Abstract
Objective
A history of complex febrile seizures can increase the risk of epilepsy, but the role of genetic factors is unclear. This analysis evaluated the relationship between febrile seizures and epilepsy.
Methods
Information on history of seizures was obtained by a questionnaire from twin pairs in the Mid-Atlantic, Danish and Norwegian Twin Registries. The information was verified using medical records and detailed clinical and family interviews. The initial study evaluated the genetic epidemiology of febrile seizures in this population. Further information was analyzed and used to evaluate genetic associations of different febrile seizure subtypes.
Results
Histories of febrile seizures were validated in 1051 twins in 900 pairs. The febrile seizure type was classified as simple, complex or febrile status epilepticus. There were 61% simple, 12% complex and 7% febrile status epilepticus. There were 78 twins who developed epilepsy. The highest rate of epilepsy (22.2%) occurred in the febrile status epilepticus group. Concordance was highest in simple group.
Conclusion
A twin with febrile status epilepticus is at the highest risk of developing epilepsy, but simple febrile seizures gave the highest risk for the unaffected twin to develop seizures or other neurological issues. These results are consistent with previous findings.
Implications
There is a subgroup of febrile seizures that can be associated with long term consequences. The subgroup can be associated with a significant financial and emotional burden. It is currently not possible to accurately identify which children will develop recurrent febrile seizures, epilepsy or neuropsychological comorbidities. Further studies are needed.
Introduction
Febrile seizures are the single most common seizure type. They occur in 2% to 5% of children younger than age 5 years, with a peak incidence in the second year of life (Shinnar 2003). The incidence and prevalence of febrile seizures are similar across numerous large epidemiologic studies. The known higher incidence populations, such as Japan and Guam, are not included in this study (Mathai et al. 1968, Stanhope et al. 1972, Tsuboi 1984). Febrile seizures are often defined as two separate categories, simple and complex. A simple febrile seizure is isolated, brief and generalized (Shinnar and Glauser 2002). A febrile seizure is considered complex if it has a focal onset, occurs more than once during a febrile illness, or lasts more than 10 to 15 minutes (Waruiru & Appleton 2004). Febrile status epilepticus is a unique group of febrile seizures, and can be considered extreme complex febrile seizures. Febrile status epilepticus describes febrile seizures that last for more than 30 minutes. For this discussion and analysis febrile status epilepticus will be a separate group.
There are multiple reported risk factors associated with an increased risk of developing an afebrile seizure after presenting with a febrile seizure. The risk factors are generalized and often debated. Currently the individual importance for these risk factors for each child is not known (Berg and Shinnar 1994). Twin studies are an excellent tool to determine the relative importance of environmental versus genetic factors in a specific disease. Previously published febrile seizure twin studies have consistently shown an approximately three-fold higher concordance rate in monozygotic twins as compared to dizygotic twins (Corey et al. 1991, Kjeldsen et al. 2002, Kjeldsen et al. 2005 and Corey et al. 2011). This study utilized one of the largest twin populations, which included twin pairs from three different twin registries (Mid-Atlantic, Norwegian and Danish). This study evaluates both genetic and environmental factors involved in febrile seizures.
Methods
The subjects analyzed for this study were twin pairs that had a history of febrile seizures. These databases were utilized because of the large number of known twin pairs and the ability to collaborate between the sites. The Mid-Atlantic Twin Registry population included all twin pairs born in Virginia and North Carolina between 1915 and 1997. The Norwegian Twin Registry included all same-sex pairs born in Norway between 1915 and 1979. The Danish Twin Registry included all pairs born in Denmark between 1952 and 1982. The twins from the three registries during these time periods were mailed a questionnaire that screened for febrile seizures. The same questionnaire was sent to all three areas and translated as needed.
