Abstract
Genital chlamydial infections lead to severe upper reproductive tract pathology in a subset of untreated women. We demonstrated previously that TNF-α producing CD8+ T cells contribute significantly to chlamydial upper genital tract pathology in female mice. Additionally, we observed minimal chlamydial oviduct pathology develops in OT-1 transgenic (OT-1) mice, wherein CD8+ T cell repertoire is restricted to recognition of the ovalbumin peptide Ova257–264, suggesting that non-Chlamydia-specific CD8+ T cells may not be responsible for chlamydial pathogenesis. In the current study, we evaluated whether antigen-specific CD8+ T cells mediate chlamydial pathology. Groups of wild type C57BL/6J (WT), OT-1 mice, and OT-1 mice replete with WT CD8+ T cells (1×106 cells/mouse intravenously) were infected intravaginally with C. muridarum (5 × 104 IFU/mouse). Serum total anti-Chlamydia antibody and total splenic anti-Chlamydia IFN-γ and TNF-α responses were comparable among the three groups of animals. However, Chlamydia-specific IFN-γ and TNF-α production from purified splenic CD8+ T cells of OT-1 mice was minimal, whereas responses in OT-1 mice replete with WT CD8+ T cells were comparable to those in WT animals. Vaginal chlamydial clearance was comparable between the three groups of mice. Importantly, the incidence and severity of oviduct and uterine horn pathology was significantly reduced in OT-1 mice but reverted to WT levels in OT-1 mice replete with WT CD8+ T cells. Collectively, these results demonstrate that Chlamydia-specific CD8+ T cells contribute significantly to upper genital tract pathology.
Keywords: Chlamydia, genital infection, upper genital tract pathology, Chlamydia-specific, antigen-specific, CD8+ T cells
INTRODUCTION
Chlamydia trachomatis is the leading cause of sexually transmitted bacterial infection worldwide 1,2. Chlamydial infections in a subset of untreated women ascend to the upper reproductive tract and induce severe immunopathology in the uterus and fallopian tubes, including pelvic inflammatory disease (PID), and complications such as ectopic pregnancy and infertility 3,4. Due to host tropism dictated by IFN-γ evasion mechanisms, C. trachomatis does not productively infect and cause severe pathologies in mice 5. Chlamydia muridarum is a mouse pathogen that causes genital infection and reproductive tract pathology in mice, similar to the effects of C. trachomatis in humans 6,7. We demonstrated previously that TNF-α–producing CD8+ T cells cause chlamydial upper genital tract (UGT) immunopathology 8, and that OT-1 mice wherein CD8+ T cell repertoire is limited to recognition of the ovalbumin peptide Ova257–264 develop minimal UGT pathology 9, suggesting that CD8+ T cells that do not recognize chlamydial antigens do not contribute significantly to such pathology. However, it remained to be demonstrated that Chlamydia-specific CD8+ T cells cause the UGT pathology.
In the current study, we compared immune responses and UGT pathology in wild type C57BL/6J (WT) mice, OT-1 mice, and OT-1 mice replete with WT CD8+ T cells at the time of Chlamydia muridarum intravaginal (i.vag) infection.
RESULTS
Immune responses following genital C. muridarum infection
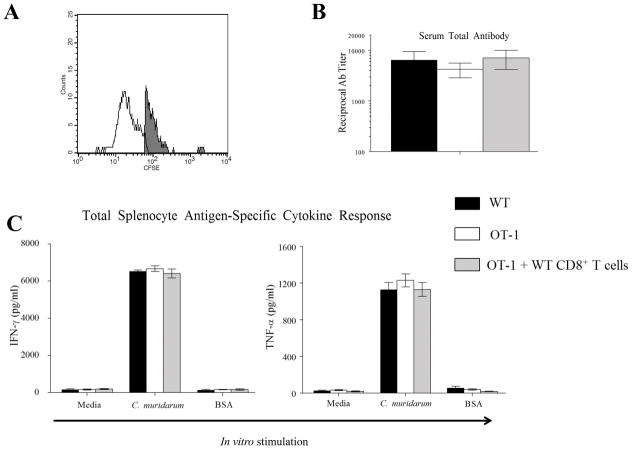
On day 4 after cellular injection, CD3+ CD8+ CFSE+ cells were enumerated in 3 OT-1 mice replete with WT CD8+ T cells and constituted 16.5±1.5 % of all CD3+ CD8+ T cells in the spleen, compared to none in control OT-1 mice (Fig 1A), confirming the success of adoptive transfer. Serum anti-Chlamydia total antibody responses were measured on day 40 in WT mice, OT-1 mice, and OT-1 mice replete with WT CD8+ T cells infected with 5 × 104 IFU of C. muridarum and were found to be comparable between the groups of mice (Fig 1B). The splenic total cellular IFN-γ and TNF-α production in response to in vitro C. muridarum stimulation also were comparable among the three groups of mice (Fig 1C).
