Abstract
The hypertrophic response to resistance training is generally attenuated with aging; yet the mechanisms regulating this phenomenon are largely unknown. Several studies to date have shown blunted translational efficiency following acute resistance exercise in older adults; however, the effects on translational capacity (i.e., ribosome biogenesis) have not yet been examined. Thus the purpose of this study was to examine changes in markers of ribosome biogenesis following an acute bout of resistance loading (RL; 9 sets × 10 repetitions of knee extensions) in younger (Y; n = 14; 39.2 ± 4.1 yr) and older (O; n = 12; 75.7 ± 5.7 yr) adults. Vastus lateralis biopsies were taken pre- and 24 h post-RL, and muscle samples were analyzed for total RNA content, 45S pre-rRNA expression, ribosomal protein content, and levels of signaling proteins that regulate ribosome biogenesis. Before RL, O had higher total RNA content (+28%; P < 0.05), a trend toward higher 45S pre-rRNA expression (+59%; P = 0.08), and greater protein content of several ribosomal components (≈ +50–80%; P < 0.05) than Y. However, 24 h post-RL, only Y increased 45S pre-rRNA expression (+34%; P < 0.01), possibly driven by higher basal p-Rb (Ser780) (+61%; P = 0.10), and a robust transcription initiation factor (TIF)-1a response (+75%; P < 0.05). RL tended to increase protein components of the 40S ribosomal subunit in Y only (≈ +20–25%; P ≤ 0.12). Overall, the data suggest blunted ribosome biogenesis in response to RL in O, which may be a potential mechanism driving the age-related attenuation of resistance training-induced hypertrophy.
Keywords: aging, skeletal muscle, ribosome, rRNA, resistance exercise
age-related loss of skeletal muscle mass (i.e., sarcopenia) results from motor neuron death and myofiber atrophy and leads to reduced strength, power, and functional mobility in older adults (reviewed in Ref. 11). Thus far, the most effective strategy to regrow atrophied muscle in older adults is resistance exercise training (RT), which results in muscle hypertrophy via increases in cellular protein accretion (10). While RT typically induces robust muscle hypertrophy in younger adults, the hypertrophic response to RT appears to be blunted in old (18, 24, 37). For example, we have previously shown that, in response to 16 wk of progressive RT, average myofiber hypertrophy is greater in younger adults compared with old (18, 29) and that nearly 40% of older adults do not realize any gains in myofiber size after 16 wk of RT (5). While the mechanisms regulating this attenuated hypertrophic response to RT in older adults are largely unknown, it is likely driven by a reduced protein synthetic response to repeated bouts of resistance exercise.
Following a bout of resistance exercise, muscle protein synthesis is increased and can remain elevated for up to 48 h (31). This acute elevation is thought to be driven primarily by increased translational efficiency via an enhanced rate of translation initiation (4). Several studies have shown that the early protein synthetic response to a single, acute bout of resistance exercise is blunted or delayed in older adults compared with young (12, 13, 19, 23), which may account for their attenuated long-term hypertrophic response to chronic RT. However, it has recently been shown that acute increases in myofibrillar protein synthesis following a single bout of resistance exercise are not correlated with the magnitude of muscle hypertrophy following long-term RT (26). In addition, we have previously shown that young subjects increase muscle protein synthesis 24 h after a single bout of resistance exercise (approximately equal to +100%), while older subjects do not; however, the older subjects still achieved significant myofiber hypertrophy following 16 wk of progressive RT (23). These data indicate that, although older adults have a blunted protein synthetic response to acute resistance exercise, it may not prevent them from attaining significant gains in muscle mass with long-term RT. Overall, it appears that the blunted protein synthetic response following an acute bout of resistance exercise may not be the sole determinant of the attenuated hypertrophic response to long-term RT that occurs in older adults.