The twins from these three databases were initially screened for a history of seizures via a mailed written questionnaire. Information regarding the questionnaire and the details of the queries can be found in Kjeldson et al. 2005. Twins that reported a seizure (febrile or afebrile) or an event that was possibly based in seizure (ex: staring) were contacted by phone. The phone interview was standardized across all three sites and was used to validate the history of the seizure. If a seizure was validated the cotwin was queried regarding seizure and neurological issues. This analysis used the information collected to analyze the relationship between febrile seizure type and development of epilepsy. The full method of patient identification, the accuracy of self-reporting and the process of validation can be found in Corey et al. 2009.
The twin pairs were evaluated for zygosity (monozygotic vs dizygotic) using either DNA serum markers when available or a validated questionnaire. Some pairs were excluded based on a deceased twin or a twin not wanting to participate. The concordance rates of febrile seizures in the twin pairs were also analyzed. The statistical analyses were conducted with SAS v.9.2. Febrile seizure type frequencies were calculated within each twin sample for all twin pairs that had available data. Full method explanation for zygosity and concordance rates are published in the Corey et al. 2011 article.
Results
A history of febrile seizures was validated in 1051 twins in 900 pairs. The febrile seizure type was classified as one of four independent groups: simple, complex, febrile status epilepticus or unclassified. There were 643 (61%) simple febrile seizures, 125 (12%) complex febrile seizures, 72 (7%) febrile status epilepticus, and 211 (20%) of unclassified. This can be seen in Figure 1. There were 40.6% of the twin pairs that had a co-twin with febrile or afebrile seizures.
Figure 1.
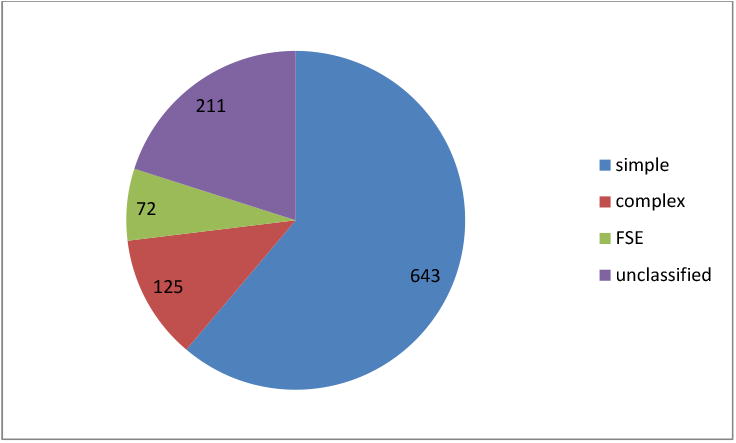
Febrile seizure semiology.
In this study, there were 78 twins who developed epilepsy. The highest rate of epilepsy was seen in the febrile status epilepticus group, which was the smallest group of twins. The development of epilepsy for the simple febrile seizure group was 2.6%, the complex febrile seizure group was 12%, the febrile status epilepticus group was 22.2% and the unclassified group was 14.2%. This can be seen in Figure 2.
Figure 2.
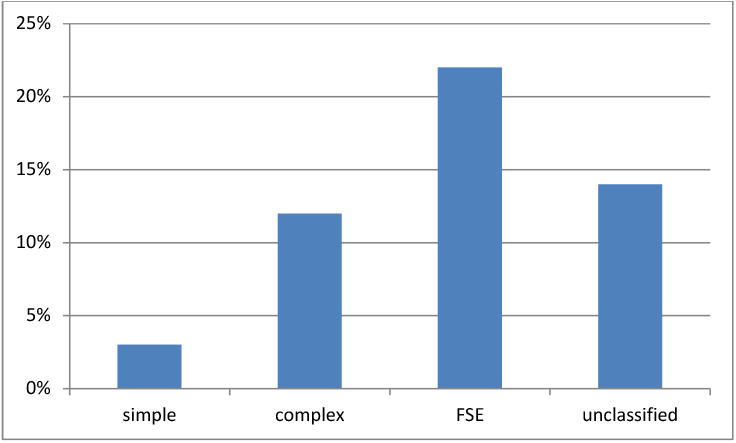
Development of epilepsy.