Figure 1. Immune responses in OT-1 mice replete with WT CD8+ T cells.
(A) A group (n = 3) of OT-1 mice were replete with CFSE labeled CD8+ T cells on day 0 and euthanized on day 4 to evaluate the frequency of CD3+ CD8+ CFSE + cells in the spleen. A representative histogram is shown with a mouse in this group to an OT-1 mouse control. Results are representative of two independent experiments. (B) Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were pre-treated (5 days prior to infection) with Depo-provera ®, and challenged (on day 0) with 5×104 IFU of C. muridarum. Mice were bled on day 40 and serum anti-chlamydial total antibody levels determined. Mean ± SEM of the reciprocal antibody titer corresponding to 50% maximal binding is shown. Results pooled from two independent experiments is shown. (C) Groups (n = 4) of mice (WT mice, OT-1 mice, and OT-1 mice replete with CD8+ T cells) were pre-treated (5 days prior to infection) with Depo-provera ®, and challenged (on day 0) with 5×104 IFU of C. muridarum. On day 14, total splenocyte IFN-γ and TNF-α production in response to in vitro stimulation with UV-inactivated C. muridarum was evaluated. Mean ± SEM of the cytokine levels in each group is shown. Results are representative of two independent experiments.
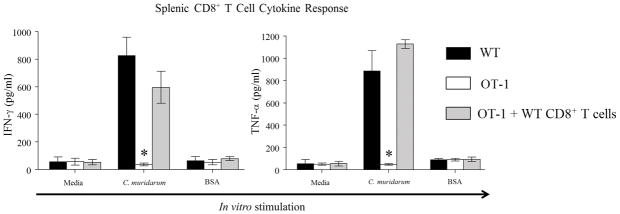
We then evaluated Chlamydia-specific cytokine response from enriched splenic CD8+ T cells from the three groups of mice. The production of Chlamydia-specific IFN-γ and TNF-α from WT CD8+ T cells was significantly higher compared to the minimal amounts produced by OT-1 CD8+ T cells (Fig 2). Importantly, CD8+ T cells from OT-1 mice replete with WT CD8+ T cells produced comparable levels of IFN-γ and TNF-α to WT cells demonstrating the restoration of the ability of these mice to respond to chlamydial infection via CD8+ T cells in an antigen-specific fashion. The ability of OT-1 CD8+ T cells to produce IFN-γ and TNF-α was confirmed by in vitro stimulation with Ova257–264 peptide of enriched CD8+ T cells from ovalbumin immunized animals (data not shown). Collectively, these results demonstrate that serum antibody response and total splenic cytokine responses were comparable between WT and OT-1 mice, suggesting that a general deficiency of immune response does not explain reduced upper genital tract pathology in OT-1 mice as reported by us previously. However, CD8+ T cells from only WT, not OT-1, mice respond to C. muridarum infection in an antigen-specific fashion. Moreover, OT-1 mice replete with WT CD8+ T cells could mount Chlamydia-specific CD8+ T cell cytokine response to a level comparable to WT animals.
Figure 2. Splenic CD8+ T cell cytokine response against chlamydial infection.
Groups (n = 4) of mice (WT mice, OT-1 mice, and OT-1 mice replete with CD8+ T cells) were pre-treated (5 days prior to infection) with Depo-provera ®, and challenged (on day 0) with 5×104 IFU of C. muridarum. On day 14, Splenic CD8+ T cell IFN-γ and TNF-α production in response to in vitro stimulation with live C. muridarum infected antigen-presenting cells. Mean ± SEM of the cytokine levels in each group is shown. * Significant (p ≤ 0.05; One-way ANOVA with Holm-Sidak method for multiple group comparisons) difference between OT-1 mice and wild type mice, and between OT-1 mice and OT-1 mice replete with WT CD8+ T cells when stimulated in vitro with chlamydial antigens. Results are representative of two independent experiments.
