Abstract
Lower body negative pressure (LBNP) is often used to simulate blood loss in humans. It is unknown if cerebral blood flow responses to actual blood loss are analogous to simulated blood loss during LBNP. Nine healthy men were studied at baseline, during three levels of LBNP (5 min at −15, −30, and −45 mmHg), and during three levels of blood loss (333, 667, and 1,000 ml). LBNP and blood loss conditions were randomized. Intra-arterial mean arterial pressure (MAP) during LBNP was similar to that during blood loss (P ≥ 0.42). Central venous pressure (2.8 ± 0.7 vs. 4.0 ± 0.8, 1.2 ± 0.6 vs. 3.5 ± 0.8, and 0.2 ± 0.9 vs. 2.1 ± 0.9 mmHg for levels 1, 2, and 3, respectively, P ≤ 0.003) and stroke volume (71 ± 4 vs. 80 ± 3, 60 ± 3 vs. 74 ± 3, and 51 ± 2 vs. 68 ± 4 ml for levels 1, 2, and 3, respectively, P ≤ 0.002) were lower during LBNP than blood loss. Despite differences in central venous pressure, middle cerebral artery velocity (MCAv) and cerebrovascular conductance were similar between LBNP and blood loss at each level (MCAv at level 3: 62 ± 6 vs. 66 ± 5 cm/s, P = 0.37; cerebrovascular conductance at level 3: 0.72 ± 0.05 vs. 0.73 ± 0.05 cm·s−1·mmHg−1, P = 0.53). While the slope of the MAP-MCAv relationship was slightly different between LBNP and blood loss (0.41 ± 0.03 and 0.66 ± 0.04 cm·s−1·mmHg−1, respectively, P = 0.05), time domain gain between MAP and MCAv at maximal LBNP/blood loss (P = 0.23) and low-frequency MAP-mean MCAv transfer function coherence, gain, and phase were similar (P ≥ 0.10). Our results suggest that cerebral hemodynamic responses to LBNP to −45 mmHg and blood loss up to 1,000 ml follow a similar trajectory, and the arterial pressure-cerebral blood velocity relationship is not altered from baseline under these conditions.
Keywords: simulated hemorrhage, cerebrovascular, hypovolemia
hemorrhage accounts for approximately one-third of all trauma-related deaths (28) and 80% of potentially survivable battlefield injuries (15). As logistical and ethical constraints have often limited comprehensive assessment of the physiological responses to hemorrhage in humans, studies investigating the early detection and prevention of blood loss in humans have often used lower body negative pressure (LBNP) to simulate the hemodynamic effects of actual blood loss. LBNP elicits progressive reductions in central blood volume, reflected by decreases in central venous pressure (CVP), stroke volume (SV), and cardiac output (CO), eliciting baroreflex-mediated increases in heart rate (HR) and total vascular resistance and the release of vasoactive and volume-regulating hormones (11, 13, 18, 26, 35, 46, 47, 53). As reviewed by Cooke et al. in 2004, many of these hemodynamic adjustments associated with LBNP are similar to those induced by hemorrhage (13). While many studies have assessed the effects of blood loss on hemodynamic responses in humans, such as arterial pressure, HR, SV, sympathetic nerve activity, and peripheral resistance (1, 2, 19, 39, 45, 50), few have investigated cerebral blood flow responses (7, 48). Inadequate cerebral blood flow and oxygenation, the final common pathway to loss of consciousness from blood loss, represent an important area of investigation. Two studies (7, 48) have demonstrated progressive reductions in cerebral oxygenation assessed via near-infrared spectroscopy (NIRS) following withdrawal of ≤500 ml of blood. No studies, to our knowledge, have investigated cerebral blood flow (or velocity) responses to hemorrhage of any magnitude in humans or whether the effects of actual blood loss on cerebral blood flow regulation are analogous to simulated blood loss during LBNP.
A direct comparison of the physiological responses to LBNP and blood loss has been performed in a baboon model (21), and from these data, the estimated loss of blood in humans was calculated; approximately −70 mmHg LBNP equated to blood loss of 17.8 ml/kg, or ∼0.25 ml·kg−1·mmHg LBNP−1. This study provided the basis for our work comparing simulated hemorrhage using LBNP with actual blood loss in adult men (25). While reductions in CVP and SV elicited by 1,000 ml of blood loss were smaller than those elicited by −45 mmHg LBNP, the CVP, SV, HR, and mean arterial pressure (MAP) responses to LBNP and blood loss were linearly related (25). Importantly, this suggests that the hemodynamic responses to central hypovolemia associated with LBNP are similar to those associated with blood loss in adult men.
During progressive central hypovolemia using LBNP, middle cerebral artery (MCA) velocity (MCAv) is initially maintained and then decreases progressively until the onset of presyncope (3, 29, 41). As the inability to maintain adequate cerebral blood flow and oxygenation can determine tolerance to central hypovolemia (7, 29), the purpose of the present study was to compare the effects of actual graded blood loss with the effects of simulated hemorrhage using progressive LBNP on cerebral blood flow (velocity) regulation in humans.
METHODS
Subjects
Nine healthy men (age 31 ± 6 yr, height 183 ± 7 cm, weight 89 ± 9 kg, body mass index 26.7 ± 1.8 kg/m2) were recruited for the study. These subjects were a subset of the 12 subjects who participated in another study focused on hemodynamic and hormonal responses to this protocol (25). All subjects reported that they were free of cardiovascular, respiratory, neurological, or metabolic disease. Subjects were nonobese (body mass index <30 kg/m2), were nonsmokers, and were not taking medication. Prior to the study day, all subjects provided written informed consent after all procedures and risks of the study were fully explained; the study was approved by the Mayo Clinic Institutional Review Board. Subjects reported to the Clinical Research Unit at the Mayo Clinic at 0700 following an overnight fast. At this time, each subject consumed a small breakfast bar (Clif Bar, Shelton, CT; 240 kcal) and drank 250 ml of water. Subjects were studied in the supine position in a temperature-controlled (20–22°C) room. To ensure subject safety, a board-certified anesthesiologist was present throughout the study day, and a member of the Mayo Clinic autologous transfusion team was in attendance during the protocol.