Concordance rate was evaluated for monozygotic and dizygotic twin pairs. There were 50 monozygotic twins that were found to have one pair member that had a febrile seizure and went on to have epilepsy and a cotwin with a history of seizures. The concordance rate for monozygotic twins was 41 of the simple febrile seizure group, 4 of the complex febrile seizure group, 1 of the febrile status epilepticus group and 4 of the unknown classification group. There were 28 dizygotic twins that were concordant, 25 that had a simple febrile seizure, 2 with complex febrile seizure, 0 with febrile status epilepticus and 1 unknown.
Discussion
The analysis of this large twin population with febrile seizures supports the conclusion that there are both genetic and environmental factors involved in febrile seizures. The high concordance rate in monozygotic twins that have simple febrile seizures suggest that simple febrile seizures indicate familial risk for seizures. This may be a separate group from seizures after febrile status epilepticus, which are influenced by environmental factors. Although previous smaller twin studies have evaluated the genetic contribution to different febrile seizure subtypes and made similar conclusions, the large population analyzed for this study makes this an important conclusion (Eckhaus et al. 2013).
This analysis concluded that twins with complex febrile seizures and febrile status epilepticus are at a higher risk to develop epilepsy. This study did not evaluate families to determine if they represented patients with generalized epilepsy and febrile seizure plus (GEFS+) or any other known syndromes. This supports the theory that risk of epilepsy after a febrile seizure is not only genetically determined, but can be related to an injury caused by the seizure. The results of this analysis are consistent with results from The Consequences of Prolonged Febrile Seizure Study (FEBSTAT), which is a multicenter prospective study that evaluated febrile seizures > 30 minutes (Hesdorffer et al. 2012). FEBSTAT has already demonstrated that children with febrile status epilepticus are at risk for acute hippocampal injury and that a substantial number also have abnormalities in hippocampal development (Shinnar et al. 2012).
A positive family history of febrile seizures can be found in 25-40% of cases when a child present with a febrile seizure (Frantzen et al.1970, Annegers et al. 1979, Hauser et al. 1985). Febrile seizures can be seen in multiple family members, but there is no single accepted mechanism. The higher concordance rate in monozygotic twins as compared to dizygotic twins in this study demonstrates a population that could give further information regarding the mechanism. This large sample size may contribute to the rate of epilepsy development compared to smaller population studies (Tsuboi 1987, Tsuboi and Endo 1991, Johnson et al. 1996, Berkovic et al. 1998). Although a limitation if the study is the group of unclassified seizures, these subjects were predominately born before the 1950s. Our findings have implications for future genetic studies that need to be performed to evaluate genetic relationship of febrile seizure and epilepsy. The specific loci involved in determining febrile seizure risk has not been identified, and further clinical and molecular evaluations are essential. The education and counseling of children with febrile seizures may need to be changed based on febrile seizure semiology.
Acknowledgments
This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (NS031564).
This research received support from award number P60MD002256 from the National Institute on Minority Health and Health Disparities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annegers JF, Hauser WA, Elveback LR, Kurland LT. The risk of epilepsy following febrile convulsions. Neurology. 1979;29(3):297–303. doi: 10.1212/wnl.29.3.297. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S. The contributions of epidemiology to the understanding of childhood seizures and epilepsy. J Child Neurol. 1994;9(2):19–26. [PubMed] [Google Scholar]