Vaginal bacterial clearance and upper genital tract pathology following genital C. muridarum infection
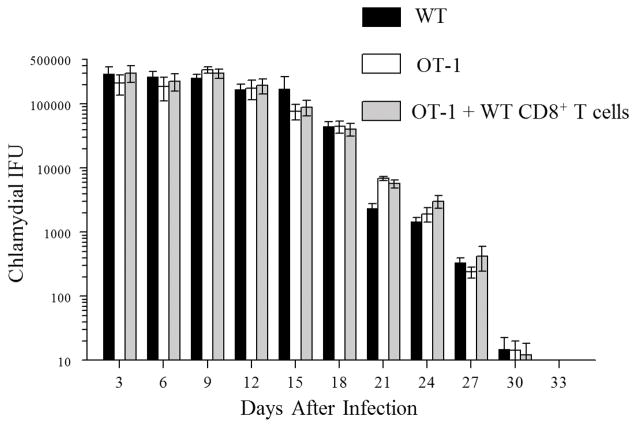
Groups of WT mice, OT-1 mice, and OT-1 mice replete with WT CD8+ T cells were infected with 5 × 104 IFU of C. muridarum. WT mice shed high numbers of bacteria at early time-periods and displayed a progressive reduction in vaginal chlamydial shedding with complete clearance by day 33 after primary chlamydial inoculation (Fig. 3). The kinetics of vaginal bacterial shedding and resolution of infection in OT-1 mice and OT-1 mice replete with WT CD8+ T cells was comparable to that in WT mice. These results suggest that antigen-specific CD8+ T cells are not required for clearance of primary intravaginal C. muridarum infection and agrees with the similar previous findings from our lab and others 8–10.
Figure 3. Chlamydial shedding after genital C. muridarum challenge.
Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were pre-treated (5 days prior to infection) with Depo-provera ®, and challenged (on day 0) with 5×104 IFU of C. muridarum. Chlamydial shedding was enumerated at the indicated timepoints for a period of 33 days. The mean ± SEM of vaginal chlamydial shedding per group at each time point is shown. Results pooled from two independent experiments is shown.
Oviduct and uterine horn pathology following genital C. muridarum infection
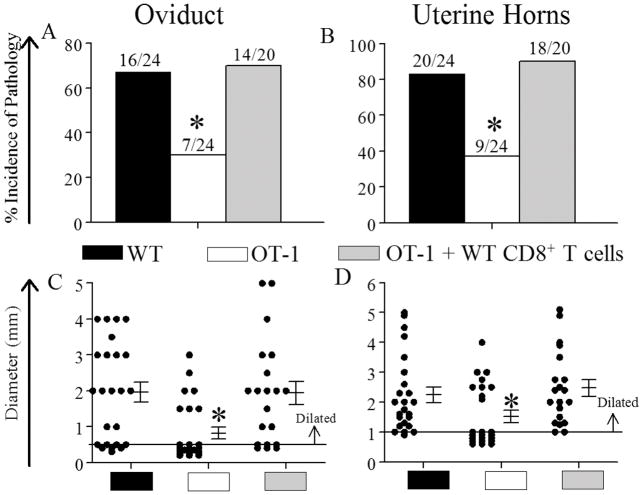
The development of hydrosalpinx (fluid-filled oviduct dilatation), a characteristic marker of reproductive tract pathological sequelae, and uterine horn dilatation was evaluated at day 80 following primary genital chlamydial inoculation in the three groups of mice. OT-1 mice displayed significant reduction in the incidence of both oviduct and uterine horn dilatation when compared to WT mice (Fig 4A and B). Importantly, OT-1 mice replete with WT CD8+ T cells displayed a significant increase in the incidence of both oviduct and uterine horn dilatation when compared to OT-1 mice, and to a level comparable to that in WT mice (Fig 4A and 4B). Furthermore, there was a significant reduction in the severity of oviduct and uterine horn dilatation as represented by the size of dilatation in OT-1 mice compared to WT animals, whereas OT-1 mice replete with WT CD8+ T cells displayed comparable severity of oviduct and uterine dilatation to WT animals (Fig 2D & 2E, respectively). Collectively, these results demonstrate that WT CD8+ T cells that are capable of mounting Chlamydia-specific cytokine responses can restore UGT pathology in OT-1 mice to a level found in WT animals.
Figure 4. Upper genital tract pathology after genital C. muridarum challenge.
Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were pre-treated (5 days prior to infection) with Depo-provera ®, and challenged (on day 0) with 5×104 IFU of C. muridarum. (A–D) On day 80, oviduct and uterine horn pathology was evaluated. The percentage and the number of pathological and normal tissues in the oviduct (A) and uterine horns (B) is shown. *, significant (p ≤ 0.05, Fisher’s exact test) difference in the incidence of dilatation within the group of OT-1 mice compared to wild type mice or OT-1 mice replete with WT CD8+ T cells. The macroscopic oviduct diameter (C) and uterine horn diameter (D) were measured. Each individual marker represents one oviduct or uterine horn, and the mean ± SEM of oviduct/uterine horn diameter is also shown. The horizontal line at 0.5 mm and 1 mm depicts the distinction between normal and dilated oviducts and uterine horns, respectively. *, significant (p ≤ 0.05, One-way ANOVA with Dunn’s test for multiple group compairsons) difference in severity of dilatation between OT-1 mice and wild type mice, and between OT-1 mice and OT-1 mice replete with WT CD8+ T cells. Results pooled from two independent experiments are shown.
DISCUSSION
We demonstrated previously that TNF-α producing CD8+ T cells contribute significantly to Chlamydia-induced upper genital tract pathology 8. Additionally, we had reported that OT-1 mice display minimal chlamydial UGT pathology 9, suggesting that CD8+ T cells that do not recognize chlamydial antigens do not contribute significantly to such pathologies. In this study, we provide compelling evidence that antigen-specific CD8+ T cells respond to chlamydial infection and cause much of the UGT pathology following primary genital C. muridarum infection.
This study is the first to demonstrate the antigen-specificity of CD8+ T cells that mediate pathological sequelae following primary genital chlamydial infection. These results confirm and extend previous evidence on the pathogenic role of CD8+ T cells in different models including the mouse model of C. muridarum infection, salpingitis in nonhuman primates, and trachoma in human individuals 8,9,11–14. These previous studies had not specifically addressed whether pathologies were mediated by antigen-specific CD8+ T cells. The results of the current study have important implications in the context of anti-chlamydial vaccine development. Vaccination regimens can elicit various adaptive immune responses including antigen-specific CD8+ T cell responses and any anti-Chlamydia vaccination protocol or composition should avoid untoward responses 15. Conversely, several studies have demonstrated a protective role for CD8+ T cells in chlamydial infections. Specifically, certain CD8+ T cell clones expressing high levels of IFN-γ have been shown to assist in genital chlamydial clearance 16 and a CXCR5+ regulatory CD8+ T cell subset has been implicated in reduction of oviduct pathology following C. muridarum infection 17. More recently, CD8+ T cells primed in the respiratory tract, not in the genital tract, were shown to mediate protective responses 18. Additionally, CD8+ T cells primed with plasmid-deficient chlamydial organisms were shown to be important in resistance against ocular infectious challenge using virulent strains of C. trachomatis 19. Therefore, various factors including but not limited to specific phenotypic subsets, inductive sites, sites of infection and multiple infections appear to affect protective versus pathological outcomes mediated by Chlamydia-specific CD8+ T cells.
In summary, the role of CD8+ T cells in genital chlamydial infections continues to be elaborated, and this study provides an important and compelling addition to the existing body of evidence. Given the significant interest in development of an anti-Chlamydia vaccine, future studies need to focus on teasing out the specific nature and mechanisms of pathogenesis and protective immunity mediated by Chlamydia-specific CD8+ T cells.
METHODS
Chlamydia muridarum and mice
Chlamydia muridarum Nigg (C. muridarum) strain was grown in HeLa 229 cells, and elementary bodies (EB) were obtained as described previously 20,21. UV inactivated organisms were generated by subjecting gradient-purified chlamydial EB to 30 min of UV irradiation. Female 4 to 6 week old C57BL/6J WT mice and OT-1 mice were purchased from the Jackson Laboratory, and bred and maintained at Midwestern University. No specific randomization protocol was followed to place animals in different experimental groups. Personnel were not blinded to the mouse groups. Food and water were supplied ad libitum and all experimental procedures used in this manuscript were approved by the Institutional Animal Care and Use Committee (IACUC) at Midwestern University.
Enrichment of CD8+ T cells from splenocytes and adoptive transfer
Spleens were collected from naïve donor mice after euthanasia, and CD8+ T cells were enriched using magnetic beads (Easysep, Stemcell Technologies, CA) as described previously 21. The enriched (>95%) cells were injected intravenously (1 × 106 cells/mouse) into recipient mice in 100 μl sterile 1X PBS 2 hours after intravaginal infection with C. muridarum. Some mice which received carboxy-fluoro-succinimidyl ester (CFSE) labeled CD8+ T cells were euthanized on day 4 and splenic CD3+ CD8+ CFSE+ cells were enumerated using flow cytometry to confirm successful transfer.