Experimental Design
LBNP and blood loss protocols were performed on the same day in a counterbalanced order. Figure 1 illustrates the study protocol. The goal of the experimental design was to elicit a wide range of CVP in both protocols. On the basis of approximations for comparing LBNP levels with blood loss (13), we chose the initial stages of the US Army Institute for Surgical Research LBNP protocol (−15, −30, and −45 mmHg chamber pressure) and stepwise reductions in blood volume that would closely mirror CVP at each stage (three 333-ml aliquots of blood). Because the order of the protocols was mixed, we were unable to closely match CVP values between LBNP and blood loss, as described by Hinojosa-Laborde et al. in their baboon study, where LBNP always followed blood loss (21). Either protocol was terminated early if 1) MAP fell by 30% compared with baseline MAP, 2) systolic blood pressure dropped below 80 mmHg, or 3) the subject began to experience symptoms of presyncope or syncope. Hematocrit was measured from arterial blood samples collected during the baseline period and at the termination of each experimental protocol.
Fig. 1.
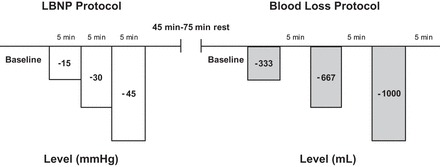
Study protocol. Lower body negative pressure (LBNP) and blood loss conditions were counterbalanced. Duration of the rest period between LBNP and blood loss depended on which protocol was performed first, with more time required after the blood loss protocol.
Measurements and Procedures
Hemodynamic monitoring.
Subjects were positioned in the supine posture on an adjustable bed. A three-lead electrocardiogram was used to continuously record HR (Cardiocap/5, Datex-Ohmeda, Louisville, CO). Arterial oxygen saturation was monitored using a finger pulse oximeter, and end-tidal CO2 (ETCO2) was collected from a nasal cannula (Cardiocap/5). A 20-gauge, 5-cm catheter was placed into the brachial artery under local anesthesia (2% lidocaine) using aseptic techniques and ultrasound guidance. The catheter was attached to a high-resolution transducer positioned at heart level to obtain continuous brachial arterial pressure waveforms. Continuous hemodynamic, oxygen saturation, and ETCO2 traces were interfaced with a data acquisition system for offline analysis (WinDaq, DATAQ Instruments, Akron, OH).
Cerebral blood velocity.
Subjects were imaged using a 2-MHz transcranial Doppler (TCD) probe (Neurovision System, Multigon, Yonkers, NY) to estimate MCAv. The basal portion of the left MCA was insonated by placement of the probe over the temporal bone just above the zygomatic arch in front of the ear. The Doppler signal was optimized by variation of the sample volume depth in incremental steps and variation of the angle of insonation to obtain the best-quality signal. Once the optimal signal was determined, the probe was secured with a headband device to maintain a constant angle throughout the protocol.
Central venous pressure.
A 16-gauge central catheter was introduced into an antecubital vein under local anesthesia (2% lidocaine) using aseptic techniques and advanced to the superior vena cava prior to its junction with the right atrium. This catheter was connected to a high-resolution transducer (FloTrac, Edwards Lifesciences, Irvine, CA) positioned at heart level and interfaced with a personal computer for continuous measurement of CVP. Correct placement of the peripherally inserted central catheter was visually confirmed by two anesthesiologists using the CVP waveform.
Blood removal.
A 14-gauge catheter was placed in an antecubital vein to facilitate blood removal for the blood loss protocol. The catheter was placed under local anesthesia (2% lidocaine) using aseptic techniques. Bags containing preservative/anticoagulant (63 ml of anticoagulant citrate phosphate dextrose solution) were placed below the level of the bed to allow transfer of blood from the subject to the blood collection bags via gravity. In two subjects, a blood pressure cuff was inflated around the upper arm to 40 mmHg to enhance the rate of blood removal; this cuff pressure was released during all subsequent hemodynamic measurements. As blood was being collected, it was weighed to determine the volume of blood removed by multiplying the weight of the blood by a factor of 1.06 ml/g. The removed blood was kept in the study room (20–22°C), the temperature of the blood was allowed to fluctuate, and the collection bags were periodically agitated to prevent clotting.
Blood loss protocol.
After a 5-min baseline period, three 333-ml aliquots of blood were removed as described above. Measurements were made for 5 min after each aliquot. Subjects were not allowed to cross their legs and were instructed to refrain from contracting lower body muscles throughout the protocol. At the end of the protocol, all shed blood was reinfused at a rate of 20 ml/min into the antecubital vein. Subjects rested quietly in the supine position for 45–75 min between protocols.
LBNP protocol.
Subjects were supine in an airtight LBNP chamber that was sealed at the iliac crest and covered the lower body. The LBNP protocol was based on the first three stages of a commonly used protocol (8–10, 20, 41, 42) consisting of a 5-min baseline period followed by 5 min at −15, −30, and −45 mmHg of chamber decompression. Subjects were not allowed to cross their legs and were instructed to refrain from contracting lower body muscles throughout the protocol.
