable precursors of depression, and efficiently deliver them to specific patients who need them.
Altered attentional patterns could constitute one mechanistic bridge from anxiety to depression. Aberrant patterns of attention to negative stimuli are posited to play a key role in both anxiety and depression across the lifespan (de Raedt & Koster, 2010; MacLeod, Mathews, & Tata, 1986; Pine, Guyer, & Leibenluft, 2008). While preferential attention towards negative stimuli is thought to promote negative affective states and maladaptive cognitions, excessive avoidance of threat represents the opposite extreme on an attentional continuum, and may be equally detrimental in that it precludes adaptive engagement with threats and concomitant habituation (Mogg, Bradley, Miles, & Dixon, 2004). While theoretical accounts have focused primarily on vigilance as an indicator of hyper-engagement with disorder-relevant information (Mathews & MacLeod, 1994), avoidance behavior is also highly clinically relevant in both depression and anxiety, manifesting as persistent avoidance of threatening contexts (e.g., school refusal), social withdrawal, and/or broad behavioral deactivation (Dimidjian, Barrera, Martell, Munoz, & Lewinsohn, 2011). Avoidance during the course of development may result in missed opportunities for threat engagement and processing, habituation, and the acquisition of adaptive, problem-oriented emotion regulation strategies. Compounded over time, the resulting emotion regulation deficits could constitute one developmental mechanism whereby anxious youth become depressed adolescents.
Experimental evidence supports the notion that both attentional extremes—vigilance and avoidance—are linked with symptoms of anxiety and depression in youth. Vigilant patterns are the more widely documented characteristic among anxious youth samples (Shechner et al., 2012) and are particularly evident when early attentional processes are assessed, such as during initial orienting to threat (Shechner et al., 2013) and following brief (e.g., 500ms) stimulus presentations (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). Vigilance towards negative stimuli has also been found in depressed youth and youth at risk for depression, particularly when using reaction time measures sensitive to slightly later stages of stimulus processing (e.g., Joormann, Talbot, & Gotlib, 2007; Salum et al., 2013). By contrast, avoidant attentional patterns have been found in depressed children when measuring the persistent direction of eye gaze over a more sustained presentation period (Harrison & Gibb, 2014). Avoidant patterns also characterize subsets of anxious samples, including anxious youth with specific (fear-related) anxiety diagnoses (Waters, Bradley, & Mogg, 2014) and adults and children with unfavorable acute outcomes to certain forms of cognitive-behavioral therapy (CBT) (Legerstee et al., 2010; Price, Tone, & Anderson, 2011; Waters, Mogg, & Bradley, 2012).
The degree to which aberrant attentional patterns are remediated by conventional treatments remains unclear. Psychotherapy, and CBT in particular, is considered a first-line treatment for pediatric anxiety, producing substantial reductions in anxiety for the majority of patients (Silverman, Pina, & Viswesvaran, 2008). However, a substantial minority of patients [e.g., 40%; (Walkup et al., 2008)] do not respond, and of those who do, some fail to maintain gains, suggesting the risk of progression from anxiety to depression remains high in these youth. While CBT for anxiety teaches skills to reduce vigilance-related cognitive biases (e.g., overestimation of risk) as well overt behavioral avoidance, it largely relies on the patient’s conscious awareness of vigilance and avoidance patterns. Such conscious behaviors may be distinct from the forms of attentional alteration described in the attentional bias literature, which occur relatively automatically on a time course of milliseconds to seconds (Buetti, Juan, Rinck, & Kerzel, 2012; Najmi, Kuckertz, & Amir, 2010). While there is some limited evidence that CBT for anxiety may reduce vigilance patterns (e.g., Lavy, van den Hout, & Arntz, 1993; Mohlman, Price, & Vietri, 2013; c.f. Waters, Wharton, Zimmer-Gembeck, & Craske, 2008), such findings at the group level imply that individuals who begin at the opposite (avoidant) end of the vigilance-avoidance continuum may either persist in this pattern, or may move even further in the avoidant direction following treatment. Avoidant attention could potentially interfere with maximal engagement in key therapy strategies thought to promote enduring reorganization of threat representations in memory [e.g., exposure and habituation; (Foa & Kozak, 1986)]. In that case, acute benefits might still be obtained through a variety of alternative (i.e., non-attentionally mediated) pathways (including both specific and non-specific factors), but a dormant risk of long-term relapse and/or progression to new symptoms (e.g. depression) could potentially endure in spite of state-of-the-art care. Progression to depression, in particular, might be likely among certain youth if anxiety-focused psychotherapy taught skills effective for the management of anxiety itself, but failed to remediate a core attentional pattern conferring risk for the emergence of depression during the key developmental stage of adolescence.
In the current study, all participants received standardized psychotherapy (CBT or client-centered therapy; CCT) in the context of a larger anxiety treatment trial and, on the whole, exhibited substantial acute decreases in anxiety during both treatments [clinical trial results are presented separately; (Silk et al., Submitted)]. If attentional features indeed predict prospective symptoms, even among individuals known to have received high-quality psychotherapy, and over and above any acute treatment benefit, this would strongly imply that existing first-line behavioral interventions are insufficient to ameliorate the specific form of risk contained in certain attentional patterns. Given mounting evidence that attentional patterns themselves are malleable using automated approaches (MacLeod & Clarke, 2015), findings could simultaneously suggest viable targets for alternative intervention/prevention efforts.
To promote the feasibility of clinical translation, we focused current prediction efforts on measures obtained using a relatively inexpensive laboratory set-up (computer, eyetracker). We assessed visual attentional patterns in eye gaze during fearful-neutral face pair presentations, focusing specifically on overall bias in dwell time (the most consistent marker of depression and depression risk) and bias in initial fixation (an early attentional marker linked to anxiety) (for review, see Armstrong & Olatunji, 2012). Eyetracking measures were selected to index attentional patterns because they provide detailed information about the time course of attention, including indices of both relatively early/automatic (i.e., initial fixation) and relatively late/controlled (i.e., dwell time) components of attention, and were more reliable than reaction time indices obtained during this version of the task (Price et al., In press). Fear-related stimuli, which are particularly relevant to anxiety disorders (Cisler & Koster, 2010; Gotlib et al., 2004), were selected to best match concurrent symptoms and treatment targets within the sample at baseline.