Evaluation of serum antibody and cellular cytokine responses
Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were bled on day 40 after infection, sera prepared, and analyzed separately for each mouse for anti-chlamydial total antibody, as described previously 21. Reciprocal serum dilution corresponding to 50% maximal binding titer was used for comparisons. Cellular cytokine production also was evaluated as described previously 21. Fourteen days after primary infection with C. muridarum, mice (8 per group; 4 for total splenocyte and 4 for CD8+ T cell cytokine response) were euthanized, spleens collected and pooled single cell suspensions for each group were made. Splenocytes (106 cells/well; quadruplicate) were stimulated with 104 UV irradiated chlamydial EB, or the unrelated protein bovine serum albumin (BSA), or medium alone, and incubated for 72 hours, and supernatants were collected. For evaluation of CD8+ T cell responses, enriched CD8+ T cells (106 cells/well; quadruplicate) were cultured with equal number of mouse antigen presenting cells pre-infected with live C. muridarum (MOI 1) or incubated with control antigens. At the end of 72 hours incubation, supernatants were collected to analyze cytokine production. All supernatants were assayed (four replicates per in vitro stimulation condition) for IFN-γ and TNF-α using Ebioscience ELISA kits (San Diego, CA), according to manufacturer’s instructions.
Intravaginal infection of mice and monitoring of bacterial shedding
Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were treated with 2.5 mg of Depo-provera® (medroxy-progesterone acetate) per mouse subcutaneously 5 days before vaginal challenge to render the mice anestrous and receptive to the genital infection. The mice were then challenged with 5 × 104 inclusion-forming units (IFU) of C. muridarum contained in 10 μl of sucrose-phosphate-glutamate (SPG) buffer placed into the vaginal vault. The course of infection was followed by swabbing the vaginal vault on the indicated days following inoculation. Chlamydial counts were determined separately for each mouse as described previously 21,22.
Estimation of oviduct and uterine horn pathology
Groups (n = 10–12) of mice (WT mice (n=12), OT-1 mice (n=12), and OT-1 mice replete with CD8+ T cells (n=10)) were infected with C. muridarum and upper genital tract pathology was evaluated on day 80 post-challenge, as described previously 21,23. Mice were euthanized and genital tracts removed. Images of tissues placed next to a standard metric ruler were photographed using a 20 mega-pixel Panasonic ZS20 camera. Dilated oviducts measuring >0.5 mm in diameter were used as an indicator of hydrosalpinx. When multiple oviduct loops were present, the one with the greatest diameter was reported. For uterine horns, the greatest cross-sectional diameter of each 5-mm longitudinal section of an individual uterine horn was measured and the average per uterine horn was used to calculate the mean per group of mice. The baseline normal mouse oviduct diameter was determined to be up to 0.5 mm and normal uterine horn diameter to be up to 1 mm by prior analysis of a group of age-matched naïve mice.
Statistical analyses
The number of animals per group for various experiments were determined based on our previous studies. Sigma Stat (Systat Software Inc., San Jose, CA) was used to perform all tests of significance. Analysis of variance (One-way ANOVA; Systat, CA) was used for all comparisons. For multiple group comparisons, Holm-Sidak method in Figure 2 and Dunn’s method Figure 4 was also used). The variance between groups compared statistical statistically was similar. The differences in incidence of oviduct pathology were compared between two groups at a time using Fisher’s exact test. Differences between groups were considered statistically significant if P values were < 0.05. All experiments were repeated at least twice, and each experiment was analyzed independently, with the exception of the serum antibody levels, vaginal chlamydial shedding and upper genital tract pathology results wherein results from two experiments were pooled and analyzed.
Acknowledgments
Funding Disclosure: This work was supported by Midwestern University College of Health Sciences Master’s thesis funds to KV and National Institutes of Health grant 1R15AI101920, American Heart Association Midwest Affiliate grant 13SDG17310011, and Midwestern University Faculty Startup Grant to AKM. The funders had no role in design of these studies.