Data and Statistical Analysis
Data were collected at 500 Hz (WinDaq) and stored on a laboratory computer for offline analysis with signal-processing software (WinCPRS, Absolute Aliens, Turku, Finland). All variables of interest (HR, blood pressure, CVP, ETCO2, and MCAv) were continuously monitored throughout both protocols, and data were analyzed and averaged over the last 3 min of each stage for statistical analysis. MAP and mean MCAv were calculated as the area under the arterial pressure and MCAv curves. SV was calculated using specialized analysis software (WinCPRS) based on the brachial arterial pressure waveform (23). CO was derived using the calculated SV and HR obtained by electrocardiogram. The HR, MAP, SV, CO, and CVP responses for 12 subjects are presented elsewhere (25). Cerebrovascular conductance (CVC) was calculated as MCAv/MAP. The gain between changes in mean MCAv and MAP was calculated to assess arterial pressure-cerebral blood velocity relationships in the time domain at the maximal level of LBNP/blood loss for each subject.
Arterial pressure-cerebral blood velocity relationships were also explored via transfer function analysis. Beat-to-beat time domain MAP and mean MCAv waveforms were processed with a fast Fourier transform. Data were made equidistant by linear interpolation and resampled at 5 Hz. Data were then passed through a low-pass filter with a cutoff frequency of 0.5 Hz. Three-minute data sets were fast Fourier-transformed with a Hanning window to obtain power spectra. Spectral power was expressed as the integrated area within the very-low-frequency (VLF) range of 0.004–0.04 Hz and low-frequency (LF) range of 0.04–0.15 Hz. We calculated the coherence between MAP and mean MCAv by dividing the squared cross-spectral densities of the two signals by the product of the individual autospectra. Transfer function gain and phase between MAP and mean MCAv represent a frequency dependence and can be used to assess dynamic cerebral blood flow-pressure relationships (17, 54). Transfer function gain and phase were considered valid and averaged in the VLF and LF ranges only when coherence values were ≥0.5.
To explore the relationships between the physiological responses from the two protocols, the amalgamated r2 value was calculated using linear regression analysis for each variable of interest (SV and CVP) for blood loss vs. LBNP, as described by Johnson et al. (25). Linear mixed-effect model analysis with repeated measures was used to assess the mean MCAv-MAP relationship across LBNP and blood loss for all subjects; ETCO2 was also included as a covariate due to the independent effects of arterial CO2 on mean MCAv and MAP. Condition × stage (2 × 4) repeated-measures ANOVAs were used to determine if values obtained during the LBNP protocol were similar to those at the corresponding stages of the blood loss protocol. A one-way repeated-measures ANOVA was used to compare the time of blood withdrawal across the three aliquots. If a significant main or interaction effect was detected, Tukey's post hoc analyses were performed to determine where differences existed. Paired t-tests were used to compare hematocrit responses within the LBNP or hemorrhage protocols and maximal MAP-mean MCAv gain responses between conditions. Group data are presented as means ± SE, unless otherwise stated. Exact P values are reported.
RESULTS
All nine subjects performed both trials. Due to presyncopal symptoms, one subject did not complete the last level of LBNP, one subject did not complete the last level of blood loss, and one subject did not complete the last level of either trial. The mean time for blood removal was 563 ± 49 s for the first 333-ml aliquot, 489 ± 56 s for the second 333-ml aliquot, and 467 ± 73 s for the final 333-ml aliquot (P = 0.195). Hematocrit increased with LBNP (from 40.6 ± 0.9% at baseline to 41.9 ± 0.9% at termination, P = 0.020) and decreased with hemorrhage (from 40.8 ± 0.9% at baseline to 39.7 ± 0.9% at termination, P = 0.001). Hemodynamic responses are shown in Table 1. MAP decreased between baseline and level 3 only during the LBNP trial (P = 0.001). There were no differences in MAP between the LBNP and blood loss trials at any level (P ≥ 0.42). At each level, CVP decreased below baseline in both LBNP and blood loss protocols, but values were consistently higher during blood loss than LBNP (P ≤ 0.003). During the LBNP trial, SV and CO were lower than baseline at every level, but for the blood loss trial SV was reduced during levels 2 and 3 only and CO did not decrease below baseline values. Consistent with the CVP responses, SV and CO were higher during the blood loss than the LBNP trial at each level of the protocol except baseline. HR was higher than baseline for levels 2 and 3 of LBNP and during level 3 of blood loss; in response to the greater reduction in central blood volume, HR was higher during levels 2 and 3 of the LBNP trial compared with the blood loss trial. The CVP and SV responses during LBNP and blood loss trials are presented in Fig. 2; both amalgamated r2 values were ≥0.80, but the slopes were <0.6, reflecting the differences in central blood volume reduction between conditions.
Table 1.