- Berkovic SF, Howell RA, Hay DA, Hopper JL. Epilepsies in twins: genetics of the major epilepsy syndromes. Ann Neurol. 1998 Apr;43(4):435–45. doi: 10.1002/ana.410430405. [DOI] [PubMed] [Google Scholar]
- Corey LA, Berg K, Pellock JM, Solaas MH, Nance WE, DeLorenzo RJ. The occurrence of epilepsy and febrile seizures in Virginian and Norwegian twins. Neurology. 1991;41(9):1433–1436. doi: 10.1212/wnl.41.9.1433. [DOI] [PubMed] [Google Scholar]
- Corey LA, Kjeldsen MJ, Solaas MH, et al. The accuracy of selfreported history of seizures in Danish, Norwegian and U.S. twins. Epilepsy Res. 2009;84:1–5. doi: 10.1016/j.eplepsyres.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey LA, Pellock JM, Kjeldsen MJ, Nakken KO. Importance of genetic factors in the occurrence of epilepsy syndrome type: a twin study. Epilepsy Res. 2011 Nov;97(1-2):103–11. doi: 10.1016/j.eplepsyres.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhaus J, Lawrence KM, Helbig I, Bui M, Vadlamudi L, Hopper JL, Scheffer IE, Berkovic SF. Genetics of febrile seizure subtypes and syndromes: a twin study. Epilepsy Res. 2013 Jul;105(1-2):103–9. doi: 10.1016/j.eplepsyres.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Frantzen E, Lennox-Buchthal M, Nygaard A, Stene J. A genetic study of febrile convulsions. Neurology. 1970;20(9):909–917. doi: 10.1212/wnl.20.9.909. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Anderson VE, Kurland LT. The risk of seizure disorders among relatives of children with febrile convulsions. Neurology. 1985;35(9):1268–1273. doi: 10.1212/wnl.35.9.1268. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Shinnar S, Lewis DV, Moshé SL, Nordli DR, Jr, et al. Design and phenomenology of the FEBSTAT study. Epilepsia. 2012;53(9):1471–1480. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WG, Kugler SL, Stenroos ES, et al. Pedigree analysis in families with febrile seizures. Am J Med Genet. 1996;61:345–352. doi: 10.1002/(SICI)1096-8628(19960202)61:4<345::AID-AJMG8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kjeldsen MJ, Kyvik KO, Friis ML, Christensen K. Genetic and environmental factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. 2002 Sep;51(1-2):167–77. doi: 10.1016/s0920-1211(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Kjeldsen MJ, Corey LA, Solaas MH, Friis ML, Harris JR, et al. Genetic factors in seizures: a population-based study of 47,626 US, Norwegian and Danish twin pairs. Twin Res Hum Genet. 2005;8(2):138–1347. doi: 10.1375/1832427053738836. [DOI] [PubMed] [Google Scholar]
- Mathai KV, Dunn DP, Kurland LT, Reeder FA. Convulsive disorders in the Mariana Islands. Epilepsia. 1968;9(2):77–85. doi: 10.1111/j.1528-1157.1968.tb05130.x. [DOI] [PubMed] [Google Scholar]
- Shinnar S. Febrile seizures and Mesial Temporal Sclerosis. Epilepsy Curr. 2003;3(4):115–118. doi: 10.1046/j.1535-7597.2003.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar S, Bello JA, Chan S, Hesdorffer DC, Lewis DV, et al. MR Imaging abnormalities following Febrile Status Epilepticus in Children: The FEBSTAT Study. Neurology. 2012;79(9):871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar S, Glauser TA. Febrile seizures. J Child Neurol. 2002;17(1):S44–S52. doi: 10.1177/08830738020170010601. [DOI] [PubMed] [Google Scholar]
- Stanhope JM, Brody JA, Brink E, Morris CE. Convulsions among the Chamorro people of Guam, Mariana Islands. II. Febrile convulsions. Am J Epidemiol. 1972;95(3):299–304. doi: 10.1093/oxfordjournals.aje.a121397. [DOI] [PubMed] [Google Scholar]
- Tsuboi T. Epidemiology of febrile and afebrile convulsions in children in Japan. Neurology. 1984;34(2):175–181. doi: 10.1212/wnl.34.2.175. [DOI] [PubMed] [Google Scholar]
- Tsuboi T. Genetic analysis of febrile convulsions: twin and family studies. Hum Genet. 1987 Jan;75(1):7–14. doi: 10.1007/BF00273830. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Endo S. Genetic studies of febrile convulsions: analysis of twin and family data. Epilepsy Res Suppl. 1991;4:119–28. [PubMed] [Google Scholar]
- Waruiru C, Appleton R. Febrile seizures: an update. Arch Dis Child. 2004;89(8):751–756. doi: 10.1136/adc.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]


