Abstract
Resin bonding to dentin requires the use of self-etching primers or acid etching to decalcify the surface and expose a layer of collagen fibrils of the dentin matrix. Acid-etching reduces the stiffness of demineralized dentin from approximately 19 GPa to 1 MPa, requiring that it floats in water to prevent it from collapsing during bonding procedures. Several publications show that crosslinking agents like gluteraladehyde, carbodiimide or grape seed extract can stiffen collagen and improve resin-dentin bond strength.
Objective
The objective was to assess a new approach for evaluating the changes in stiffness of decalcified dentin by polar solvents and a collagen cross-linker.
Methods
Fully demineralized dentin beams and sections of etched coronal dentin were subjected to indentation loading using a cylindrical flat indenter in water, and after treatment with ethanol or ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). The stiffness was measured as a function of strain and as a function of loading rate from 1 to 50 µm/sec.
Results
At a strain of 0.25% the elastic modulus of the fully demineralized dentin was approximately 0.20 MPa. It increased to over 0.90 MPa at strains of 1%. Exposure to ethanol caused an increase in elastic modulus of up to four times. Increasing the loading rate from 1 to 50 µm/sec caused an increase in the apparent modulus of up to three times in both water and ethanol. EDC treatment caused increases in the stiffness in fully demineralized samples and in acid-etched demineralized dentin surfaces in situ.
Significance
Changes in the mechanical behavior of demineralized collagen matrices can be measured effectively under hydration via indentation with cylindrical flat indenters. This approach can be used for quantifying the effects of bonding treatments on the properties of decalcified dentin after acid etching, as well as to follow the loss of stiffness over time due to enzymatic degradation.
Keywords: collagen, crosslinking, dentin bonding agents, durability, EDC, endogenous proteinases, stiffness
INTRODUCTION
Mineralized dentin contains about 50% mineral by volume. If it is acid-etched with phosphoric acid in preparation for resin-dentin bonding, approximately the top 10 µm of mineralized dentin is completely demineralized (1). After rinsing off the excess acid, the rinse water extracts the solubilized mineral and leaves the insoluble collagen fibrils floating in approximately 70 vol% water. The goal in adhesive dentistry is to replace that volume of water with adhesive resin monomers. However, the monomer concentration is low (3–4 moles/L) when compared to the water concentration (56 moles/L). Consequently, it is impossible for dentists to remove all of the water using solvated comonomer mixtures in the relatively short period (60–90 sec) that most dentists generally allot for monomer infiltration.
The presence of excess residual water (2) in demineralized dentin is problematic. Application of bonding blends that contain dimethacrylates like BisGMA, TEGDMA or UDMA that are insoluble in water, undergo phase changes when they encounter that residual water (3,4). Consequently, some collagen fibrils remain infiltrated by water instead of monomers (5,6). These collagen fibrils also contain bound endogenous proteases (MMPs and cathepsins) that become uncovered and activated by acid-etching (e.g. 5–10). They are hydrolases that use the residual water to slowly destroy the collagen fibrils, which are critical to anchor the adhesive and resin composite to the underlying mineralized dentin.
If the water is removed from demineralized dentin, there is a rapid, spontaneous development of new interpeptide hydrogen bonds between adjacent collagen peptides. Formation of these bonds increases collagen stiffness (11, 12) and causes shrinkage (13). Both changes are rapidly reversible if the collagen is rehydrated because the Hoy’s solubility parameter describing the hydrogen bonding cohesive forces (δh) is 40 (J/cm2)½, which is much greater than that of collagen peptides 14.8 (J/cm2)½ (14). Water preferentially H-bonds to collagen peptides and prevents interpeptide H-bonds from forming, which is how water plasticizes collagen and synthetic polymers (14).
Solvents like acetone and ethanol produce reversible interpeptide hydrogen bonds, which also cause reversible increases in matrix stiffness (11, 15, 16). However, proanthocyanidin agents like Grape Seed Extract (GSE) and tannic acid produce covalent cross-links and irreversible increases in matrix stiffness (17–19) and strength (20). Such cross-linking may also make the dentin too stiff to undergo complete shrinkage if the matrix is inadvertently air-dried. The increase in stiffness of the demineralized dentin reflects the degree of interfibrillar cross-linking achieved within the collagen matrix (21). Consequently, measurements of the changes in matrix stiffness provide a quantitative means for assessing the degree of cross-linking and the treatment’s effectiveness (1,22). Recently, Liu et al. (23) showed that addition of 2 wt% GSE to 20 vol% phosphoric acid produced an acid-etched dentin matrix that was apparently stabilized against the action of microbial collagenase within 15–20 sec. Nevertheless, they did not measure the increase in stiffness of the demineralized matrix caused by the GSE/phosphoric acid mixture. That would require an approach capable of measuring the stiffness of the etched layer of dentin in situ with adequate sensitivity.
The changes in stiffness of demineralized dentin after application of cross-linkers or other treatments reflects the degree of intermolecular bonding achieved. Thus, quantifying the changes matrix stiffness is important. The stiffness of demineralized dentin collagen matrices has been measured using atomic force microscopy (24, 25, 26) and flexure methods (11, 17–20, 27). While AFM is a potent method for evaluating the stiffness of collagen, it is generally applied to evaluate single fibrils and not the matrix as a continuum (28). Quantifying the degree of collagen cross-linking requires measures at the meso-scale, not at the fibril level. Existing flexure and tensile testing methods are macro-scale and not capable of measuring the small changes in stiffness of a 1 to 10 µm layer of demineralized dentin matrix in situ that results from application of polar solvents. Therefore, the purpose of this investigation was to examine a new approach for evaluating the stiffness of demineralized dentin collagen matrices. Here we attempt to perform macroscopic “indentations” with a cylindrical flat indenter to measure reversible and irreversible changes in stiffness of demineralized dentin collagen matrices resulting from application of various clinically-relevant treatments. The null hypothesis of the investigation is that macroscopic indentations are not capable of quantifying the stiffness of decalcified layers of dentin resulting from acid etching in situ.
MATERIALS AND METHODS
Dentin Specimens
Human third molars were obtained from the Oral Surgery Clinics of the Georgia Regents University with signed informed consent using a document approved by the GRU Human Assurance Committee. The teeth came from young patients (18–24 yrs of age) and were examined at receipt under light microscopy for signs of caries or cracks. Those teeth without signs of carious lesions or mechanical degradation were stored under refrigeration (4°C) until ready for use.
The occlusal enamel and superficial dentin were removed using an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA) at right angles to the long axis of the tooth. A second, parallel section was made to create a disk of mid-coronal dentin of either 1.0 or 2.0 mm thick thickness (±0.1 mm). The 2 mm thick disks were further sectioned to prepare beams approximately 3×2×5 mm. Three beams and three disks were prepared, all from different teeth. After preparation the samples were stored in 0.9% NaCl containing 0.02% sodium azide to prevent microbial growth.