Aside from acute increases in protein synthesis driven by enhanced translational efficiency, resistance exercise can also increase translational capacity (i.e., ribosome biogenesis) to promote muscle growth. Several studies have shown that markers of ribosome biogenesis are increased during load-induced muscle hypertrophy in both rodents and humans (reviewed in Ref. 8), likely in an effort to sustain the enhanced rate of protein synthesis following repeated bouts of loading. Ribosome biogenesis is a complicated and energetically-costly process; formation of a single translational-competent ribosome requires production of four ribosomal RNAs (rRNAs) and the synthesis of ∼80 ribosomal proteins. By increasing the number of ribosomes following a mechanical loading event, more protein can be synthesized per given mRNA template, and thus, hypertrophy will be facilitated. It is unknown whether ribosome biogenesis is required for muscle hypertrophy in humans; however, it appears that increases in ribosome biogenesis can occur within the first 24 h following a single bout of resistance exercise (16, 28). In addition, we have previously shown that individuals who have an extreme hypertrophic response to long-term RT are able to significantly increase muscle RNA content [indicative of rRNA transcription, a rate-limiting step of ribosome biogenesis (21)] 24 h following an acute bout of resistance exercise, while individuals who have only a modest or no hypertrophic response do not to increase muscle RNA content at this time point (16). If the ability to effectively increase ribosome biogenesis following a bout of resistance exercise is necessary for maximal hypertrophy, it would be interesting to see whether older adults can activate ribosome biogenesis to the same extent as young during RT and if the inability to do so is a possible cause of their attenuated hypertrophic response to RT.
Few studies to date have examined the effects of load-induced skeletal muscle ribosome biogenesis with regard to aging. Haddad and Adams (15) found that skeletal muscle from older rats has more RNA content (normalized to tissue weight) compared with young at baseline, indicating higher rRNA levels. Interestingly, they also found that only young are able to significantly increase muscle RNA content 24 h following a bout of isometric resistance exercise. The older rats were able to increase total RNA levels but not until 48 h following the resistance exercise bout, indicating a delay in rRNA synthesis. Similarly, a recent study from Kirby et al. (17) showed that, following synergist ablation, rRNA expression was greatly increased in skeletal muscle from young mice, while the increase in rRNA expression was blunted in aged mice (+50% vs. 2.5-fold). Based on these findings, we hypothesize that a possible mechanism regulating the attenuated RT-induced hypertrophic response in older adults is a reduced ability to activate ribosome biogenesis following resistance exercise. To test this hypothesis, we examined several markers of ribosome biogenesis in untrained younger and older adults before and 24 h after intensive resistance exercise. We found indexes of heightened ribosome biogenesis in the resting muscles of old vs. young. Additionally, we found that younger adults increase markers of skeletal muscle ribosome biogenesis following acute resistance exercise while old do not, suggesting a blunted response with advancing age.
METHODS
Subjects.
Twenty-six younger (Y; n = 14; 39.2 ± 4.1 yr) and older (O; n = 12; 75.7 ± 5.7 yr) adults were recruited from the Birmingham, Alabama, metropolitan area. Both Y and O were balanced by gender (7 M, 7 F in Y; 6 M, 6 F in O). All subjects completed health history and physical activity readiness questionnaires. Subjects in the O group were also screened by comprehensive physical exam by a physician and a graded exercise stress test with 12-lead ECG. Subjects were excluded for a history of RT, musculoskeletal, or other disorder that might influence testing or risk of injury, obesity (body mass index ≥30), or any current medications that might influence test results. The study was approved by the Institutional Review Boards of the University of Alabama at Birmingham and the Birmingham Veterans Affairs Medical Center, and all subjects provided written, informed consent before participation.
Body composition and muscle mass.
Body composition and muscle mass were assessed via dual-energy X-ray absorptiometry (DXA; Lunar Prodigy model no. 8743; GE Lunar, Madison, WI) as previously described (25, 30). In brief, limb (bilateral arm + leg) muscle mass and thigh muscle mass (TMM) were measured using enCORE 2002 software (version 6.10.029), according to the manufacturer's instructions. Measures of muscle mass were normalized to height for standardization across subjects. Skeletal muscle index was calculated as limb muscle mass (kg)/height (m)2. Bilateral TMM (kg) was also adjusted by height (m)2, and we refer to this adjusted value as TMM. Body composition and muscle mass data are reported in Table 1.