While eyetracking indices provide a direct assessment of visual attentional mechanisms in the presence of threat, pupilometry was used to provide complementary information on covert neural-attentional processes occurring in the wake of threat stimuli, during a sustained post-threat period. Pupil dilation is a peripheral marker of neural engagement that provides a summative index of cognitive and affective processing load. We have previously reported sustained pupil alterations in the aftermath of fearful-neutral face pairs among anxious youth that persisted for >8s after threat stimuli were removed (Price et al., 2013), possibly signifying attentional alterations that endure beyond the presence of threat and therefore cannot be measured via conventional behavioral (e.g. eye gaze) patterns. Sustained pupil alterations (increases or decreases) in the aftermath of negative stimuli have also been observed in anxious adults (Oathes, Siegle, & Ray, 2011) and depressed adults and youth (Siegle, Granholm, Ingram, & Matt, 2001; Siegle, Steinhauer, Carter, Ramel, & Thase, 2003b; Silk et al., 2007). Like conventional attentional bias markers, pupillary markers can reflect both vigilant (i.e., preferential neural engagement with negative information) and avoidant (preferential engagement with neutral information) patterns, with detrimental effects posited in each case. Increased neural engagement with previously presented negative stimuli may represent a perseverative form of negative attentional orientation [e.g, rumination (Siegle, Steinhauer, et al., 2003b)], while relatively increased engagement following neutral stimuli may represent persistent attempts at avoidance (Oathes et al., 2011). Pupil dilation persisting in the aftermath of negative stimuli has been shown to prospectively predict acute treatment outcomes in depression with high accuracy (Siegle et al., 2014; Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011), and may also have strong reliability (Siegle et al., 2014), making it an attractive candidate for predicting outcomes at the level of individual patients.
In summary, efforts to identify biomarkers of prospective depression risk among youth have so far been limited. Here we describe findings from one of the first studies to prospectively follow anxious youth (without primary depression at baseline) over a key 2-year developmental window during the transition to adolescence. Depression was assessed on a continuum, consistent with a dimensional approach to uncovering developmental mechanisms of psychological distress (Nigg, 2015) and allowing for incorporation of subclinical forms of depression, which are both impairing and prognostic of subsequent depressive disorders (Compas, Ey, & Grant, 1993). We hypothesized that attentional markers (i.e., eye gaze and pupilometry, indexing peri-threat and post-threat attention, respectively) would confer prospective risk of depression during the transition to adolescence, in spite of state-of-the-art treatment for anxiety during youth. This would suggest a key transdiagnostic mechanism that is not remediated by existing first-line treatments.
Methods
Participants
Sixty-seven youth (ages 9–13; 29 female) with DSM-IV diagnoses of generalized anxiety disorder, separation anxiety disorder, and/or social phobia were recruited for a larger psychotherapy trial. Results of the treatment trial will be presented separately (Silk et al., Submitted); for the present report, we focused on prediction of depressive symptoms that emerge in spite of treatment. 67 youth had usable data from: a) baseline (pre-treatment) attentional measures and b) clinical variables including baseline depression and anxiety symptoms, anxiety symptoms assessed immediately after the acute treatment phase, and depression and anxiety symptoms assessed approximately 2 years following the conclusion of the acute treatment phase (cognitive-behavioral therapy or client-centered therapy; see Table 1 and below for further details). Of these, 53 youth also had depressive symptoms assessed acutely post-treatment. Informed consent/assent and study procedures were approved by the University of Pittsburgh IRB.
Table 1.
Descriptive Characteristics of the Sample
| Anxious Youth (n=67) | |
|---|---|
| Age | 11.1 (1.4) |
| Female , n (%) | 36 (53.7%) |
| Caucasian, n (%) | 61 (91.0%) |
| Baseline diagnoses a, n (%) | |
| Separation anxiety disorder | 13 (19.4%) |
| Social phobia | 17 (25.4%) |
| Generalized anxiety disorder | 49 (73.1%) |
| Specific phobia | 11 (16.4%) |
| Major depressive disorder | 1 (1.5%) |
| Baseline SCARED | 37.8 (11.01) |
| Baseline MFQ | 20.4 (11.32) |
| 2-year SCARED | 17.51 (11.57) |
| 2-year MFQ | 11.0 (10.1) |
Note: Data presented as mean (SD) unless otherwise noted. SCARED= Screen for Child Anxiety Related Emotional. Disorders—child report; MFQ=Mood and Feelings Questionnaire—child report
Diagnostic groups are partially overlapping due to inclusion of comorbid patients. Primary/principle diagnoses were not designated, meaning that percentages for the 3 diagnostic inclusion groups will not sum to 100.
Clinical Assessments, Treatment, and Sample Composition
Data come from a large treatment outcome study of pediatric anxiety (clinicaltrials.gov NCT00774150)(Silk et al., Submitted). In brief, following a brief phone screen, an intake assessment occurred during which a structured diagnostic interview was administered to the child and his/her parent to confirm presence of an anxiety disorder. Diagnoses were made by trained interviewers using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; (Kaufman et al., 1997). Parents and youth were interviewed separately, with interviewers integrating data from both informants to arrive at final diagnoses. Diagnoses were reviewed and supervised by a psychiatrist (NR). Participants were excluded if they demonstrated an IQ below 70 as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI; (Wechsler, 1999), required current ongoing treatment with psychoactive medications including anxiolytics and antidepressants, were acutely suicidal or at risk for harm to self or others, failed to meet MRI safety requirements, or had previously completed a course of cognitive-behavioral therapy. Participants were excluded from the study if they had current, primary major depressive disorder at baseline. Co-morbid depressive disorders secondary to anxiety in terms of functional impact were allowed (n=1 in current analyses; no reported results affected by excluding this individual). Additional diagnostic exclusionary criteria included current diagnosis of obsessive-compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence, attention deficit/hyperactivity disorder combined type or predominantly hyperactive-impulsive type, or lifetime diagnosis of autism or Asperger syndrome (as assessed by the Child Asperger Syndrome Test; (Allison et al., 2007), bipolar disorder, psychotic depression, schizophrenia, or schizoaffective disorder.
Patients were randomized to receive 16 sessions (14 with the child, plus 2 parent sessions) of Cognitive Behavioral Therapy (CBT) or Client-Centered Therapy (CCT) in a 2:1 ratio. Masters’ and doctoral level therapists administered both treatments (therapists and treatment were fully crossed). In brief, CBT was delivered using the Coping Cat therapist manual (Kendall & Hedtke, 2006a) and child workbook (Kendall & Hedtke, 2006b). The first 8 sessions focused on anxiety-management skills; the second 8 sessions involved the therapist guiding the child through a hierarchy of exposure tasks. CCT (Cohen, Deblinger, Mannarino, & Steer, 2004) is a manualized nondirective, supportive psychotherapy based on humanistic principles. Acceptance, reflection, and nondirective problem-solving are key techniques. The intervention was developed to be analogous to typical supportive psychotherapy that anxious children and adolescents receive in the community. Further details of the study protocol and treatment conditions are provided elsewhere (Silk et al., Submitted).