Physiological responses to LBNP and blood loss
| Hypovolemic Stress |
||||
|---|---|---|---|---|
| Baseline | Level 1 | Level 2 | Level 3 | |
| LBNP, mmHg | −15 | −30 | −45 | |
| Blood loss, ml | −333 | −667 | −1,000 | |
| MAP, mmHg | ||||
| LBNP | 94 ± 3 | 91 ± 3 | 87 ± 5 | 86 ± 4† |
| Blood loss | 93 ± 3 | 92 ± 2 | 90 ± 3 | 91 ± 3 |
| CVP, mmHg | ||||
| LBNP | 7.4 ± 0.9 | 2.8 ± 0.7† | 1.2 ± 0.6† | 0.2 ± 0.9† |
| Blood loss | 6.5 ± 0.8 | 4.0 ± 0.8*† | 3.5 ± 0.8*† | 2.1 ± 0.9*† |
| SV, ml | ||||
| LBNP | 81 ± 4 | 71 ± 4† | 60 ± 3† | 51 ± 2† |
| Blood loss | 85 ± 5 | 80 ± 3* | 74 ± 3*† | 68 ± 4*† |
| HR, beats/min | ||||
| LBNP | 57 ± 3 | 60 ± 2 | 67 ± 3† | 76 ± 4† |
| Blood loss | 57 ± 3 | 58 ± 2 | 61 ± 2* | 65 ± 3*† |
| CO, l/min | ||||
| LBNP | 4.6 ± 0.3 | 4.2 ± 0.2† | 3.9 ± 0.2† | 3.8 ± 0.2† |
| Blood loss | 4.8 ± 0.3 | 4.7 ± 0.3* | 4.5 ± 0.2* | 4.4 ± 0.3* |
| Mean MCAv, cm/s | ||||
| LBNP | 70.0 ± 4.2 | 69.3 ± 4.3 | 65.2 ± 4.3 | 61.5 ± 5.8† |
| Blood loss | 69.5 ± 5.1 | 69.6 ± 5.3 | 67.7 ± 5.0 | 66.5 ± 5.2 |
| CVC, cm·s−1·mmHg−1 | ||||
| LBNP | 0.75 ± 0.04 | 0.77 ± 0.05 | 0.76 ± 0.05 | 0.72 ± 0.05 |
| Blood loss | 0.75 ± 0.04 | 0.75 ± 0.05 | 0.75 ± 0.04 | 0.73 ± 0.05 |
| ETCO2, mmHg | ||||
| LBNP | 40 ± 2 | 40 ± 2 | 39 ± 2 | 38 ± 3 |
| Blood loss | 41 ± 2 | 40 ± 2 | 39 ± 2 | 38 ± 3† |
| Respiration rate, n | ||||
| LBNP | 15 ± 1 | 13 ± 1† | 13 ± 1† | 14 ± 1† |
| Blood loss | 13 ± 1* | 13 ± 1 | 13 ± 1 | 12 ± 1 |
Values are means ± SE. Data are calculated from the final 3-min of each level. LBNP, lower body negative pressure; MAP, mean arterial pressure; CVP, central venous pressure; SV, stroke volume; HR, heart rate; CO, cardiac output; MCAv, middle cerebral artery velocity; CVC, cerebral vascular conductance; ETCO2, end-tidal CO2.
P < 0.05 vs. LBNP at the same level.
P < 0.05 vs. baseline of the same protocol.
Fig. 2.
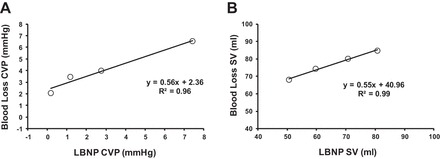
Linear regression for amalgamated values for central venous pressure (CVP; A) and stroke volume (SV; B) responses between LBNP and blood loss conditions.
Cerebral blood velocity and CVC responses to LBNP and blood loss are shown in Table 1. Mean MCAv decreased by 11 ± 3% and 3 ± 4% for the LBNP and blood loss protocols (P = 0.44) but was statistically distinguishable from baseline at the final level of the LBNP protocol only (P = 0.002). CVC did not change, and responses were similar between LBNP and blood loss trials (P ≥ 0.47). ETCO2 decreased at level 3 for the blood loss trial only, and respiration rate decreased for the LBNP trial only.
Individual mean MCAv and MAP responses are presented in Fig. 3. There was intersubject variability in these responses, and as a group, the slope of the line between MCAv and MAP was lower with LBNP than blood loss (0.41 ± 0.03 vs. 0.66 ± 0.04 cm·s−1·mmHg−1, P = 0.05). The time domain gain between maximal changes in mean MCAv and MAP was similar between LBNP and blood loss (1.2 ± 0.2 vs. 4.3 ± 2.4 cm·s−1·mmHg−1, P = 0.23). LF and VLF power spectral density for MAP and mean MCAv are shown in Fig. 4. There were no differences from baseline (P ≥ 0.13) in power spectral density for MAP LF and VLF or for MCAv LF and VLF in either trial or in these responses between the LBNP and blood loss conditions (P ≥ 0.23). Similarly, there was no effect of condition or level for MAP-MCAv LF coherence, gain, or phase (P ≥ 0.10; Fig. 5). Because VLF coherence was consistently <0.5 for both conditions across all levels, phase and gain are not reported.
Fig. 3.
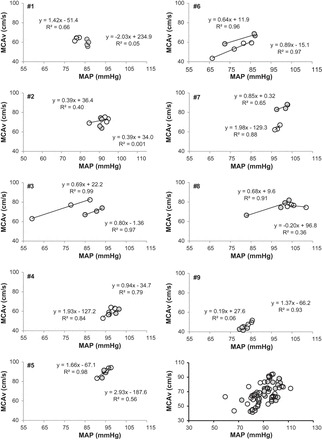
Individual plots of mean arterial pressure (MAP) vs. mean middle cerebral artery velocity (MCAv) for all 9 subjects for LBNP (white circles) and blood loss (gray circles). Group responses are presented at bottom right (n = 9).
Fig. 4.
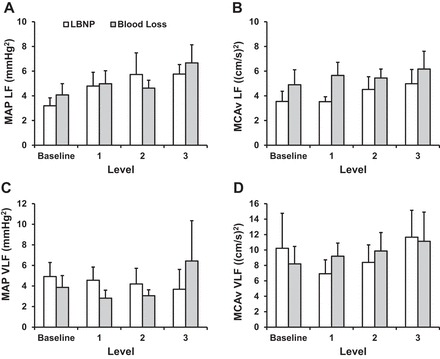
Low-frequency (LF) and very-low-frequency (VLF) power spectral density for MAP (A and C) and mean MCAv (B and D) during LBNP and blood loss. Values are means ± SE.