This work was supported by Midwestern University College of Health Sciences Master’s thesis funds to KV and National Institutes of Health grant 1R15AI101920, American Heart Association Midwest Affiliate grant 13SDG17310011, and Midwestern University Faculty Startup Grant to AKM. We thank Mr. Ryan Incrocci and Mr. William Kay for technical assistance.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Lewis DA, Latif AS, Ndowa F. WHO global strategy for the prevention and control of sexually transmitted infections: time for action. Sex Transm Infect. 2007;83:508–509. doi: 10.1136/sti.2007.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belland R, Ojcius DM, Byrne GI. Chlamydia Nat Rev Microbiol. 2004;2:530–531. doi: 10.1038/nrmicro931. [DOI] [PubMed] [Google Scholar]
- 3.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 4.Westrom L, Mardh PA. Chlamydial salpingitis. Br Med Bull. 1983;39:145–150. doi: 10.1093/oxfordjournals.bmb.a071806. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DE, Virok DP, Wood H, Roshick C, Joshnson RM, Whitmire WM, et al. Chlamydial IFN-gamma immune evasion is linked to host infection tropism. Proc Natl Acad Sci U S A. 2005;102:10658–10663. doi: 10.1073/pnas.0504198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rank RG. Animal models for urogenital infections. Methods Enzymol. 1994;235:83–93. doi: 10.1016/0076-6879(94)35133-3. [DOI] [PubMed] [Google Scholar]
- 7.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–2751. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, et al. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun. 2011;79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manam S, Nicholson BJ, Murthy AK. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis. 2013;67:221–224. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000;68:6979–6987. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igietseme JU, He Q, Joseph K, Eko FO, Lyn D, Ananaba G, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009;200:926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimani J, Maclean IW, Bwayo JJ, MacDonald K, Oyugi J, Maitha GM, et al. Risk factors for Chlamydia trachomatis pelvic inflammatory disease among sex workers in Nairobi, Kenya. J Infect Dis. 1996;173:1437–1444. doi: 10.1093/infdis/173.6.1437. [DOI] [PubMed] [Google Scholar]
- 13.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Voorhis WC, Barrett LK, Sweeney YT, Kuo CC, Patton DL. Analysis of lymphocyte phenotype and cytokine activity in the inflammatory infiltrates of the upper genital tract of female macaques infected with Chlamydia trachomatis. J Infect Dis. 1996;174:647–650. doi: 10.1093/infdis/174.3.647. [DOI] [PubMed] [Google Scholar]
- 15.Murthy AK, Arulanandam BP, Zhong G. Chlamydia Vaccine; Progress and Challenges. ASM Press; 2012. pp. 311–333. [Google Scholar]
- 16.Igietseme JU, Magee DM, Williams DM, Rank RG. Role for CD8+ T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect Immun. 1994;62:5195–5197. doi: 10.1128/iai.62.11.5195-5197.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J, Champion CI, Wei B, Liu G, Kelly KA. CD8(+)CXCR5(+) T cells regulate pathology in the genital tract. Infect Dis Obstet Gynecol. 2013;2013:813238–813245. doi: 10.1155/2013/813238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogueira CV, Zhang X, Giovannone N, Sennott EL, Starnbach MN. Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae. J Immunol. 2015;194:2319–2329. doi: 10.4049/jimmunol.1402675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivares-Zavaleta N, Whitmire WM, Kari L, Sturdevant GL, Caldwell HD. CD8+ T cells define an unexpected role in live-attenuated vaccine protective immunity against Chlamydia trachomatis infection in macaques. J Immunol. 2014;192:4648–4654. doi: 10.4049/jimmunol.1400120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy AK, Chambers JP, Meier PA, Zhong G, Arulanandam BP. Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production. Infect Immun. 2007;75:666–676. doi: 10.1128/IAI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manam S, Thomas JD, Li W, Maladore A, Schripsema JH, Ramsey KH, et al. Tumor Necrosis Factor (TNF) Receptor Superfamily Member 1b on CD8+ T Cells and TNF Receptor Superfamily Member 1a on Non-CD8+ T Cells Contribute Significantly to Upper Genital Tract Pathology Following Chlamydial Infection. J Infect Dis. 2015;211(12):2014–2022. doi: 10.1093/infdis/jiu839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine. 2007;25:3773–3780. doi: 10.1016/j.vaccine.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manam S, Chaganty BK, Evani SJ, Zafiratos MT, Ramasubramanian AK, Arulanandam BP, et al. Intranasal vaccination with Chlamydia pneumoniae induces cross-species immunity against genital Chlamydia muridarum challenge in mice. PLoS One. 2013;8:e64917. doi: 10.1371/journal.pone.0064917. [DOI] [PMC free article] [PubMed] [Google Scholar]