Dot-probe Task
Participants completed the dot-probe task with concurrent eyetracking and pupilometry assessment, as previously described (Price et al., 2013). After an initial fixation cross in the middle of the screen (500ms), a fearful and a neutral face pair from the NimStim battery (Tottenham et al., 2009) were presented simultaneously on the top and bottom of the screen for either a short (200ms) or long (2000ms) interval, followed by a probe (dot) replacing one of the faces (“congruent” trials=fearful face location; “incongruent” trials=neutral face location). Participants responded as quickly as possible with a keyboard press to indicate the location of the probe. The dot remained on-screen for the remainder of the trial irrespective of when a response was made (10.6s seconds for short stimulus trials, 8.8s for long stimulus trials; each trial=11.3s total), allowing for continuous pupilometry assessment of covert attentional processes occuring in the wake of threat (without interference from changes in screen luminescence). For consistency within all analyses, data were restricted to the 32 trials per participant with long (2000ms) fearful-neutral face pair presentations, as they provided sufficient time for meaningful eyetracking analyses [while 200ms presentations do not reliably allow for completion of a single eye movement (Henderson & Hollingworth, 1998)].
Attentional Predictor Variables
Eyetracking. An ISCAN RK-786, affixed to a table top, was used to track eye movements and pupillary motility continuously at 60Hz. Eye fixations were defined as eye positions stable within 1° of visual angle for at least 100ms and were used to calculate 2 bias scores (difference scores) representing the following gaze patterns: 1) percentage of trials with initial fixations falling within regions of interest defined by the fearful vs. neutral face locations (an “early” index of initial attentional capture); 2) percentage of dwell time spent fixating on fearful vs. neutral faces (an index of overall attentional preference throughout the face presentation).
Both eyetracking indices reflect a continuum from avoidance of threat (i.e. gaze preference for neutral information) to vigilance (gaze preference for threat information), and were calculated such that larger scores indicate greater vigilance and smaller (negative) scores indicate avoidance of threat. Trials with incorrect responses, comprised of >50% blinks, or with no detectable fixations prior to manual response were excluded prior to analysis (16% of trials). Participants (n=3) were excluded from the reported sample if they had <=10 usable trials. Excluded participants did not differ from included participants on any clinical or demographic variable in Table 1 (p’s>.1).
Pupillary motility. Pupil diameter values were cleaned using our lab’s standard procedures to remove blinks, as previously described (Price et al., 2013). Pupillary responses were baseline-corrected within each trial by subtracting mean pupil diameter during the first 10 samples (167ms) from the remainder of the trial. Baseline-corrected pupil diameter values were then averaged across all trials during a temporal window of interest corresponding to post-threat probe presentation, i.e. from probe onset until the conclusion of the trial (an 8.8s window; incongruent and congruent trials averaged separately). Resulting means were outlier-corrected prior to analysis using a Winsorizing approach in which values outside 1.5 inter-quartile ranges from the 25th or 75th percentiles of the distribution were rescaled to the last valid value within that range .
For consistency with eyetracking indices, which represent bias towards/away from threat in a single measure, pupil bias scores were quantified on the basis of the dot-probe attentional manipulation, which orients attention towards (congruent trials) or away from (incongruent trials) the previous threat location. Pupil bias was calculated as: average pupil diameter (expressed as change from baseline), averaged across the probe period, for congruent – incongruent trials. Akin to the eyetracking bias scores, larger values indicate relatively greater neural engagement (pupil dilation) with the location of threat (i.e., post-threat vigilance), while smaller (negative) values indicate relatively greater neural engagement with the neutral location (post-threat avoidance).
For post hoc pupilometry timeseries analyses, a test statistic (specifically, correlation with 2-year depression; or independent samples t-test in the extreme-groups comparison analysis) was calculated at every timepoint within the mean pupil waveform. To hold type I error at p<.05 across all timepoints, Guthrie and Buchwald’s (Guthrie & Buchwald, 1991) Monte Carlo simulation technique was used to identify the duration of the temporal window over which a series of contiguous point-by-point t-tests or correlation coefficients could be considered significant given the observed temporal autocorrelation of the waveform, as described previously (Siegle, Steinhauer, Carter, Ramel, & Thase, 2003a; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003; Siegle, Steinhauer, & Thase, 2004). A minimum duration of 2.97s (178 samples) was identified using this technique.
An additional post hoc analysis of pupil data was used to aid in interpreting pupil findings through neural “source localization” in a subset of subjects (n=43) with usable functional magnetic resonance imaging (fMRI) data collected during an identical task (see Supplement). While prediction analyses were intentionally constrained to measures obtainable with a relatively inexpensive laboratory set-up, fMRI data were used to provide potentially disambiguating information regarding the interpretation of primary pupil findings.
Dependent Variables
The primary outcome was depressive symptoms 2-years post-treatment, assessed on a continuum via the Mood and Feelings Questionnaire-Child report [MFQ; (Kent, Vostanis, & Feehan, 1997)]. To assess specificity for progression to depression, anxiety symptoms at 2-years post-treatment were also assessed via the child-report Screen for Child Anxiety Related Emotional Disorders [SCARED; (Birmaher et al., 1997)].
Covariates
Baseline MFQ and SCARED scores, acute post-treatment SCARED scores (a primary marker of therapy response), and therapy condition (CBT or CCT) were controlled in all regression analyses. Because the acute treatment phase targeted anxiety specifically and primary depression constituted a study exclusion, acute post-treatment MFQ scores were inconsistently obtained and were available from only a subset (n=53) of participants. Regression analyses were repeated controlling for post-treatment MFQ within this subsample (see Supplement). In exploratory analyses, pre-treatment age, gender, and the age*gender interaction (a potential proxy for pubertal development, given that girls enter puberty earlier than boys) were explored as additional developmental covariates.
Analytic Strategy
Bivariate correlations were used for preliminary interrogation of relationships between attentional predictors, covariates, and dependent variables. For primary prediction analyses, hierarchical linear regression was used to identify predictors of MFQ scores at 2-years post-treatment. For comparison, SCARED scores at 2-years were used as a secondary endpoint. Unless otherwise noted, predictors were entered as follows—Step 1: baseline depression (MFQ) and anxiety (SCARED) scores, post-treatment anxiety (SCARED), and therapy condition; Step 2: eyetracking bias measures (dwell time bias and initial fixation bias); Step 3: pupil bias.