Dentin demineralization
To create completely demineralized dentin, single beams or dentin disks were suspended in 10 ml of 10 wt% phosphoric acid (PA) in screw top tubes. Multiple tubes were tumbled at room temperature for 16 hrs to completely demineralize the specimens after (29). At the end of demineralization the enamel periphery had been completely removed from the coronal disks. Completely demineralized dentin disks have a modulus of elasticity of ≤4 MPa (25). Partially demineralized dentin disks have higher values of stiffness (1,000–12,000 MPa). The fact that none of our experimental stiffness values reached 4 MPa indicates they were all completely demineralized.
An additional group of 1 mm thick mineralized dentin disks (N=5) were prepared and etched using 37% phosphoric acid (Scotchbond, 3M ESPE) to achieve a layer of demineralized dentin of approximately 10 µm. The depth of demineralization was measured using Confocal Laser Scanning Microscopy (CLSM) as described in Scheffel et al (1). Specifically, flat dentin surfaces were acid-etched with 37% phosphoric acid for 15 sec, rinsed with water for 10 sec and then immersed in 1% w/v Fluorescein Isothiocyanate (FITC) in DMSO and 1 % w/v xylenol orange (XO) in water for 5 hours. After rinsing with water, the stained disks were examined by CLSM. Exposed collagen fluoresced a green color while underlying mineralized dentin was red, as described in Scheffel et al (1). The average thickness of the demineralized layer was 8 µm in Scheffel et al., 2014, but was 10–15 µm in our current study. Many of the teeth had deep occlusal grooves that carried enamel deep into the dentin. When the section is made deep enough to avoid all traces of enamel, the dentin surface is sometimes in deep dentin that contains more tubules than more superficial dentin, allowing etchants to penetrate more deeply into the dentin matrix and increasing the thickness of the demineralized matrix. After completion of the period of etching the samples were rinsed with excess water and then maintained in the 0.9% NaCl solution at room temperature for approximately 1 hour.
EDC treatment
For the 1 mm thick disks of completely demineralized dentin, the stiffness was initially evaluated in the solution of 0.9% NaCl. The samples were then subjected to a cross-linking treatment using an experimental solution of 0.5 M ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) in water for 60 sec. The dentin stiffness was then measured by indentation as the samples were maintained within a droplet of the EDC treatment solution. Similarly, for the fully mineralized dentin disks that were subjected to acid etching of the surface, the stiffness of the etched layer of dentin collagen was evaluated in situ under hydration with the 0.9% NaCl solution. Then the samples were subjected to 0.5 M EDC crosslinking treatment for 30 sec and evaluated again.
Measurement of Stiffness
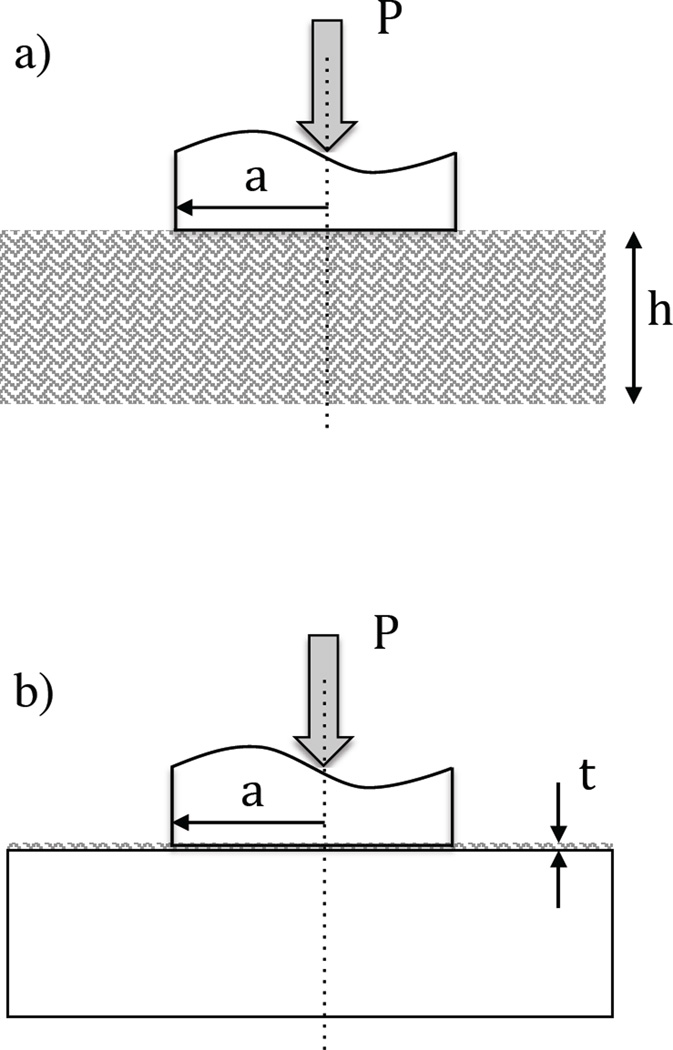
Measurement of the stiffness was performed using a commercial universal testing machine (BOSE, Model Electroforce 3100, Eden Prairie, MN, USA). The system was equipped with a special load cell with full scale range of 2.5 N and sensitivity of 0.01%. This system has a resolution in displacement control of 1 µm and a resolution in displacement measurement of 0.1 µm. Loading of the demineralized dentin was performed in indentation mode (compression) as shown in Figure 1 using a specially designed stainless steel cylindrical indenter with 1.9 mm diameter and flat face. The demineralized dentin specimens (Figure 1a) were placed on a loading platform of UHMW polyethylene with the pulp side down after Walton et al. (30). The specimen was covered within a droplet of 0.9% NaCl containing 0.02% sodium azide to maintain the hydration of the specimen during the indentation process.
Figure 1.
Schematic diagrams describing indentation of the dentin substrates using a cylindrical indenter with flat face. a) indentation of the fully demineralized dentin specimens; b) indentation of the acid-etched mineralized dentin specimens. The variable P, a, and h represent the indentation load, indenter radius and thickness of the demineralized substrate, respectively. The variable t in (b) represents the thickness of the etched dentin layer.
To achieve loading of the demineralized samples, the indenter was lowered within the droplet until developing a preload of 2 mN. For the beams, the point of contact was centered within the 3 mm beam width and along the 5 mm length. At this point, the system was converted to displacement control actuation and loading was conducted in displacement control to a maximum displacement of 20 µm. Loading was conducted at displacement rates of 1, 10 or 50 µm/sec. The acquisition of the load and displacement responses was monitored over the range of displacement. Each sample was loaded in three different locations over the surface area. After evaluation in water, the samples were placed in a bath of ethanol and maintained with agitation for a period of 30 seconds. Thereafter, the samples were placed on the UHMW polyethylene stage covered with a droplet of ethanol. The samples were load as previously described using the BOSE systems at one of the three displacement rates. Following completion of the ethanol treatment and loading, the samples were returned to the 0.9% NaCl solution and maintained at room temperature. After approximately 15 minutes the samples were evaluated again within a droplet of the NaCl solution using the aforementioned universal testing system and loading approach. Contact loading of the demineralized disk was conducted in exactly the same manner before and after EDC treatment.