Fig. 5.
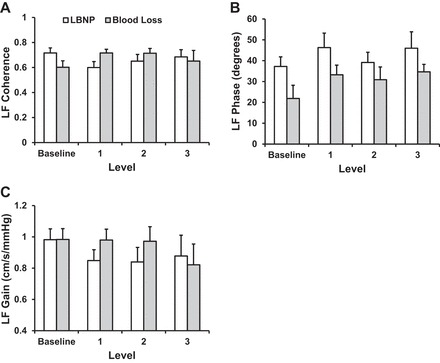
LF coherence, phase, and gain between MAP and mean MCAv during LBNP and blood loss. Values are means ± SE.
DISCUSSION
This is the first study to systematically compare cerebral blood velocity responses during LBNP and actual hemorrhage in healthy human subjects. The key findings from this investigation are that 1) LBNP up to −45 mmHg elicited greater reductions in central blood volume than hemorrhage up to ∼1,000 ml (as indicated by comparisons of SV, CO, and CVP); 2) the subsequent cerebral blood velocity responses reflected these differences in central blood volume, but the trajectories of the cerebral blood velocity and CVC responses were similar during LBNP and blood loss; and 3) neither the LBNP nor the blood loss protocol induced changes in the MAP-mean MCAv relationship as determined by gain calculations in the time domain and via transfer function analysis.
In 1940, Ebert and Stead (16) reported the sequestration of ∼15% of total blood volume into the extremities (2 legs and 1 arm) following rapid application of tourniquets as a potential alternative to phlebotomy for treatment of congestive heart failure. Over 20 years later, a number of investigators introduced LBNP as a method to further decrease central blood volume to simulate the cardiovascular effects of hemorrhage and orthostasis (6, 46). Direct comparison of the hemodynamic responses to LBNP and removal of 450 ml of blood (i.e., 1 unit) from human volunteers suggested equivalency between 1 unit of blood loss and −10 to −20 mmHg LBNP determined by reductions in CVP (39) and SV (19) and subsequent reflex increases in sympathetic nerve activity (39). Recently, studies comparing the cardiovascular and neurohumoral responses to LBNP and blood loss >1 unit (i.e., >500 ml) were performed in baboons (21) and humans (25). Based on the results reported by Hinojosa-Laborde et al. (21), LBNP elicits a reduction in central blood volume (indexed by SV) of ∼0.25 ml·kg−1·mmHg LBNP−1, equating to blood loss of ∼450, 1,000, and 1,600 ml with LBNP of −30, −60, and −90 mmHg in a 70-kg human.
While protection of cerebral perfusion and oxygenation is essential for maintaining consciousness under hypotensive conditions of actual or simulated hemorrhage, few studies have measured these responses to actual blood loss, and none have compared responses between blood loss and LBNP. In studies assessing cerebral oxygen saturation responses (via NIRS) to blood loss of ≤500 ml, Colier et al. (7) and Torella et al. (48) reported increases in deoxyhemoglobin concentration and decreases in oxyhemoglobin concentration and cerebral oxygen saturation. As NIRS measures a sample volume consisting of ∼25% arterial and 75% venous blood (33, 38), decreases in oxyhemoglobin and increases in deoxyhemoglobin suggest an increase in oxygen extraction, most likely to compensate for reduced blood flow supplying the cerebral tissues; measures of cerebral blood flow (or velocity), however, were not reported in either of these investigations. The current study is the first, to our knowledge, to report cerebral blood velocity responses to actual hemorrhage (up to ∼1,000 ml) in humans and to compare these responses with LBNP. As reported for a larger group of subjects (n = 12) (25), greater reductions in central blood volume are elicited by LBNP to −45 mmHg than by 1,000 ml of blood loss. As a consequence, mean MCAv was reduced by ∼11% with LBNP compared with ∼3% with blood loss, MAP decreased by ∼8% with LBNP and ∼2% with blood loss, and the gain between mean MCAv and MAP was lower for LBNP compared with blood loss (Fig. 3). We speculate that continued blood loss would eventually elicit similar cerebral blood velocity responses between conditions. Based on the cerebral blood velocity data presented in Table 1 and Fig. 3 and the hemodynamic data presented by Johnson et al. (25), blood loss of 1,000 ml implemented in the present protocol appears to be equivalent to LBNP of −15 to −30 mmHg. This is in contrast to estimations using SV responses from baboons exposed to both LBNP and hemorrhage (0.25 ml·kg−1·mmHg−1) (21), where −45 mmHg LBNP would be equivalent to 1,000 ml of blood loss in the subjects used in the present investigation (i.e., ∼90 kg body wt). Prospective matching of both CVP responses and the time course of blood withdrawal and LBNP exposure between the two protocols, as described by Hinojosa-Laborde et al. (21), may address these differences in central hypovolemia observed in the current investigation and allow more accurate calculations of equivalency.