Results
Bivariate Relationships
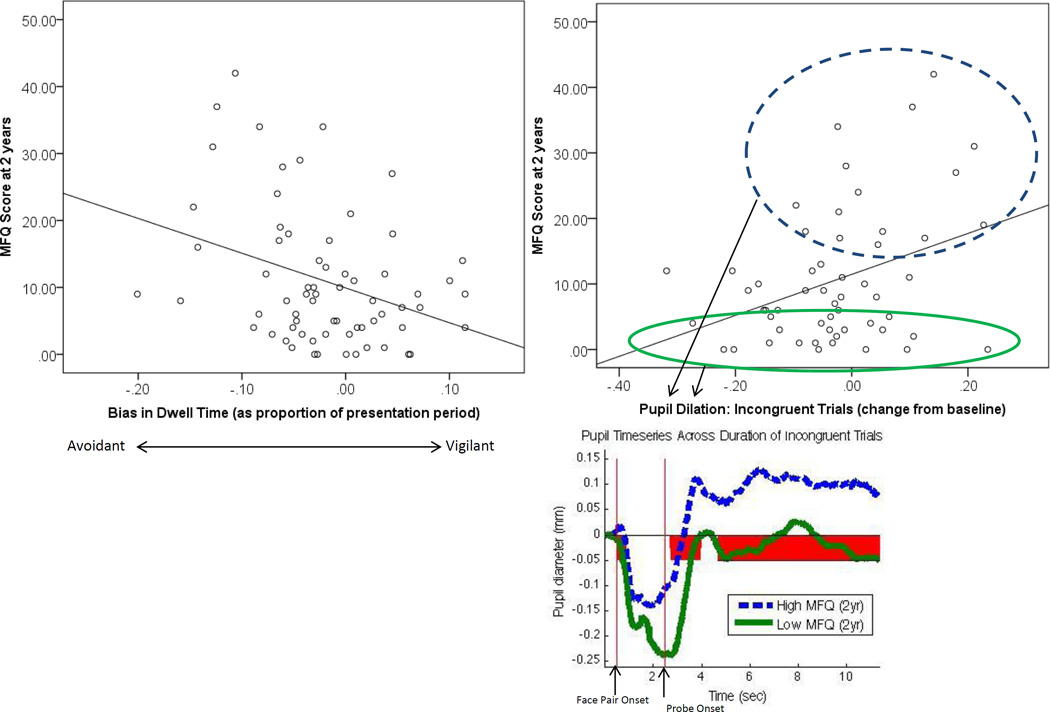
The correlation matrix for predictor variables, covariates, and dependent variables is presented in Table 2. Higher depressive symptoms at 2 years (the primary outcome) were associated with two indices of avoidant attention (see Figure 1): more avoidant eye gaze patterns across the trial (dwell time bias: r=−.341, p=.005) and lesser differential pupil diameter (pupil bias) for congruent compared to incongruent trials (r=−.317; p=.01), which was driven by a relationship with increased pupil diameter during incongruent trials (r=.338; p=.005).
Table 2.
Correlation matrix
| Attentional Features |
Covariates |
Dependent Variables |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ Pupil diameter (congruent trials) |
Δ Pupil diameter (incongruent trials) |
Pupil bias (probe period) |
Dwell time bias |
Initial fixation bias |
Baseline age |
Gender | Baseline age*Gender |
Baseline MFQ |
Baseline SCARED |
Post- treatment MFQ |
Post- treatment SCARED |
2-year MFQ | 2-year SCARED |
|
| Δ Pupil diameter (congruent trials) | 1.00 | .354** | .618** | −.068 | .158 | .073 | −.203 | −.201 | −.074 | .099 | .063 | .062 | −.036 | −.092 |
| Δ Pupil diameter (incongruent trials) | 1.00 | −.517** | −.209 | .238 | .072 | −.073 | −.055 | −.046 | .065 | .249 | .182 | .338** | .085 | |
| Pupil bias (probe period) | 1.00 | .113 | −.055 | .006 | −.125 | −.138 | −.029 | .036 | −.152 | −.096 | −.317** | −.156 | ||
| Dwell time bias | 1.00 | .183 | .034 | −.030 | −.010 | −.161 | −.160 | −.045 | −.104 | −.341** | −.134 | |||
| Initial fixation bias | 1.00 | .123 | −.037 | −.005 | .047 | .061 | −.076 | −.019 | −.102 | −.121 | ||||
| Baseline age | 1.00 | −.153 | −.022 | .039 | .111 | .160 | .152 | .164 | .065 | |||||
| Gender | 1.00 | .984** | −.115 | .157 | .196 | .262* | .273* | .385** | ||||||
| Baseline age*Gender | 1.00 | −.115 | .162 | .208 | .292* | .293* | .402** | |||||||
| Baseline MFQ | 1.00 | .474** | .357** | .210 | .069 | .051 | ||||||||
| Baseline SCARED | 1.00 | .184 | .459** | .265* | .364** | |||||||||
| Post-treatment MFQ | 1.00 | .558** | .331* | .320* | ||||||||||
| Post-treatment SCARED | 1.00 | .419** | .603** | |||||||||||
| 2-year MFQ | 1.00 | .658** | ||||||||||||
| 2-year SCARED | 1.00 | |||||||||||||
Note. MFQ=Mood and Feelings Questionnaire; SCARED=Screen for Child Anxiety Related Emotional Disorders;
=Correlation is significant at the 0.01 level (2-tailed).
=Correlation is significant at the 0.05 level (2-tailed)
Figure 1.
Scatterplots for attentional variables predicting 2-year depression. Timeseries plot depicts pupil diameter (expressed as change from baseline) in top and bottom MFQ (2-year) quartiles, with red shading indicating timepoints with significant group difference. Initial dip during face presentation is driven by pupillary light reflex. MFQ=Mood and Feelings Questionnaire (child report)
To explore the timing of the observed pupil correlation during incongruent trials, a post hoc time series analysis was conducted. Significant correlations between pupil diameter and 2-year depression began at the point of probe onset and continued almost continuously throughout the remaining 8.8s of the trial, with the peak correlation coefficient (r=.35) occurring 4.1s after probe onset (i.e., >3s after the average manual response time of 840ms). Similarly, when comparing the top and bottom quartile of 2-year MFQ scorers, significant pupil differences commenced shortly after probe onset and were sustained nearly continuously throughout the remaining 8.8s of the trial (Figure 1).