Table 1.
Descriptive characteristics
| Younger (7 M, 7 F) | Older (6 M, 6 F) | P Value | |
|---|---|---|---|
| Age, yr | 39.2 ± 4.1 | 75.7 ± 5.7 | |
| BMI, kg/m2 | 24.9 ± 1.8 | 23.9 ± 2.7 | P = 0.27 |
| TMM, kg/m2 | 3.95 ± 0.46 | 3.40 ± 0.76 | P < 0.05 |
| SMI, kg/m2 | 7.82 ± 1.10 | 6.91 ± 1.52 | P = 0.09 |
All values are means ± SD. M, male; F, female; BMI, body mass index = weight (kg)/height (m)2; TMM, bilateral thigh muscle mass (kg) adjusted by height (m)2; SMI, skeletal muscle index = (bilateral arm + leg lean mass)/height (m)2.
Resistance loading protocol and tissue collection.
The resistance loading (RL) protocol we used has been described in detail previously (25, 35). In brief, subjects performed 9 sets of 10 repetitions of unaccustomed, dynamic, bilateral knee extensions against a resistance load equal to ≈65% of one-repetition maximum strength. Subjects performed the concentric phase of each repetition explosively, followed by a controlled eccentric lowering phase. The RL protocol induced modest muscle damage in these subjects (≈60% increase in serum creatine kinase and no change in serum myoglobin 24 h post-RL; see Ref. 25 for methodology).
Vastus lateralis muscle biopsies were performed in a fasted state at rest and 24 h after the RL bout according to previously established procedures (6). Muscle samples were obtained under local anesthetic (1% lidocaine) by percutaneous needle biopsy, and the contralateral limb was used for the post-RL biopsy. Muscle samples were snap frozen in liquid nitrogen and stored at −80°C until further analysis.
Muscle RNA and protein isolation.
Frozen muscle samples (≈30 mg) were pulverized and total RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH) in accordance with the manufacturer's instructions. RNA quantity and quality were determined using a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Rockford, IL). Total RNA content/tissue weight was used as a surrogate of rRNA abundance, as >85% of skeletal muscle RNA is comprised of rRNA (38). Due to tissue availability, muscle samples from 8 Y (4 M, 4 F) and 10 O (5 M, 5 F) subjects were used for protein analyses. Muscle samples were pulverized and homogenized in 6 μl/mg muscle of ice cold lysis buffer with protease and phosphatase inhibitors and then centrifuged at 15,000 g for 40 min at 4°C according to previously established procedures (25). The supernatant was stored at −80°C until assayed for protein content using the bicinchoninic acid technique with BSA as a standard.
Quantitative PCR.
Transcript levels of 45S pre-rRNA were assessed before and 24 h after the RL bout using quantitative RT-PCR. cDNA was synthesized via reverse transcription using the SuperScript VILO cDNA Synthesis kit (Invitrogen, Carlsbad, CA), and real-time RT-PCR was performed using a StepOne System (Applied Biosystems, Foster City, CA). Forward and reverse primers designed to recognize the 5′-external transcribed spacer (5′-ETS) of 45S pre-rRNA were 5′-CCTGCTGTTCTCTCGCGCGTCCGAG-3′ and 5′-AACGCCTGACACGCACGGCACGGAG-3′ and have been previously validated by Northern blot (14). All samples were run in triplicate, and GAPDH (Hs02758991_g1) expression served as an internal control. GAPDH expression did not differ between age groups and did not change from pre- to post-RL. Relative amounts of 45S pre-rRNA (i.e., ΔCT values) were determined using the comparative threshold cycle method via StepOne software version 2.2.2 (Applied Biosystems).
Immunoblotting.