LF oscillatory power for both MAP and mean MCAv did not change from baseline during LBNP or blood loss. The stability of MAP LF was unexpected based on previously observed increases in MAP LF with LBNP of similar magnitude and duration (4, 5, 41, 55). LF oscillations in arterial pressure are primarily modulated by the baroreflex, evidenced by a strong association with LF power in muscle sympathetic nerve activity (MSNA), which in turn, is related to higher absolute MSNA (12, 27). As such, baroreflex-mediated sympathoexcitation with LBNP-induced hypotension increases MSNA and LF power in both MSNA and arterial pressure (12). The very mild reductions in MAP (−8 and −2 mmHg) by the final level of LBNP and blood loss in the current study may not have been sufficient to elicit increases in MSNA; hence, there was no increase in MSNA LF or, subsequently, MAP LF. This speculation is supported, in part, by an increase in circulating norepinephrine with LBNP, but not with blood loss, as reported by Johnson et al. (25). The small subject number, combined with high intersubject variability in MAP LF responses under both protocols, also contributes to this finding. As oscillations in arterial pressure are the primary factor driving increased MCAv oscillations, it is not surprising that MCAv LF power did not change under either protocol.
Assessment of the arterial pressure-cerebral blood velocity relationship via transfer function analysis in the VLF and LF ranges has been interpreted as an index of cerebral autoregulation (54). The low coherence between MAP and mean MCAv in the VLF range (<0.5) across time and condition indicates an independence of flow from pressure within this frequency range (54). While coherence between MAP and mean MCAv was consistently >0.5 in the LF range, transfer function gain and phase did not change with LBNP or blood loss and were not different between conditions. In contrast, other studies show a reduction (41) or an increase (55) in MAP-mean MCAv gain during LBNP of similar magnitude. In particular, Zhang et al. (55) suggested that simultaneous increases in the magnitude of oscillations in arterial pressure and cerebral blood velocity and the subsequent increase in MAP-mean MCAv gain represented attenuated cerebral autoregulation that may, in turn, predispose individuals to presyncope. The stability of MAP-mean MCAv gain and phase reported in the current investigation is most likely associated with the stability of MAP and mean MCAv LF oscillations and the high intersubject variability inherent in transfer function estimates of cerebral pressure-flow relationships, further compounded by the small sample size utilized in this study. In the time domain, cerebral autoregulation can also be assessed as the gain between changes in arterial pressure and cerebral blood velocity (36, 40); in the present study this relationship was not altered under either condition and was not statistically distinguishable between conditions. Together, these data suggest that cerebral pressure-flow relationships across multiple time scales (fast component via transfer function analysis and slow component via time domain analysis) were not affected by the magnitude of central hypovolemia induced by LBNP or blood loss. Other factors, including small reductions in arterial CO2 and increased sympathetic drive, may also contribute to the small decrease in MCAv with LBNP and blood loss.
Methodological Considerations
Many of the key methodological considerations associated with the design of this study have been addressed by Johnson et al. (25), including removal of absolute blood volumes (i.e., 333, 666, and 1,000 ml) rather than a percentage of total blood volume, the inability to match CVP responses due to the random order of the protocols, restricting exposure to LBNP and blood loss to submaximal levels, differences in the time course of blood removal vs. LBNP exposure, and inclusion of only male subjects. There are some additional issues specific to the data included in this study that should be considered.
As we used TCD for assessment of cerebral blood velocity within the MCA, we assume that the measurement of velocity is equivalent to flow as long as the caliber of the MCA does not change over the course of the intervention. While recent studies have indicated changes in MCA cross-sectional area with both increases (ETCO2 ≥9 mmHg above baseline) and decreases (ETCO2 ≥13 mmHg below baseline) in arterial CO2 (14, 49), the magnitude of hypocapnia induced with both LBNP and blood loss in the current investigation (2–3 mmHg below baseline) was well below these levels. Additionally, sympathoexcitation with the hypotensive stimuli of both LBNP and blood loss could result in cerebral vasoconstriction, which may also invalidate the assumption of constant arterial diameter. MCA diameter is constant, however, with LBNP up to −40 mmHg (44), and the mild hypotensive stimulus elicited with both LBNP and blood loss in the current study renders this limitation unlikely. Future assessment of cerebral blood flow of the extracranial feeding arteries (e.g., internal carotid artery and vertebral artery) (22, 37, 43, 52) and/or use of transcranial color-coded Doppler ultrasound (34, 51) during this type of investigation would allow for direct assessment of cerebral blood flow without reliance on the assumption of constant arterial caliber. Furthermore, recent investigations have revealed potential regional differences in cerebral blood flow regulation, where the posterior circulation may be more sensitive to hypotension and hypocapnia than the anterior circulation (31), indicating inclusion of these measurements in future studies.
While maintenance of cerebral blood flow is crucial for the delivery of oxygen to the cerebral tissues, the ability of the brain to extract and utilize this oxygen may be of greater importance. This issue has been highlighted by a number of studies demonstrating that protection of absolute cerebral blood flow (or velocity) does not necessarily provide insight into tolerance to central hypovolemia (24, 30, 32, 41). Cerebral oxygenation, oxygen extraction, and/or cerebral oxygen metabolism measurements would be valuable additions to comparisons of LBNP and hemorrhage to address this important issue.
Conclusion
The findings from the present investigation indicate that cerebral blood velocity responses to central hypovolemia induced by LBNP to −45 mmHg and actual blood loss up to 1,000 ml follow a similar trajectory, and the arterial pressure-cerebral blood velocity relationship is not altered under these conditions. Careful matching of the magnitude of central hypovolemia (e.g., via CVP) and the time course of blood loss vs. LBNP exposure and inclusion of additional cerebral blood flow and oxygenation measurements in future studies will facilitate a more comprehensive understanding of these responses. This study represents an important step in understanding cerebral blood flow responses to hemorrhage and provides evidence for the continued use of LBNP as a model of hemorrhage in healthy, conscious volunteer subjects.