Hierarchical Regressions Predicting 2-year Outcomes
In primary prediction analyses, after controlling for baseline depression and anxiety, treatment group, and post-treatment anxiety (Step 1: ΔR2=.19, ΔF4,62 =3.7, p=.01), eyetracking bias measures (Step 2) explained significant additional variance in 2-year MFQ scores (ΔR2=.09, ΔF2,60 =3.9, p=.03). Likewise, pupil bias explained significant variance in 2-year MFQ scores at Step 2 after controlling for clinical covariates at Step 1 (ΔR2=.07, ΔF7,59 = 4.5, p=.01). In a final hierarchical model, pupil bias was added at Step 3 (after controlling eyetracking bias indices at Step 2) and explained further significant variance in 2-year MFQ scores, above both clinical and eyetracking measures (ΔR2=.07, ΔF1,59 =5.9, p=.02). At Step 3, 35% of variance was explained (adjusted R2=.27; F7,59 =4.5, p<.001), and greater 2-year MFQ scores were predicted by more avoidant eyetracking bias (β=−.26, [95% CI: −.48, −.04], p=.02) and more avoidant pupil bias (β=−.26 , [95% CI: −.48, −.05], p=.02), controlling for all other variables.
All significant findings above were upheld when post-treatment depression was covaried in participants with available data (see Supplement for details).
Parallel regression analyses for 2-year anxiety revealed no significant effects for attentional predictors at any step (dwell time bias, initial fixation bias, pupil bias; p’s>.2). As above, baseline depression and anxiety, treatment group, and post-treatment anxiety collectively predicted 2-year anxiety (Step 1: ΔR2=.39, ΔF4,62 =10.0, p<.001) but Steps 2 and 3 did not improve prediction (Step 2: ΔR2=.02, ΔF2,60 =.81, p=.45; Step 3: ΔR2=.01, ΔF1,592 =1.41, p=.24), and no interaction effects were significant (see Supplement).
Interaction effects among covariates, and between covariates and attentional features, were not significant predictors of 2-year depression or anxiety, suggesting covariates in the model were valid (see Supplement). Of particular note, relationships between attentional features and 2-year depression were not moderated by psychotherapy treatment condition (see Supplement).
Developmental Factors
Exploratory regression analyses examining the influence of age, gender, and age*gender interactions suggested observed relationships were not better explained by these demographic factors. Consistent with the clinical developmental literature, female gender and the age*gender interaction (i.e., older girls, relative to younger girls and boys at any age) were associated with greater symptomatology (depression and anxiety) at 2-years (see Table 1). However, although age, gender and age*gender interactions collectively explained 12% of variance in 2-year depression (Step 1: ΔR2=.12, ΔF3,63 =2.81, p=.05), these indices were no longer significant once clinical and attentional variables were entered (as above; p’s>.1), while both avoidant dwell time bias (β=−.27 , [95% CI: −.49, −.05], p=.02) and avoidant pupil bias (β=−.24 , [95% CI: −.46, −.03], p=.03) remained predictive in the final model. Age and gender did not moderate any attentional feature to predict 2-year depression or anxiety (see Supplement).
Discussion
More than two years after a laboratory assessment of attention to threat among anxious youth, youth depression scores were predicted by avoidant gaze patterns, over and above variance explained by baseline clinical and demographic measures, and in spite of treatment-related effects (therapy condition, acute post-treatment symptoms). Depression severity was further predicted by a sustained, avoidant pattern of pupil dilation in the aftermath of threat, suggesting that persistent cognitive efforts at post-threat avoidance further compounded this risk. Findings were specific for depression, while anxiety at 2 years was related to clinical but not attentional measures. Although the total variance explained in the final model was modest (35%; 20% for attentional/pupilometry measures alone), it is nevertheless notable that measures taken during a simple laboratory task were predictive of prospective depressive scores in spite of an intervening 2-year period of life events and developmental/pubertal progress. This long-term pattern suggests the attentional measures captured a key mechanism in the progression from pediatric anxiety to adolescent depression.
The detrimental impact of avoidance patterns was consistent across both peri-threat (eyetracking) and post-threat (pupilometry) indices, in spite of quite distinct forms of information obtained with these methods. During incongruent trials, which encourage an avoidant response by orienting attention away from the previous location of threat, elevated pupil dilation in youth at highest depression risk was sustained across a >8s post-stimulus period (Figure 1). In conjunction with explicitly avoidant eye gaze patterns, this pattern suggests possible persistent cognitive efforts to sustain attention (e.g., mental focus) in the “safe” (i.e., previously neutral) relative to the “dangerous” (i.e., previously threatening) context, even when actual visual attentional avoidance of threat was no longer necessary or, indeed, possible (given that no threat remained on-screen). Avoidant pupillary responses (i.e., reduced responses to negative relative to neutral trial types) have previously been reported in adults with high self-reported worry (Oathes et al., 2011) and depressed youth (Silk et al., 2007). Just as in the current dataset, the alterations began in the wake of stimuli, and persisted for several seconds after stimuli were removed from view and responses were made. Such patterns could indicate preferential recruitment of elaborative processing and/or cognitive control mechanisms in the wake of neutral relative to negative information. To promote clinical translation, we opted to restrict primary regression analyses to measures that can be obtained quickly and inexpensively in a relatively simple laboratory set-up (and, increasingly, using handheld and/or mobile devices). However, in a supplementary analysis conducted among a subset of participants who performed the same task during functional neuroimaging (see Supplement), pupil values during incongruent trials tracked with larger responses in a right posterior parietal region implicated in sustaining visual attention (Malhotra, Coulthard, & Husain, 2009). This statistical relationship across individuals further links the observed pupil pattern to an attentional control mechanism pertinent to sustained attention, helping to rule out alternative explanations (e.g., arousal, emotional responding).
Given that dwell time and pupil bias explained distinct variance in outcome, youth at highest prospective depression risk exhibited the cumulative burden of behavioral avoidance during threat presentation, plus sustained avoidance attempts in the wake of threat. These two avoidant tendencies, in aggregate, could serve to prohibit active engagement with threats, while decreasing availability of cognitive resources for learning and applying more adaptive forms of emotion regulation in the wake of a threatening encounter (e.g., problem solving, consideration of actual rather than feared outcomes). When combined with increasing stress sensitivity and normative psychosocial changes during the peri-pubertal period, avoidant attention could propagate more widespread withdrawal from an increasingly “threatening” (e.g., interpersonally) environment, thereby setting the stage for depression to emerge in adolescence.