For the etched mineralized dentin sample, contact loading was performed to evaluate the stiffness of the thin demineralized layer in situ (Figure 1b). Due to the limited depth of the demineralized layer (ca. 10–15 µm) and range of the load cell (2.5 N), displacement control loading was conducted at a rate of 1µm/sec to a maximum displacement of 10 µm. Following completion of the tests, the samples of water-saturated demineralized dentin were subjected to cross-linking using the EDC treatment for 30 seconds and loaded again via cylindrical indentation in the EDC solution. Three indentations were performed on each sample.
For both sample configurations the load-displacement curves were used in evaluating the indentation stiffness of the demineralized dentin. These quantities were then used to estimate the elastic modulus at various strains of interest. Under cylindrical indentation, the contact pressure (Pr) can be described as a function of penetration depth (h) using Sneddon’s solution for indentation of an elastic half-space by a flat-ended cylindrical punch according to (31)
| (1) |
where E, a and ν are the elastic modulus, indenter radius and Poisson’s ratio, respectively. Expanding the contact pressure in terms of the contact force (P) and area, the apparent elastic modulus can be estimated in terms of the indentation stiffness according to
| (2) |
In the case of a thin demineralized layer of collagen developed by etching of mineralized dentin, the elastic modulus of the demineralized section is substantially lower than that of the mineralized portion. For small displacements that are limited to the demineralized layer thickness, the reaction force is attributed to the demineralized collagen layer only. Thus, the elastic response can be evaluated using Eqn. 2. However, the thin layer of hydrated collagen is restrained by the fully mineralized dentin foundation through the collagen fibril anchors. Hayes et al (32) developed a model for indentation of layered substrates to be applied in assessing the elastic modulus of articular cartilage, which is described by
| (3) |
where the term corresponds to a corrective term that accounts for the restraint of the compressible material. In considering the dimensions of a thin layer of demineralized dentin that results from acid etching, it could be considered a thin film. Yang (33) derived an expression for indentation of a compressible elastic thin film by cylindrical flat punch, where the elastic modulus is given by
| (4) |
This expression applies for a thin film mounted on a rigid substrate, where t is the film thickness (Figure 1b).
For all three of the aforementioned approaches, the indentation modulus may be determined from the indentation stiffness, indenter radius and Poisson’s ratio of the substrate. Although the Poisson’s ratio for the demineralized dentin collagen matrix has not been reported, evaluations performed on articular cartilage show that a reasonable value for the hydrated network is 0.175 (e.g. 34,35). That value was adopted for estimating the elastic modulus of the demineralized dentin. The estimated elastic moduli were compared using a one-way analysis of variance with repeated measures and Tukeys HSD. Significant differences were identified by p ≤ 0.05.
RESULTS
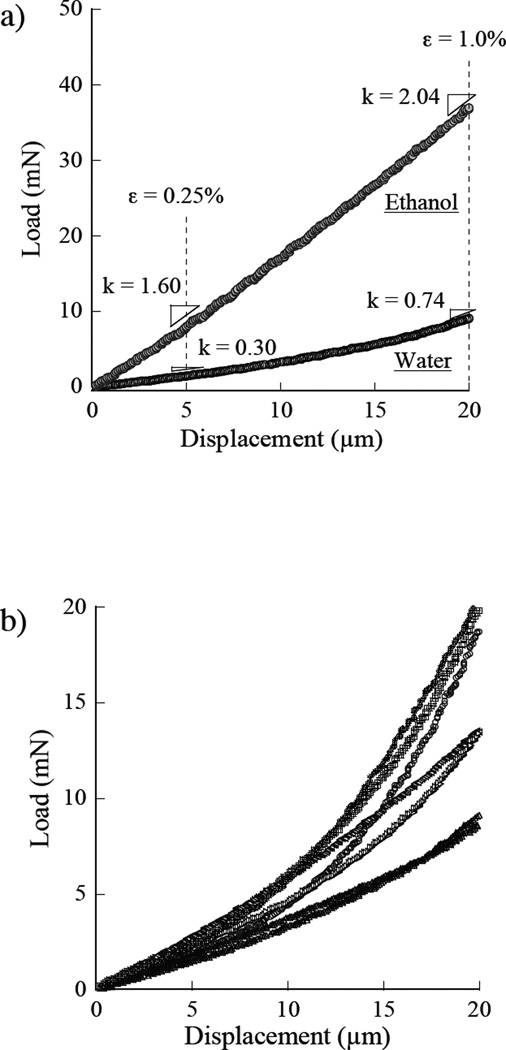
A representative load-displacement response obtained from indentation loading of the demineralized dentin beams at a displacement rate of 1 µm/sec is shown in Figure 2a. Examples are shown for water hydration and in ethanol. Measures of the apparent stiffness are highlighted at the displacements corresponding to indentation strains of 0.25% and 1%. All of the responses obtained for indentation of the water-hydrated collagen are shown in Figure 2b. As evident from these figures, the stiffness and corresponding elastic modulus increases with degree of indentation. The average elastic modulus of the demineralized matrices in water estimated using Eqn 2 at strains of 0.25% and 1% deformation were 0.21±0.04 MPa and 0.91±0.37 MPa, respectively.
Figure 2.
Load-displacement responses for indentation loading of the completely demineralized dentin samples in water. a) a single specimens with stiffness measurements at 0.25% and 1.0% strains; b) distribution of the stiffness responses obtained from the samples in water.
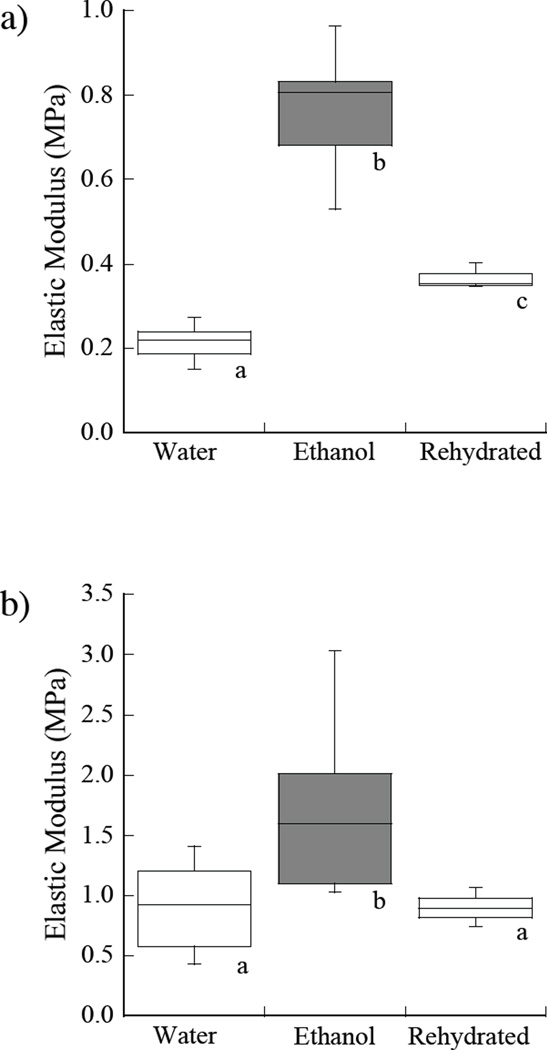
A comparison of the average elastic moduli estimated from indentation loading of the demineralized dentin beams within water, ethanol and after rehydration in water are shown in Figure 3. The responses are shown separately for strains of 0.25% and 1% in Figures 3a and 3b, respectively. The ethanol treatment resulted in a significant increase in elastic modulus at both measures of strain. The average elastic modulus in ethanol at 0.25% and 1% strains were 0.77±0.14 MPa and 1.68±0.68 MPa. Rehydrating the samples in water for 30 minutes caused a significant decrease in the elastic modulus in comparison to the ethanol treatment condition as evident in Figure 3. After rehydration in water, the modulus returned to that of the original hydrated condition.