GRANTS
This work was funded by US Army Medical Research and Materiel Command Combat Casualty Care Research Program Grants W81XWH-11-1-0823 (M. J. Joyner) and W81XWH-11-2-0137 (C. A. Rickards), National Institute on Aging Grant AG-038067 (J. N. Barnes), and American Heart Association Midwest Affiliate Grants 13POST-14380027 (B. D. Johnson) and 14PRE-18040000 (R. E. Harvey). This publication was made possible by National Center for Advancing Translational Sciences Clinical and Translational Science Award UL1 TR-000135 and the US Army.
DISCLAIMERS
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official Department of the Army position, or decision, unless so designated by other official documentation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health, the Department of the Army, or the Department of Defense.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.A.R., B.D.J., V.A.C., M.J.J., and J.N.B. developed the concept and designed the research; C.A.R., B.D.J., and J.N.B. analyzed the data; C.A.R., B.D.J., R.E.H., V.A.C., M.J.J., and J.N.B. interpreted the results of the experiments; C.A.R. and J.N.B. prepared the figures; C.A.R. and J.N.B. drafted the manuscript; C.A.R., B.D.J., R.E.H., V.A.C., M.J.J., and J.N.B. edited and revised the manuscript; C.A.R., B.D.J., R.E.H., V.A.C., M.J.J., and J.N.B. approved the final version of the manuscript; B.D.J., R.E.H., M.J.J., and J.N.B. performed the experiments.
ACKNOWLEDGMENTS
The authors thank Shelly Roberts, Sarah Wolhart, Timothy Curry, John Eisenach, Christopher Johnson, Pamela Engrav, Branton Walker, Jennifer Taylor, and Luke Matzek for continued assistance throughout the project and Dr. Yu Chieh Tzeng for valuable advice on the linear mixed-effect models analysis.
REFERENCES
- 1.Barcroft H, Edholm OG, McMichael J, Sharpey-Schafer EP. Posthaemorrhagic fainting study by cardiac output and forearm flow. Lancet 1: 489–491, 1944. [Google Scholar]
- 2.Bassin R, Vladeck BC, Kark AE, Shoemaker WC. Rapid and slow hemorrhage in man. I Sequential hemodynamic responses. Ann Surg 173: 325–330, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondar RL, Kassam MS, Stein F, Dunphy PT, Fortney S, Riedesel ML. Simultaneous cerebrovascular and cardiovascular responses during presyncope. Stroke 26: 1794–1800, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Brown CM, Dutsch M, Hecht MJ, Neundorfer B, Hilz MJ. Assessment of cerebrovascular and cardiovascular responses to lower body negative pressure as a test of cerebral autoregulation. J Neurol Sci 208: 71–78, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brown CM, Dutsch M, Ohring S, Neundorfer B, Hilz MJ. Cerebral autoregulation is compromised during simulated fluctuations in gravitational stress. Eur J Appl Physiol 91: 279–286, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Brown E, Goei JS, Greenfield AD, Plassaras GC. Circulatory responses to simulated gravitational shifts of blood in man induced by exposure of the body below the iliac crests to sub-atmospheric pressure. J Physiol 183: 607–627, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colier WN, Binkhorst RA, Hopman MT, Oeseburg B. Cerebral and circulatory haemodynamics before vasovagal syncope induced by orthostatic stress. Clin Physiol 17: 83–94, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Convertino VA, Grudic G, Mulligan J, Moulton S. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J Appl Physiol 115: 1196–1202, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Convertino VA, Rickards CA, Lurie KG, Ryan KL. Hyperventilation in response to progressive reduction in central blood volume to near syncope. Aviat Space Environ Med 80: 1012–1017, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Convertino VA, Ryan KL, Rickards CA, Cooke WH, Metzger A, Holcomb JB, Adams BD, Lurie KG. Inspiratory resistance maintains arterial pressure during central hypovolemia: implications for treatment of patients with severe hemorrhage. Crit Care Med 35: 1145–1152, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Convertino VA, Sather TM. Vasoactive neuroendocrine responses associated with tolerance to lower body negative pressure in humans. Clin Physiol 20: 177–184, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cooke WH, Rickards CA, Ryan KL, Kuusela TA, Convertino VA. Muscle sympathetic nerve activity during intense lower body negative pressure to presyncope in humans. J Physiol 587: 4987–4999, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooke WH, Ryan KL, Convertino VA. Lower body negative pressure as a model to study progression to acute hemorrhagic shock in humans. J Appl Physiol 96: 1249–1261, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 117: 1090–1096, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma Acute Care Surg 73: S431–S437, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Ebert RV, Stead EA. The effect of the application of tourniquets on the hemodynamics of the circulation. J Clin Invest 19: 561–567, 1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giller CA. The frequency-dependent behaviours of cerebral autoregulation. Neurosurgery 27: 362–368, 1990. [DOI] [PubMed] [Google Scholar]
- 18.Greenleaf JE, Petersen TW, Gabrielsen A, Pump B, Bie P, Christensen NJ, Warberg J, Videbaek R, Simonson SR, Norsk P. Low LBNP tolerance in men is associated with attenuated activation of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 279: R822–R829, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Hanson JM, Van Hoeyweghen R, Kirkman E, Thomas A, Horan MA. Use of stroke distance in the early detection of simulated blood loss. J Trauma 44: 128–134, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hinojosa-Laborde C, Rickards CA, Ryan KL, Convertino VA. Heart rate variability during simulated hemorrhage with lower body negative pressure in high and low tolerant subjects. Front Physiol 2: 85, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinojosa-Laborde C, Shade RE, Muniz GW, Bauer C, Goei KA, Pidcoke HF, Chung KK, Cap AP, Convertino VA. Validation of lower body negative pressure as an