The existing literature in both anxiety and depression suggests that timecourse is a key factor in attentional bias effects (de Raedt & Koster, 2010; Mogg et al., 2004). Our methodology enables separation of distinct components of visual attention (initial fixation, dwell time bias throughout the “intermediate” 2s stimulus presentation, and sustained post-threat processing). Findings suggest that depression was predicted by a pervasive pattern of avoidance at intermediate and late stages of processing, while initial orienting at the earliest stages did not prospectively predict depression or anxiety. Concurrent anxiety has been most reliably linked to early attentional features [e.g., response times to brief 500ms stimuli, initial fixations in eye gaze (Bantin, Stevens, Gerlach, & Hermann, 2016; Bar-Haim et al., 2007; Gamble & Rapee, 2010; Price, Siegle, & Mohlman, 2012)], with a smaller literature suggesting a switch to avoidance of threat occurring at later processing stages (Mogg et al., 2004). Depression has been linked primarily to later stages of attention [e.g., response times to stimuli presented for 1000ms or longer, dwell time bias, disengagement difficulty; (Kellough, Beevers, Ellis, & Wells, 2008; Leyman, De Raedt, Schacht, & Koster, 2007)], although a recent metaanalysis found that both early and late attentional components may be equally affected (Peckham, McHugh, & Otto, 2010)]. Nevertheless, difficulty disengaging from negative stimuli is often considered the most reliable pattern among currently depressed adults, and may also characterize groups of children and adults at elevated risk of depression (Gotlib & Joormann, 2010). Vigilance towards negative stimuli, particularly during late stages of processing, is therefore a key depression marker.
However, using methods more akin to the present study, currently depressed children have shown avoidant eye gaze patterns (specifically, avoidance of sad faces) persisting continuously for up to 20s of face viewing (Harrison & Gibb, 2014). This avoidant pattern in eye gaze has been posited to be developmentally mediated. Specifically, while avoidance of sad faces may be a normative feature of infancy (Montague & Walker-Andrews, 2001), providing effective mood regulation at this age (Termine & Izard, 1988), depressed children may continue to apply this strategy without the same benefit (possibly due to increasing capacity for abstract cognition), while depressed adults may no longer be able to resist attending towards negative information at all (resulting in vigilant patterns). Within the constrained age range of the current study, age (and gender) did not better explain the relationship between avoidance and prospective depression. The sample was specifically selected to capture the high-risk peri-pubertal window where depression often emerges, meaning that youth may have undergone relatively homogenous (though substantial) changes across the follow-up period. A broader developmental perspective may be required to reveal whether developmental factors do indeed moderate the nature of attentional risks for depression. Nevertheless, developmental changes over time were an important element of the current findings, as avoidant tendencies manifested in depressive symptoms only after the passage of time and maturational progress.
Previous studies in currently depressed individuals suggest attentional biases pertain specifically to sad/dysphoric stimuli, while threat-related stimuli elicit attentional biases in currently anxious individuals (Gotlib et al., 2004). Our study included assessment in a currently anxious cohort using threat-related stimuli (fearful faces). It is notable that attentional features were nevertheless predictive of depression, suggesting threat-related stimuli may have greater relevance to depression when placed within a developmental framework, just as anxiety itself is predictive of future depression in spite of separable foci of symptoms and cognition. However, the failure to include dysphoric stimuli in the current study means that we cannot speak to the specificity of our findings to fearful faces and may have missed attentional patterns with even greater relevance to depression development. In addition, our study used fearful faces as a threat cue because they reliably activate fear-processing regions of the brain (Whalen et al., 2001) and have transdiagnostic relevance to fear perception through the implication that a generic, unspecified threat is present. This design decision stands in contrast to many studies using angry or disgusted faces to connote a social form of threat directed at the participant; however, there is evidence that fearful and angry faces elicit comparable eyetracking bias patterns during the dot-probe (Mogg, Garner, & Bradley, 2007). In the future, inclusion of multiple stimulus types would help to disambiguate the relative relevance of various threatening and dysphoric cues in the transition from anxiety to depression.
In summary, while our findings diverge in some respects from attentional patterns described previously in depression, they were obtained using an individual differences approach and prospective symptom assessment. These design features extend the literature, which is dominated by cross-sectional, group-level comparisons. Findings suggest that it is a persistent pattern of avoidance across the majority of a 2s timecourse, coupled with further sustained avoidance in the wake of threat, that confers the highest prospective risk. While first-line psychotherapies could effectively ameliorate and/or compensate for some of the attentional aberrations observed in cross-sectional studies, sustained avoidance of threat may have a specific, insidious effect over time (e.g., lost opportunities for habituation and adaptive problem solving, compounded over the course of development), leaving youth vulnerable to depression even when they show acute treatment benefits.
Previous studies of acute psychotherapy outcome have suggested that anxious individuals showing avoidant attentional patterns are not as well-suited for certain forms of psychotherapy as youth at the vigilant end of the spectrum (Legerstee et al., 2010; Price et al., 2011; Waters et al., 2012). Consistent with this, one possible interpretation of the current findings is that avoidant youth did poorly in psychotherapy and were therefore less buffered against subsequent depression. However, several pieces of evidence argue against this interpretation. The current effects were specific for depression (whereas the treatments targeted anxiety), and were not apparent immediately after treatment (Table 1), suggesting the longer-term transition to adolescence (and concomitant increased depression risk) were key. Furthermore, whereas both treatment conditions in the current trial produced substantial decreases in primary anxiety outcomes (Silk et al., Submitted), the impact of attentional features persisted after controlling for these treatment-related effects. Findings therefore suggest that the attentional assessment captured a long-term form of risk that was relatively impervious to the acute beneficial effects of the treatments. In other words, anxious youth exhibiting avoidant attentional features continued to be at risk for prospective depression, irrespective of how well the treatment ameliorated their symptoms acutely. Clinically, this suggests that alternative and/or adjunctive treatments may be needed for this subset of anxious youth, even when psychotherapy succeeds in reducing symptoms. These youth may be at particular risk of falling through the cracks of clinical care, as they may show initial reductions in symptoms that fail to protect them from further development of depression once developmental risk factors (e.g., increased stress reactivity, psychosocial stressors) are brought to bear.