Figure 3.
Influence of exposure to ethanol on the apparent stiffness of the demineralized dentin collagen matrix. The samples were initially evaluated in water. Then they were placed in a bath of 100% ethanol for 30 seconds and evaluated in ethanol. Thereafter, the samples were removed from ethanol, rehydrated in water for 15 minutes and evaluated again in water. a) comparison of the elastic modulus at 0.25% strain; b) comparison of the elastic modulus at 1% strain. The boxes for each condition represent the average and standard deviation. The error bars encompass the total range in responses and boxes with different letters indicate significant differences (p≤0.05).
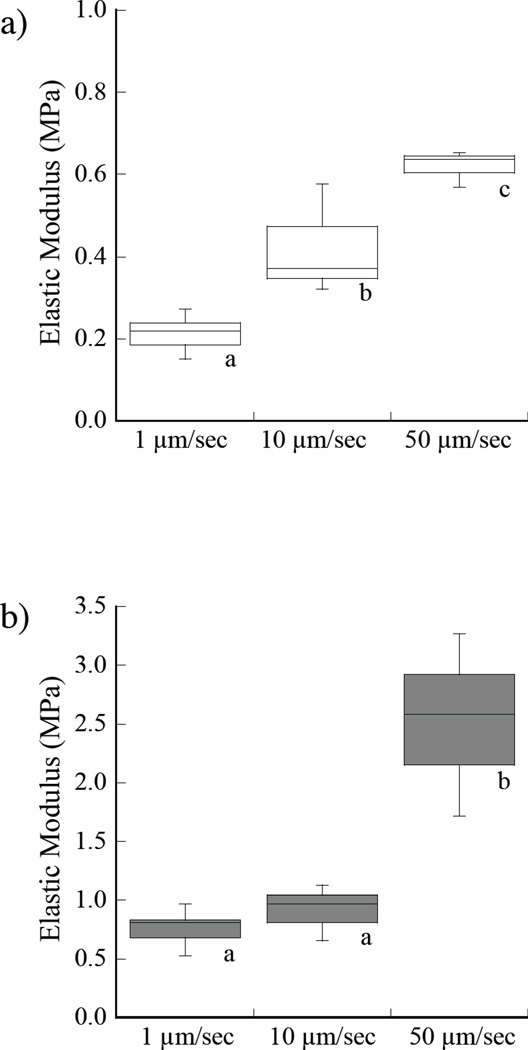
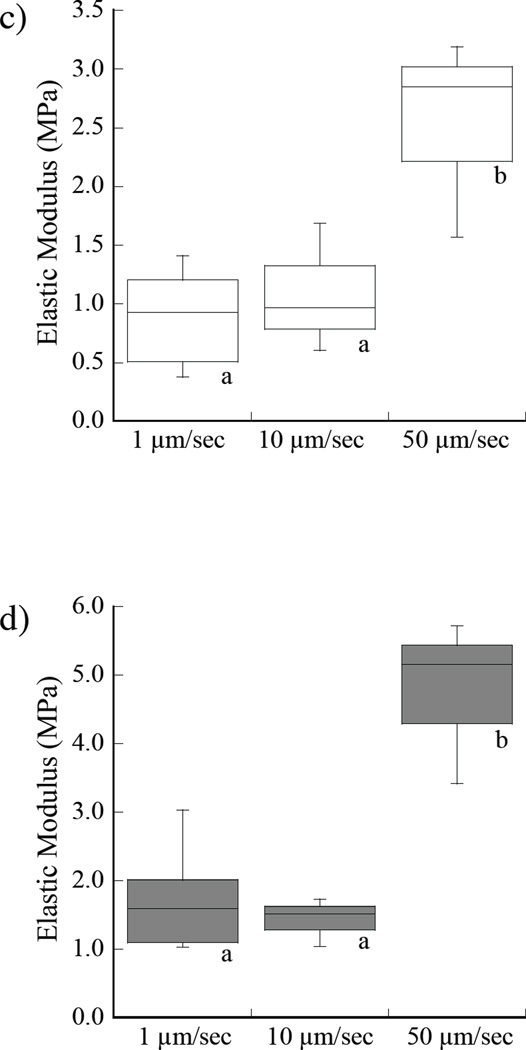
A comparison of the indentation responses obtained for loading rates of 1, 10 and 50 µm/sec in water are shown in Figure 4. The average elastic moduli in water at 0.25% strain are shown in Figure 4a. As evident from the responses, there is a significant increase in the stiffness with loading rate. The average elastic modulus in ethanol at the three rates of loading is shown in Figure 4b. In water, there was an increase in the elastic modulus with loading rate from 0.21 MPa to 0.62 MPa at 0.25% strain. When placed in ethanol, the average elastic modulus increased from 0.77±0.14 MPa to 2.52 MPa over the range in loading rate. Similar results for the elastic behavior in water and ethanol at 1% strain are shown in Figures 4c and 4d, respectively. There was a significant increase in elastic modulus with loading rate for both the water and ethanol environments and at each of the two measures of deformation.
Figure 4.
Influence of loading rate on the elastic modulus of the demineralized dentin. Indentation tests were performed at loading rates of 1, 10 and 50 µm/sec. a) at 0.25% strain in water; b) at 0.25% strain in ethanol, c) at 1% strain in water, d) at 1% strain in ethanol. Note the difference in scales used for presenting the data in (a) – (d). Boxes with different letters indicate significant differences (p≤0.05).