experimental model of hemorrhage. J Appl Physiol 116: 406–415, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SY, Moore LG, McCullough RE, McCullough RG, Micco AJ, Fulco C, Cymerman A, Manco-Johnson M, Weil JV, Reeves JT. Internal carotid and vertebral arterial flow velocity in men at high altitude. J Appl Physiol 63: 395–400, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Jellema WT, Imholz BP, Van Goudoever J, Wesseling KH, Van Lieshout JJ. Finger arterial versus intrabrachial pressure and continuous cardiac output during head-up tilt testing in healthy subjects. Clin Sci (Lond) 91: 193–200, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Jeong SM, Shibata S, Levine BD, Zhang R. Exercise plus volume loading prevents orthostatic intolerance but not reduction in cerebral blood flow velocity after bed rest. Am J Physiol Heart Circ Physiol 302: H489–H497, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BD, van Helmond N, Curry TB, van Buskirk CM, Convertino VA, Joyner MJ. Reductions in central venous pressure by lower body negative pressure or blood loss elicit similar hemodynamic responses. J Appl Physiol 117: 131–141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res 70: 12–21, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 60: S3–S11, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 90: 298–306, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Lewis NC, Bain AR, MacLeod DB, Wildfong KW, Smith KJ, Willie CK, Sanders ML, Numan T, Morrison SA, Foster GE, Stewart JM, Ainslie PN. Impact of hypocapnia and cerebral perfusion on orthostatic tolerance. J Physiol 592: 5203–5219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis NC, Smith KJ, Bain AR, Wildfong KW, Numan T, Ainslie PN. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (Lond) 129: 169–178, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Lucas RA, Pearson J, Schlader ZJ, Crandall CG. Hypercapnia-induced increases in cerebral blood flow do not improve lower body negative pressure tolerance during hyperthermia. Am J Physiol Regul Integr Comp Physiol 305: R604–R609, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol 58: 541–560, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Martin PJ, Evans DH, Naylor AR. Measurement of blood flow velocity in the basal cerebral circulation: advantages of transcranial color-coded sonography over conventional transcranial Doppler. J Clin Ultrasound 23: 21–26, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Murray RH, Thompson LJ, Bowers JA, Albright CD. Hemodynamic effects of graded hypovolemia and vasodepressor syncope induced by lower body negative pressure. Am Heart J 76: 799–811, 1968. [DOI] [PubMed] [Google Scholar]
- 36.Novak V, Novak P, Spies JM, Low PA. Autoregulation of cerebral blood flow in orthostatic hypotension. Stroke 29: 104–111, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Ogoh S, Sato K, Nakahara H, Okazaki K, Subudhi AW, Miyamoto T. Effect of acute hypoxia on blood flow in vertebral and internal carotid arteries. Exp Physiol 98: 692–698, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Stoddart HF. Validation in volunteers of a near-infrared spectroscope for monitoring brain oxygenation in vivo. Anesth Analg 82: 269–277, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Rea RF, Hamdan M, Clary MP, Randels MJ, Dayton PJ, Strauss RG. Comparison of muscle sympathetic responses to hemorrhage and lower body negative pressure in humans. J Appl Physiol 70: 1401–1405, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Rickards CA, Cohen KD, Bergeron LL, Burton BL, Khatri PJ, Lee CT, Ryan KL, Cooke WH, Doerr DF, Convertino VA. Cerebral blood flow response and its association with symptoms during orthostatic hypotension. Aviat Space Environ Med 78: 653–658, 2007. [PubMed] [Google Scholar]
- 41.Rickards CA, Ryan KL, Cooke WH, Convertino VA. Tolerance to central hypovolemia: the influence of oscillations in arterial pressure and cerebral blood velocity. J Appl Physiol 111: 1048–1058, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Ryan KL, Cooke WH, Rickards CA, Lurie KG, Convertino VA. Breathing through an inspiratory threshold device improves stroke volume during central hypovolemia in humans. J Appl Physiol 104: 1402–1409, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Sato K, Fisher JP, Seifert T, Overgaard M, Secher NH, Ogoh S. Blood flow in internal carotid and vertebral arteries during orthostatic stress. Exp Physiol 97: 1272–1280, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Shenkin HA, Cheney RH, Govons SR, Hardy JD, Fletcher AG. On the diagnosis of hemorrhage in man—a study of volunteers bled large amounts. Am J Med Sci 208: 421–436, 1944. [Google Scholar]
- 46.Stevens PM, Lamb LE. Effects of lower body negative pressure on the cardiovascular system. Am J Cardiol 16: 506–515, 1965. [DOI] [PubMed] [Google Scholar]
- 47.Summers RL, Ward KR, Witten T, Convertino VA, Ryan KL, Coleman TG, Hester RL. Validation of a computational platform for the analysis of the physiologic mechanisms of a human experimental model of hemorrhage. Resuscitation 80: 1405–1410, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torella F, Cowley RD, Thorniley MS, McCollum CN. Regional tissue oxygenation during hemorrhage: can near infrared spectroscopy be used to monitor blood loss? Shock 18: 440–444, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, Van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra high-field MRI. J Appl Physiol 117: 1084–1089, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Wallace JP, Sharpey-Schafer EP. Blood changes following controlled hemorrhage in man. Lancet 2: 393–395, 1941. [Google Scholar]
- 51.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. [DOI] [PubMed] [Google Scholar]
- 52.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolthuis RA, Bergman SA, Nicogossian AE. Physiological effects of locally applied reduced pressure in man. Physiol Rev 54: 566–595, 1974. [DOI] [PubMed] [Google Scholar]
- 54.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol 85: 1113–1122, 1998. [DOI] [PubMed] [Google Scholar]