Our findings could have implications for novel treatment development, particularly in light of growing interest in mechanistic treatments targeting attentional patterns (e.g., Attentional Bias Modification; ABM) (MacLeod & Clarke, 2015). The vast majority of ABM studies in anxious patients have trained attention away from threat (toward neutral information), invoking an attentional goal state akin to the pattern that conferred risk in our study. Although several studies showed immediate and short-term (e.g., at 4-month follow-up) benefits on anxiety measures (Amir et al., 2009; Schmidt, Richey, Buckner, & Timpano, 2009), more recent findings have been mixed (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015), and no published study has examined clinical effects (depression or anxiety) at longer-term follow-up. In the context of pediatric anxiety, it may be important to consider the possible detrimental effects of training in an avoidance pattern. However, the attentional pattern instilled by ABM (designed to remediate relatively early/automatic aspects of attention) may differ substantively from the risk pattern observed here (i.e., sustained avoidance in eye gaze and pupilometry persisting across a ~10s period). In addition, avoidant attentional patterns may be detrimental only when they arise spontaneously, but not when they occur as the result of a specific attentional training procedure. Avoidant patterns that arise on their own may be far more generalized, potentially indexing an innate, widespread, and pervasive tendency to withdraw from emotional cues (e.g., potentially both positive and negative cues), across longer spans of time. Even so, our findings could implicate the need for an alternative form of ABM tailored to the individual (e.g., training towards threat in avoidant individuals, particularly for late/sustained time points).
More broadly, findings highlight the importance and detrimental impact of attentional avoidance of threat, which has received less attention in the literature than the opposing pattern [vigilance towards threat; (Bar-Haim et al., 2007)], but is paramount in clinical manifestations of both anxiety and depression (behavioral and emotional avoidance attempts, social withdrawal). Attentional avoidance is posited to maintain emotional difficulties over time due to decreased elaborative processing of threat, decreased active coping, and reduced opportunities for fear habituation/extinction (Foa & Kozak, 1986; Mogg et al., 2004). By focusing on a key maturational window when risk of depression onset is high, the current study suggests these missed opportunities for threat engagement and processing, compounded over time, may constitute one developmental mechanism whereby anxious youth progress to depression.
Limitations
The assessment of biased attention in laboratory studies is constrained by suboptimal reliability (Price et al., In press), which limits power and risks underestimation of the true impact of attentional mechanisms. This limitation further constrains the ability to infer clinically meaningful information about individual patients. By contrast, pupilometry is reliable (Siegle et al., 2014) and has been used to classify clinical outcomes of individual patients with high accuracy (Siegle et al., 2011); however, given diffuse neural inputs (Beatty, 1986), its meaning can be difficult to interpret in isolation. Here, we sought to combine the strengths of each approach. However, modest effect sizes for prediction suggest additional measures and/or refinements of current measures would be needed to facilitate clinical translation. In particular, inclusion of dysphoric stimuli (rather than threat-related alone) may improve relevance for depression. Findings may not generalize beyond the specific task design (e.g., 2s fearful-neutral face pairs) and clinical population recruited here. Finally, the present findings await replication in a larger sample with a wider range of anxiety presentations and depression-related outcomes and a more naturalistic, treatment-free follow-up period.
Conclusions
Among anxious youth, laboratory assessments of attention—using measures feasible to obtain in a typical clinical setting—were predictive of depression scores at a delay of more than 2 years. Persistent avoidance of threat, both during and after threat presentation, emerged as a robust mechanism conferring risk for the transition from pediatric anxiety to adolescent depression, in spite of state-of-the-art behavioral treatment. Anxious youth exhibiting attentional avoidance might benefit from increased clinical attention and preventative efforts, including potential attempts to directly remediate avoidant attentional patterns. Such efforts could help forestall the onset of depression, a condition that is costly, disabling, and highly enduring across the lifespan (Weissman et al., 1999).
Supplementary Material
General Scientific Summary.
This paper suggests that anxious youth who show attentional patterns characterized by avoidance of threatening information are at higher risk of depression symptoms two years later.
Acknowledgments
Supported by National Institutes of Health grants MH091327, MH080215, MH18269 and MH082998. Dr. Price is supported by a Career Development Award from NIMH (1K23MH100259).
Footnotes
The authors have no conflicts of interest.
References
- Allison C, Williams J, Scott F, Stott C, Bolton P, Baron-Cohen S, et al. The Childhood Asperger Syndrome Test (CAST): test-retest reliability in a high scoring sample. Autism. 2007;11(2):173–185. doi: 10.1177/1362361307075710. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, et al. Attention training in individuals with generalized social phobia: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77(5):961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child and adolescent psychiatric clinics of North America. 2006;15(4):919–937. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Armstrong T, Olatunji BO. Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clinical psychology review. 2012;32(8):704–723. doi: 10.1016/j.cpr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantin T, Stevens S, Gerlach AL, Hermann C. What does the facial dot-probe task tell us about attentional processes in social anxiety? A systematic review. Journal of Behavior Therapy and Experimental Psychiatry. 2016;50:40–51. doi: 10.1016/j.jbtep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beatty J. The pupil system. In: Coles M, Donchin E, Porges S, editors. Psychophysiology: Systems, Processes, and Application. New York: Guilford; 1986. pp. 43–50. [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Buetti S, Juan E, Rinck M, Kerzel D. Affective states leak into movement execution: automatic avoidance of threatening stimuli in fear of spider is visible in reach trajectories. Cognition & Emotion. 2012;26(7):1176–1188. doi: 10.1080/02699931.2011.640662. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical psychology review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Deblinger E, Mannarino AP, Steer RA. A multisite, randomized controlled trial for children with sexual abuse-related PTSD symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(4):393–402. doi: 10.1097/00004583-200404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Ey S, Grant KE. Taxonomy, assessment, and diagnosis of depression during adolescence. Psychological Bulletin. 1993;114(2):323–344. doi: 10.1037/0033-2909.114.2.323. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Archives of general psychiatry. 2000;57(1):21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- de Raedt A, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10:50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Jr, Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Gamble AL, Rapee RM. The time-course of attention to emotional faces in social phobia. Journal of behavior therapy and experimental psychiatry. 2010;41(1):39–44. doi: 10.1016/j.jbtep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow Ba, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;113:386–398. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Green H, McGinnity Á, Meltzer H, Ford T, Goodman R. Mental health of children and young people in Great Britain, 2004. Palgrave Macmillan Basingstoke; 2005. [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Harrison AJ, Gibb BE. Attentional Biases in Currently Depressed Children: An Eye-Tracking Study of Biases in Sustained Attention to Emotional Stimuli. Journal of Clinical Child and Adolescent Psychology. 2014:1–7. doi: 10.1080/15374416.2014.930688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JM, Hollingworth A. Eye movements during scene viewing: An overview. In: Underwood G, editor. Eye guidance in reading and scene perception. Oxford, England: Elsevier; 1998. pp. 269–283. [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological review. 2008;115(2):291. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of abnormal psychology. 2007;116(1):135. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADSPL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–987. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav Res Ther. 2008;46(11):1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Hedtke K. Cognitive-behavioral therapy for anxious children: Therapist manual. Ardmore, PA: Workbook Publishing; 2006a. [Google Scholar]