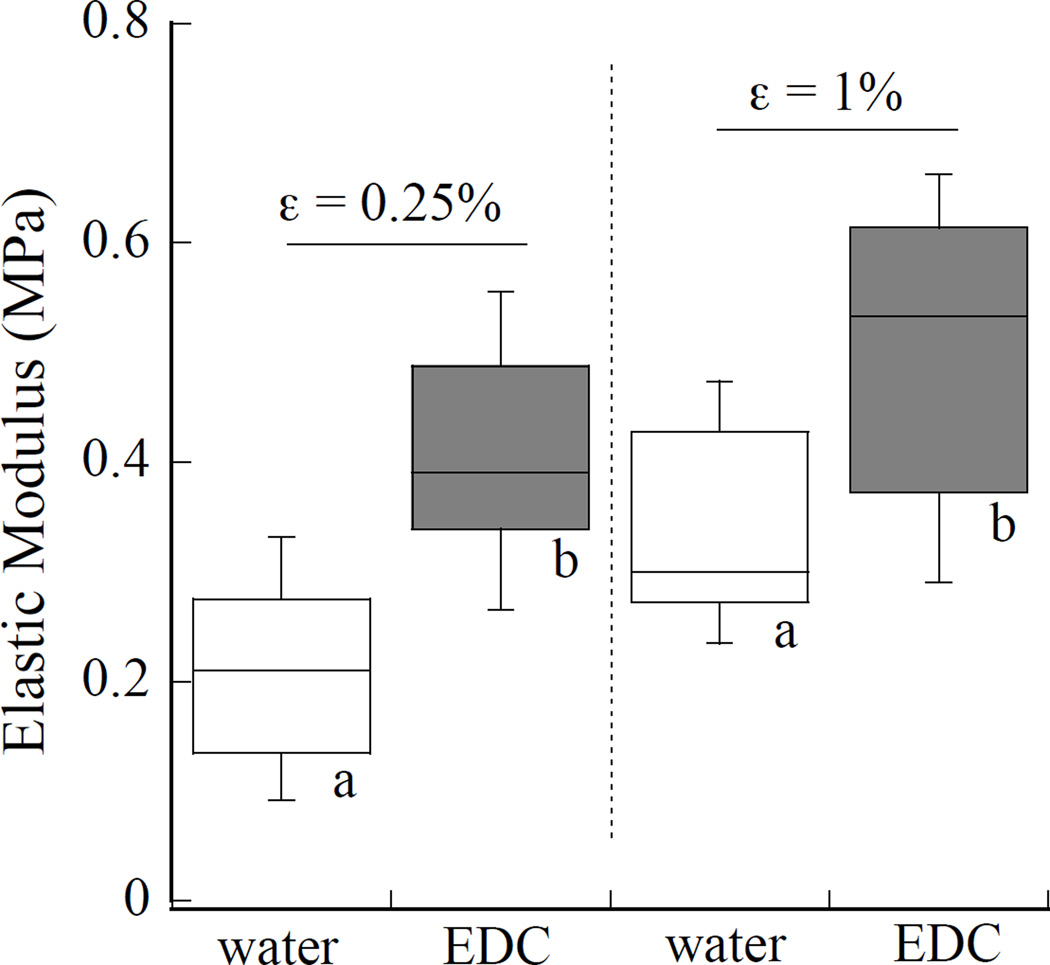
The influence from 60 sec of EDC crosslinking treatment on the elastic modulus of the demineralized dentin matrices was also evaluated by indentation. Results obtained at indentation strains of 0.25% and 1% are shown in Figure 5. Values obtained for the average elastic modulus in water before and after EDC treatment at 0.25% strain were 0.21±0.08 MPa and 0.42±0.09 MPa. At 1% strain the measure of elastic modulus in the two environments were 0.34±0.1 MPa and 0.50±0.14 MPa. The EDC treatment caused a significant (p<0.05) increase in the elastic modulus at both of the values of incremental strain.
Figure 5.
Changes in stiffness of the demineralized collagen with EDC treatment for 1 minute. A comparison is made between the water and EDC responses at indentation strains of 0.25% and 1% (Figure 2(a)). Boxes with different letters indicate significant differences (p≤0.05).
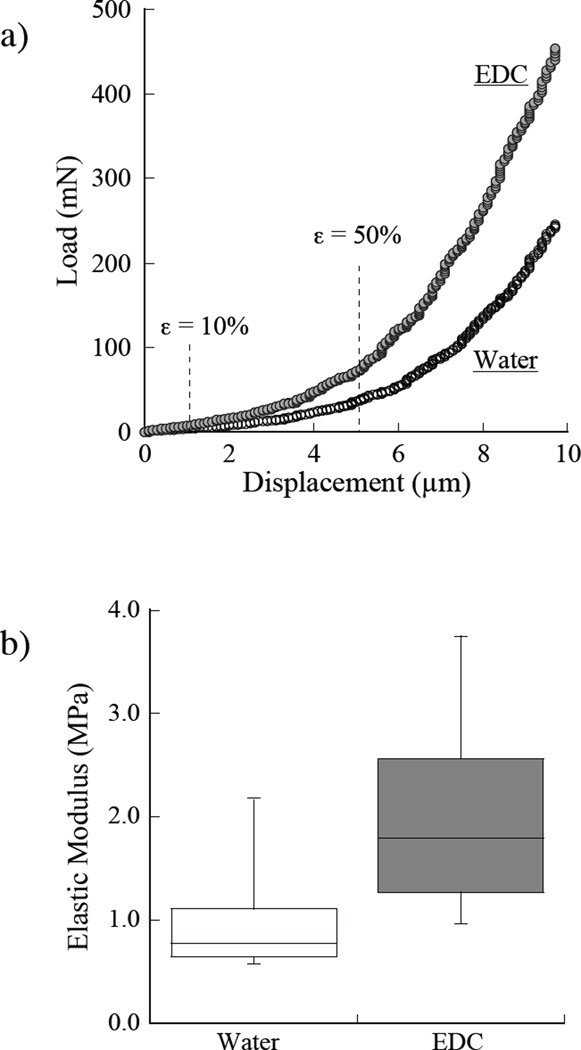
Figure 6 shows results for indentation loading of the acid-etched demineralized collagen in situ. Specifically, Figure 6a shows a comparison of the load-displacement responses obtained for a representative sample in water and then after EDC treatment. The displacements corresponding to indentation strains of 10% and 50% strain are highlighted in this figure. Here, the average demineralized layer thickness was assumed to be 10 µm, which results in a 10% compressive strain at 1 µm of indentation displacement. The elastic modulus was estimated from the stiffness measurements according to the relationships described by Eqn. 2, 3 and 4. The results obtained using Eqn.’s 3 and 4 resulted in moduli on the order of 10 kPa, which suggests that restraint from the mineralized foundation was not a concern. Therefore, the elastic modulus of the demineralized layer of dentin collagen was estimated using Eqn. 2. A comparison of the average elastic modulus from stiffness measurements at 10% strain for water and EDC treatment conditions is shown in Figure 6b. The average elastic modulus for these two conditions was 1.02±0.58 MPa and 2.02±1.00 MPa, respectively. The difference in elastic modulus between the measurements obtained in water and after EDC treatment was nearly a factor of two, but not significant (p=0.08). Presumably, had the crosslinking reaction been allowed to continue longer, the stiffness would have continued to increase (36).
Figure 6.
The apparent stiffness of the etched dentin layer measured in situ under cylindrical indentation. a) comparison of the load-displacement responses for a sample evaluated in water and then after EDC treatment; b) elastic modulus measurements at 10% strain. According to a one-way ANOVA, the difference in elastic modulus between the two conditions is approaching significance (p=0.08).
DISCUSSION
Results from the experiments showed that the stiffness and elastic modulus of demineralized dentin matrices could be assessed successfully by macroindentations using a cylindrical flat indenter. Measurements of the elastic modulus ranged from roughly 0.2 MPa to nearly 6 MPa and exhibited a strong dependence on the magnitude of strain, the rate of loading and the solution treatments. The indentation approach was also used successfully to characterize the stiffness of the decalcified layer of dentin resulting from acid etching in situ for the first time. Those accomplishments allow rejection of the null hypothesis.
The elastic modulus estimated for the fully demineralized dentin collagen in water by macroscopic indentation is similar to the values reported by Balooch et al (24), which were obtained by micro-indentation. However, the modulus estimated by indentations (Fig. 3) is nearly a factor of magnitude lower than those values reported in evaluations using flexure and tensile loading, which are approximately 6 MPa (11,17–19). One reason for this difference is the strain at which the modulus was estimated. The previous estimates obtained from flexure loading were measured at strains near 3%, whereas the values measured by indentation for the fully demineralized samples were obtained at compressive strains of 0.25% and 1%. If the load-displacement curves in Figure 2 for water hydration are extrapolated to 3% strain, the average modulus is approximately 2 MPa, which is of reasonable agreement with the values reported earlier. Another important difference is that the modulus estimates from flexure and tension reflect the tensile response of the matrix, whereas the modulus from indentations is derived from the compressive response.
It can be argued that resin-bonded specimens in normal function are subjected to low strains (11). While the matrix may be subjected to large strains at loads near fracture, the portion of the matrix stiffness most relevant to resin infiltration during dentin bonding is that corresponding to small strains. The estimated modulus of the demineralized dentin collagen in water is very consistent with that reported for articular cartilage in unconfined compression (37). Similar to cartilage, the elastic response of demineralized dentin matrix may best be described as a range rather than a single value, which depends on location and magnitude of strain. Another consideration in comparing estimates of the modulus is the relative collagen orientation and direction of loading as noted in cartilage (38). Indeed, there is a preferred arrangement of dentin collagen in the crown, which corresponds to planes arranged perpendicular to the tubules (39,40). Future studies should be conducted to examine the spatial variations in elastic modulus of dentin collagen and the importance of anisotropy.
To the authors’ knowledge the elastic modulus for the acid-etched dentin evaluated in situ are the first reported of this type (Fig. 6). It is difficult to compare these results with those obtained for the fully demineralized samples as the measured modulus was obtained at much larger strains. Due to the small thickness of the acid etched layer, a displacement of 1 µm was equivalent to a 10% compressive strain of the demineralized collagen, which was the smallest increment of strain control. Nevertheless, the in situ measures are in agreement with estimates extrapolated from results of the fully demineralized samples for a strain of 10%.
There is a distinct difference in characteristics of results for the acid-etched dentin and fully demineralized samples. Specifically, results for the in-situ experiments exhibit a much larger degree of variation in both the water and ethanol environments (Fig. 6b). Supplemental experiments showed that the variability was not associated with the testing approach or equipment. A replication of in situ indentation measurements performed at a single location of samples showed that the estimated modulus was very repeatable (Appendix), with Coefficient of Variation (COV) of 0.15. In contrast, the COV estimated from indentations at multiple locations and samples was nearly 0.5. It was noted in some of the in situ measurements that there was a rapid increase in stiffness when approaching the maximum displacement. This behavior may be indicative of variability in the depth of demineralized layer and lower depth of acid penetration in some samples. Clearly the effectiveness of etching and consequent degree of penetration is influenced by the sample microstructure, including the coronal depth in which the sample was sectioned (e.g. 41) and tooth donor age (e.g. 42). In estimating the modulus at specific strains, it was necessary to assume that the depth of demineralized layer was constant over the indentation area, and that the depth of the demineralized layer was the same in all samples. That could be a concern in application of the indentation approach when comparing in situ measurements where variations in microstructure are considered.
Exposing the demineralized dentin samples to ethanol caused stiffening, which results from the spontaneous formation of interpeptide hydrogen bonding in the absence of water (14). Such stiffening is rapidly reversed when the ethanol is replaced by water (11). When interpeptide H-bonds develop in dentin collagen, they cause a large degree of shrinkage (13). The interfibrillar spaces (ca. 20–30 nm) that are needed for infiltration of liquid monomers into demineralized dentin will disappear by such H-bonding, which can prevent resin-infiltration of dry, demineralized dentin (43). The shrinkage occurring with ethanol treatment could actually be observed during preloading of the samples in ethanol with the flat cylindrical indenter via a reduction in load. Hence, it may be possible to study the rate of H-bonding with ethanol treatment using the indentation approach.
Stiffening of the demineralized dentin also resulted from the EDC treatment, which occurs through the development of newly created irreversible covalent bonds. This could help preserve the architecture of the hydrated matrix during bonding, which is essential for resin infiltration (36). The increase in stiffness caused by EDC treatment was significant at both measures of strain. The largest increase was approximately a factor of two and corresponded to 0.25% indentation strain. The change in stiffness of the PA-etched dentin with EDC treatment was similar in magnitude, but not significant (p=0.08). Longer treatment times would be expected to promote more extensive covalent bonding and changes in stiffness.
Carrilho et al. (44) measured the initial stiffness of completely demineralized dentin beams (0.9 × 0.75 × 6 mm) using 3-point flexure. The beams were then incubated in 1 mL of simulated body fluid for up to 4 weeks. Stiffness was measured each week. The results showed a steady decrease in stiffness over time (p<0.05). An analysis of the incubation medium for collagen peptide fragments revealed increases in hydroxyproline over time (p<0.05). When 2 mass % chlorhexidine was added to the medium, there was less decrease in stiffness (p<0.05) and less hydroxyproline released. Chlorhexidine is known to inhibit matrix metalloproteins (MMPs) 2, 8 and 9 (45). Decreases in the stiffness of demineralized dentin matrices can be used as an indirect and nondestructive measure of the rate of degradation of demineralized dentin matrices. This is a very important clinical problem. A measurement approach capable of performing precise measurement of stiffness in situ would serve as a powerful tool for monitoring the effectiveness of envisioned treatments for retarding degradation of collagen by endogeneous dentin proteases.