- Kendall PC, Hedtke K. The Coping Cat Workbook. Ardmore, PA: Workbook Publishing; 2006b. [Google Scholar]
- Kent L, Vostanis P, Feehan C. Detection of major and minor depression in children and adolescents: evaluation of the Mood and Feelings Questionnaire. Journal of Child Psychology and Psychiatry. 1997;38(5):565–573. doi: 10.1111/j.1469-7610.1997.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Merikangas KR. Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry. 2001;49(12):1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Lavy E, van den Hout M, Arntz A. Attentional bias and spider phobia: conceptual and clinical issues. Behaviour Research and Therapy. 1993;31(1):17–24. doi: 10.1016/0005-7967(93)90038-v. [DOI] [PubMed] [Google Scholar]
- Legerstee JS, Tulen JH, Dierckx B, Treffers PD, Verhulst FC, Utens EM. CBT for childhood anxiety disorders: differential changes in selective attention between treatment responders and non-responders. Journal of Child Psychology and Psychiatry. 2010;51(2):162–172. doi: 10.1111/j.1469-7610.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- Leyman L, De Raedt R, Schacht R, Koster EH. Attentional biases for angry faces in unipolar depression. Psychol Med. 2007;37(3):393–402. doi: 10.1017/S003329170600910X. [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. 2015 doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Clarke P. The Attentional bias modification approach to anxiety intervention. Clinical Psychological Science. 2015;3(1):58–78. [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132(Pt 3):645–660. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annual Review of Psychology. 1994;45:25–50. doi: 10.1146/annurev.ps.45.020194.000325. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18(5):689–700. [Google Scholar]
- Mogg K, Garner M, Bradley BP. Anxiety and orienting of gaze to angry and fearful faces. Biological Psychology. 2007;76:163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlman J, Price RB, Vietri J. Attentional bias in older adults : Effects of generalized anxiety disorder and cognitive behavior therapy. Journal of Anxiety Disorders. 2013;27:585–591. doi: 10.1016/j.janxdis.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague DP, Walker-Andrews AS. Peekaboo: a new look at infants’ perception of emotion expressions. Developmental psychology. 2001;37(6):826. [PubMed] [Google Scholar]
- Najmi S, Kuckertz JM, Amir N. Automatic avoidance tendencies in individuals with contamination-related obsessive-compulsive symptoms. Behavior Research and Therapy. 2010;48(10):1058–1062. doi: 10.1016/j.brat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Editorial: The shape of the nosology to come in developmental psychopathology. Journal of Child Psychology and Psychiatry. 2015;56(4):397–399. doi: 10.1111/jcpp.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oathes DJ, Siegle GJ, Ray WJ. Chronic worry and the temporal dynamics of emotional processing. Emotion. 2011;11:101–114. doi: 10.1037/a0021781. [DOI] [PubMed] [Google Scholar]
- Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Guyer AE, Leibenluft E. Functional magnetic resonance imaging and pediatric anxiety. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1217–1221. doi: 10.1097/CHI.0b013e318185dad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Tone EB, Anderson PL. Vigilant and avoidant attention biases as predictors of response to cognitive behavioral therapy for social phobia. Depression and Anxiety. 2011;28:349–353. doi: 10.1002/da.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. doi: 10.1037/pas0000036. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle G, Mohlman J. Emotional Stroop performance in older adults: Effects of habitual worry. American Journal of Geriatric Psychiatry. 2012;20(9):798–805. doi: 10.1097/JGP.0b013e318230340d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle G, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. Sustained neural alterations in anxious youth performing an attentional bias task: A pupilometry study. Depression and Anxiety. 2013;30:22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, et al. Looking under the hood of the dot-probe task: An fMRI study in anxious youth. Depression and Anxiety. 2014;31:178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Gadelha A, Pan P, Tamanaha AC, et al. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychological Medicine. 2013;43(04):733–745. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Richey JA, Buckner JD, Timpano KR. Attention training for generalized social anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):5–14. doi: 10.1037/a0013643. [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Perez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, et al. Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety. 2012;29(4):282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Britton JC, Leibenluft E, Pine DS, Nelson EE. ATTENTION BIAS OF ANXIOUS YOUTH DURING EXTENDED EXPOSURE OF EMOTIONAL FACE PAIRS: AN EYE-RACKING STUDY. Depression and anxiety. 2013;30(1):14–21. doi: 10.1002/da.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones N, Ghinassi F, Painter T, Thase ME. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clinical Psychological Science. 2014;2(4):455–471. [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003b;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biological Psychiatry. 2011;69(8):726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003;20(1):114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the Stroop task in depression. International Journal of Psychophysiology. 2004;52(1):63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Silk J, Tan P, Ladouceur C, Meller S, Siegle G, McMakin D, et al. Benefits of individual cognitive behavioral therapy for child anxiety over active comparison in breadth, generalization, and durability of treatment response. (Submitted). [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. American Journal of Psychiatry. 2007;164(12):1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WK, Pina AA, Viswesvaran C. Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2008;37(1):105–130. doi: 10.1080/15374410701817907. [DOI] [PubMed] [Google Scholar]
- Termine NT, Izard CE. Infants’ responses to their mothers’ expressions of joy and sadness. Developmental Psychology. 1988;24(2):223. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, et al. Cognitive Behavioral Therapy, Sertraline, or a Combination in Childhood Anxiety. New England Journal of Medicine. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Bradley BP, Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus ‘fear’ diagnostic categorization. Psychological Medicine. 2014;44(3):607–616. doi: 10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Waters AM, Mogg K, Bradley BP. Direction of threat attention bias predicts treatment outcome in anxious children receiving cognitive-behavioural therapy. Behaviour Research and Therapy. 2012;50:428–434. doi: 10.1016/j.brat.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Waters AM, Wharton TA, Zimmer-Gembeck MJ, Craske MG. Threat-based cognitive biases in anxious children: comparison with non-anxious children before and after cognitive behavioural treatment. Behaviour Research and Therapy. 2008;46(3):358–374. doi: 10.1016/j.brat.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, et al. Depressed adolescents grown up. JAMA: the Journal of the American Medical Association. 1999;281(18):1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.