It is important to comment on the benefits and drawbacks of the indentation approach for evaluating the stiffness of demineralized dentin collagen. Similar to 3-pt bending, the indentation approach does not require gripping of the small compliant specimens. It is also non-destructive and enables repeated measurements to be performed on samples, including before and after selected treatments. There are also additional advantages over the use of flexure testing, including the ability to evaluate the stiffness in multiple locations, the reduced need for precise measurement of specimen cross-section dimensions, and the larger relative area of evaluation. The larger cross-section area of the flat indenter increases the reaction force resulting from loading, which is favorable for sensitivity, and also homogenizes the response over the measurement area. Lastly, until now, a meso-scale method for measuring the stiffness of the demineralized collagen layer resulting from acid etching in situ has not been reported. Hence, the indentation technique could be considered a breakthrough. It will enable future studies on the structural behavior of dentin collagen and the effectiveness of treatments considered for maximizing the durability of dentin bonds.
There are potential disadvantages to the indentation approach as well. One of the most important is that it requires an instrument with precise displacement control, particularly when applied to the acid-etched layers of dentin samples. Few universal testing systems provide this degree of resolution. Instrumented nanoindenters have the required displacement resolution, but their load range is generally far too low for this application. Alignment of the indenter’s face and sample surface is important in this mode of evaluation to achieve uniform contact over the indentation area. That requires careful preparation of samples with constant thickness and attention to the alignment between the cylindrical indenter face and sample stage.
An additional concern for indentation measurements is that the reaction load is a function of the collagen stiffness and the displacement of the water with advancement of the indenter. The relative contribution of the collagen and the water displacement to the total reaction force is unknown. The contribution of the water movement is a function of the hydraulic conductance of the water. For conditions where the fluid expression does not exceed the hydraulic conductance of those spaces, the resistance to loading is composed of the collagen fibril matrix stiffness. But if the rate of loading of the matrix exceeded the hydraulic conductance of those interconnected spaces, additional resistance to loading might be detected. More research needs to be done in that area.
CONCLUSION
On the basis of the experimental results, it was demonstrated that the method of macro-indentation using a cylindrical flat indenter is an effective approach for evaluating the elastic behavior of demineralized dentin collagen within a fluid. Application of the approach to samples of demineralized coronal dentin in water provided an average elastic modulus (at the onset of deformation) of approximately 0.21±0.04 MPa. When the water was replaced with ethanol, the elastic modulus increased to 0.77±0.14 MPa (p≤0.05). In addition, the elastic modulus increased significantly with loading rate in both the water and ethanol solutions. The method of indentation loading was also be used for evaluating the elastic modulus of acid-etched etched dentin layers in situ. Preliminary results were obtained at 10% strain and resulted in an average elastic modulus of hydrated collagen of 1.02±0.58 MPa. After the demineralized layer was cross-linked with 0.5 M carbodiimide (EDC) for 60 sec, the elastic modulus was 2.02±1.00 MPa (p=0.08).
ACKNOWLEDGEMENTS
The authors acknowledge support from the National Institutes of Health (NIDCR R01 DE015306-06 P.I. Pashley) and the National Science Foundation (NSF DMR 1337727 Takacs). The authors also gratefully acknowledge 3M ESPE for their generous donation of bonding supplies and resin composite.
APPENDIX
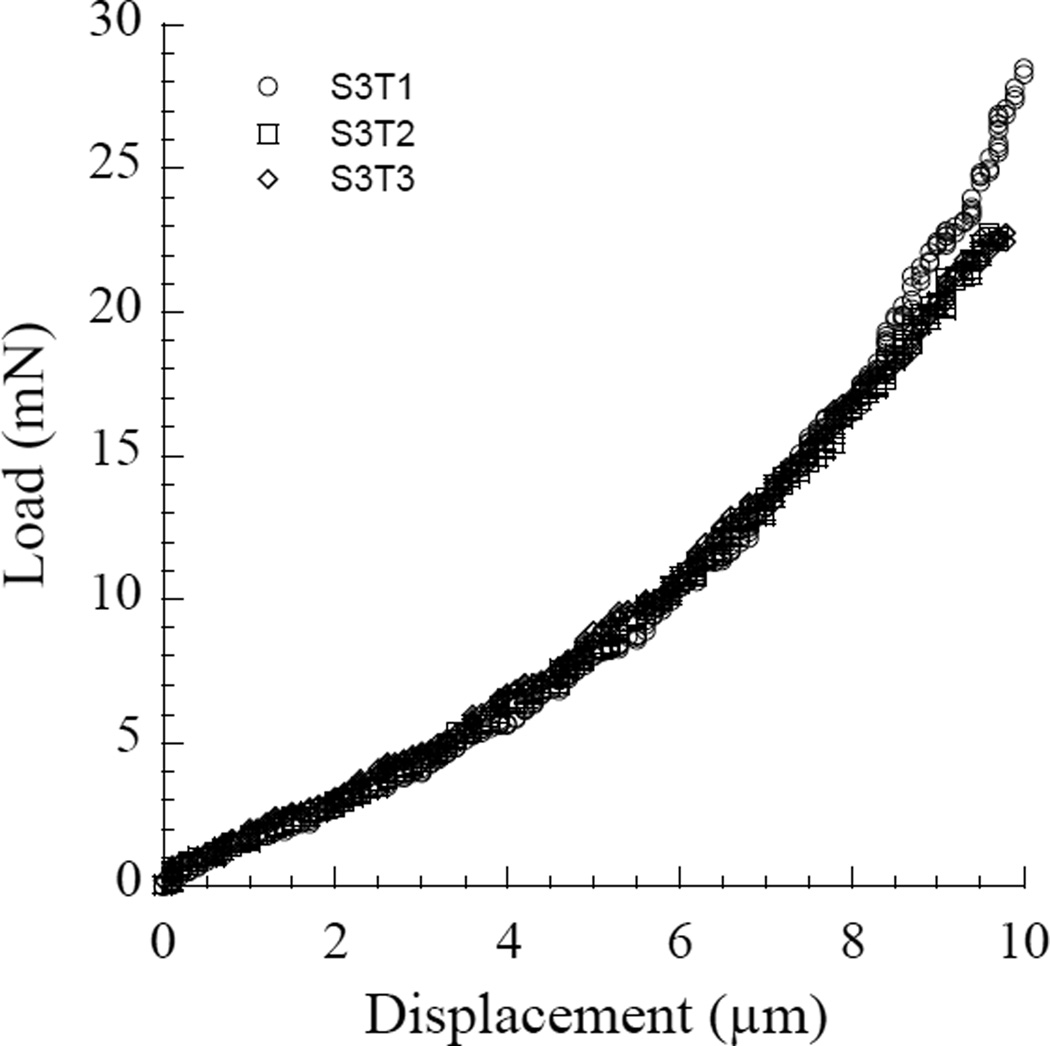
Figure A1 Comparison of indentation responses for a single etched sample of dentin in water. In this sequence of loading, three indentations were conducted with order S3T1, S3T2 and S3T3. All three were conducted at exactly the same location. Apart from the slightly greater peak stiffness in the first indentation made (S3T1), the stiffness throughout the majority of the displacement responses is strikingly consistent. The average elastic modulus estimated for these three measurements at 10% strain is 0.67±0.10 MPa.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scheffel DL, Hebling J, Scheffel RH, Agee K, Turco G, de Souza Costa CA, Pashley D. Inactivation of matrix-bound matrix metalloproteinases by cross-linking agents in acid-etched dentin. Oper Dent. 2014;39(2):152–158. doi: 10.2341/12-425-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yiu CKY, Pashley EL, Hirashi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26:6863–6872. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79(7):1458–1463. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 4.Hiraishi N, Yiu CK, King NM, Tay FR, Pashley DH. Chlorhexidine release and water sorption characteristics of chlorhexidine-incorporated hydrophobic/hydrophilic resins. Dent Mater. 2008;24(10):1391–1399. doi: 10.1016/j.dental.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29(1):116–135. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol ILS, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer: A review. Dent Mater. 2013;29:999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjäderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Mannello F, Tjäderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90(8):953–968. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maciel KT, Carvalho RM, Ringle RD, Preston CD, Russell CM, Pashley DH. The effects of acetone, ethanol, HEMA and air on the stiffness of human demineralized dentin. J Dent Res. 1996;75:1851–1858. doi: 10.1177/00220345960750110601. [DOI] [PubMed] [Google Scholar]
- 12.Pashley DH, Agee KA, Carvalho RM, Lee KW, Tay FR, Callison TE. Effects of water and water-free polar solvents on the tensile properties of demineralized dentin. Dent Mater. 2003;19(5):347–352. doi: 10.1016/s0109-5641(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 13.Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001;56(2):273–281. doi: 10.1002/1097-4636(200108)56:2<273::aid-jbm1095>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to wet-bonding to ethanol wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–21. [PubMed] [Google Scholar]
- 15.Pashley DH, Agee KA, Wataha JC, Rueggeberg FA, Ceballos L, Itou K, Yoshiyama M, Carvalho RM, Tay FR. Viscoelastic properties of demineralized dentin matrix. Dent Mater. 2003;19:700–706. doi: 10.1016/s0109-5641(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 16.Garcia FC, Otsuki M, Pashley DH, Tay FR, Carvalho RM. Effects of solvents on the early stage stiffening rate of demineralized dentin matrix. J Dent. 2005;33(5):371–377. doi: 10.1016/j.jdent.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Bedran-Russo AKB, Pashley DH, Agee KA, Drummond JL, Miescka KJ. Changes in the stiffness of demineralized dentin following application of collagen cross-linkiners. J Biomed Mater Res Appl Biomater. 2008;86B:330–334. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 18.Castellan CS, Pereira PN, Grande RH, Bedran-Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26(10):968–973. doi: 10.1016/j.dental.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedran-Russo AK, Yoo KJ, Ema KC, Pashley DH. Mechanical properties of tannic-acid-treated dentin matrix. J Dent Res. 2009;88(9):807–811. doi: 10.1177/0022034509342556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B Appl Biomater. 2007;80(1):268–272. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 21.Macedo GV, Yamauchi M, Bedran-Russo AK. Effects of chemical cross-linkers on caries-affected dentin bonding. J Dent Res. 2009;88(12):1096–1100. doi: 10.1177/0022034509351001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekambaram M, Yiu CK, Matinlinna JP. Effect of solvents on dentin collagen cross-linking potential of carbodiimide. J Adhes Dent. 2015;17(3):219–226. doi: 10.3290/j.jad.a34137. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Dusevich V, Wang Y. Addition of grape seed extract renders phosphoric acid a collagen-stabilizing etchant. J Dent Res. 2014;93(8):821–827. doi: 10.1177/0022034514538972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balooch M, Wu-Magidi I-C, Balazs A, Lundkvist AS, Marshall SJ, Marshall GW, Siekhaus WJ. Viscoelastic properties of demineralized human dentin measured in water with atomic force microscope (AFM)-based indentation. J Biomed Mater Res. 1998;40:539–544. doi: 10.1002/(sici)1097-4636(19980615)40:4<539::aid-jbm4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. J Struct Biol. 2008;162(3):404–410. doi: 10.1016/j.jsb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habelitz S, Balooch M, Marshall SJ, Balooch G, Marshall GW., Jr In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J Struct Biol. 2002;138(3):227–236. doi: 10.1016/s1047-8477(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Majd H, Weir MD, Arola DD, Xu HH. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent Mater. 2015;31(3):284–292. doi: 10.1016/j.dental.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenger MP, Bozec L, Horton MA, Mesquida P. Mechanical properties of collagen fibrils. Biophys J. 2007;93(4):1255–1263. doi: 10.1529/biophysj.106.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90(4):535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walton RE, Outhwaite WC, Pashley DH. Magnification – an interesting optical property of dentin. J Dent res. 1976;55:639–642. doi: 10.1177/00220345760550041601. [DOI] [PubMed] [Google Scholar]
- 31.Sneddon IN. The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int J Engr Sci. 1965;3(1):47–57. [Google Scholar]
- 32.Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5(5):541–551. doi: 10.1016/0021-9290(72)90010-3. [DOI] [PubMed] [Google Scholar]
- 33.Yang F. Asymptotic solution to axisymmetric indentation of a compressible elastic thin film. Thin Solid Films. 2006;515(4):2274–2283. [Google Scholar]
- 34.Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30(3):235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 35.Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Töyräs J, Jurvelin JS. Collagen network primarily controls Poisson's ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24(4):690–699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 36.Tezvergil-Mutluay A, Mutluay MM, Agee KA, Seseogulari-Dirihan R, Hoshika T, Cadenaro M, Breschi L, Vallittu P, Tay FR, Pashley DH. Carbodiimide cross-linking inactivates soluble and matrix-bound MMPs in vitro. J Dent Res. 2012;91:192–196. doi: 10.1177/0022034511427705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korhonen RK, Laasanen MS, Töyräs J, Rieppo J, Hirvonen J, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35(7):903–909. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 38.Chahine NO, Wang CC, Hung CT, Ateshian GA. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech. 2004;37(8):1251–1261. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- 40.Kinney JH, Marshall SJ, Marshal GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14:13–29. doi: 10.1177/154411130301400103. [DOI] [PubMed] [Google Scholar]
- 41.Giannini M, Carvalho RM, Martins LR, Dias CT, Pashley DH. The influence of tubule density and area of solid dentin on bond strength of two adhesive systems to dentin. J Adhes Dent. 2001;3(4):315–324. [PubMed] [Google Scholar]
- 42.Prati C, Chersoni S, Mongiorgi R, Montanari G, Pashley DH. Thickness and morphology of resin-infiltrated dentin layer in young, old, and sclerotic dentin. Oper Dent. 1999;24(2):66–72. [PubMed] [Google Scholar]
- 43.Pashley DH, Ciucchi B, Sano H, Horner JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–631. [PubMed] [Google Scholar]
- 44.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjäderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res Part B: Appl Biomater. 2009;90B:373–380. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8 and 9 by chlorhexidine. Clin Diag Lab Immun. 1999;6:437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]