Thirty-five micrograms of mixed muscle protein lysate were resolved on 4–12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Protein lysate samples from Y and O subjects were ran consecutively on the same gels to standardize exposure time between groups. To determine levels of select proteins of the small (40S) and large (60S) ribosomal subunits, antibodies against ribosomal protein (rp) S3, rpS6, rpL3, and rpL7a were used. To assess signaling proteins that regulate ribosome biogenesis, antibodies against transcription initiation factor (TIF)-1a, phosphorylated (Thr421/Ser424) and total p70S6K, phosphorylated (Ser2448) and total mammalian target of rapamycin (mTOR), phosphorylated (Ser780) and total retinoblastoma (Rb), nucleolin, c-myc, and upstream binding factor (UBF) were used. Antibodies were purchased from Cell Signaling Technologies (product no. cs- 9538, 2217, 2403, 9204, 2708, 2971, 2972, 3590, and 9309; Danvers, MA) except for rpL3, c-myc, and UBF, which were purchased from Santa Cruz Biotechnology (product no. sc- 86828, 788, and 13125; Dallas, TX), and TIF-1a, which was purchased from Sigma-Aldrich (product no. SAB4502266; St. Louis, MO). Antibodies were used at a 1:1,000 dilution (except c-myc and UBF, which were 1:500) in 5% goat serum (monoclonal antibodies) or 2% milk + 2% BSA (polyclonal antibodies). Horseradish peroxidase-conjugated secondary antibody (Thermo Scientific, Rockford, IL) was used at 1:50,000 (wt/vol) dilution, followed by chemiluminescent detection in a Bio-Rad (Hercules, CA) ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One software (version 4.5.1).
Statistical analysis.
Unpaired student's t-tests were used to compare baseline differences in body composition/muscle mass, total muscle RNA content, 45S pre-rRNA expression, ribosomal protein content, and signaling protein content between Y and O. A 2 × 2 repeated measures ANOVA (group × time) was used to examine differences between groups in total muscle RNA content, 45S pre-rRNA expression, ribosomal protein content, and signaling protein content from pre- to 24 h post-RL, with Fisher's least significant difference post hoc analysis used to examine interaction effects. Data are reported as means ± SE, with P < 0.05 being considered statistically significant.
RESULTS
Total RNA content and 45S pre-rRNA expression.
Total RNA content normalized for tissue weight did not change from pre- to post-RL in either group. Overall, O had ≈28% higher RNA content pre-RL and ≈16% higher post-RL (P < 0.05 for both) compared with Y (Fig. 1A). Similarly, O tended to have higher basal expression of 45S pre-rRNA (+59%; P = 0.08). There was a significant effect of time on 45S pre-rRNA expression, indicating that 45S pre-rRNA expression increased from pre- to 24 h post-RL. Post hoc analysis revealed that only Y increased 45S pre-rRNA expression significantly (+34%; P < 0.01), while a trend was noted in O (+21%; P = 0.08; Fig. 1B).
Fig. 1.
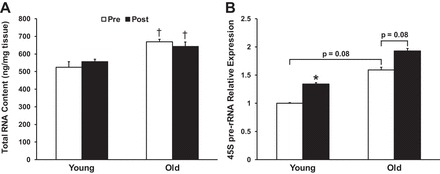
Effects of age and resistance exercise on total muscle RNA content normalized to tissue weight (A) and 45S pre-rRNA expression (B). *P < 0.05, significant change from pre- to postresistance exercise. †P < 0.05, significantly different from younger group at respective time point.
Ribosomal protein content.
Similar to total RNA content and 45S pre-rRNA expression, O muscle had higher baseline levels of ribosomal proteins compared with Y. For example, basal levels of rpS3, rpL3, and rpL7a were higher in O (+52, +69, and +81%, respectively; P < 0.05 for all), and rpS6 tended to be higher in O (+37%; P = 0.07; Fig. 2). Levels of rpL3 and rpL7a did not change in either group from pre- to post-RL. However, there was a group × time interaction effect for rpS3 and a trend toward a group × time effect (P = 0.07) for rpS6. Post hoc analysis suggested that Y tended to increase rpS3 (+24%; P = 0.12) and rpS6 (+20%; P = 0.09) levels from pre- to post-RL.
Fig. 2.
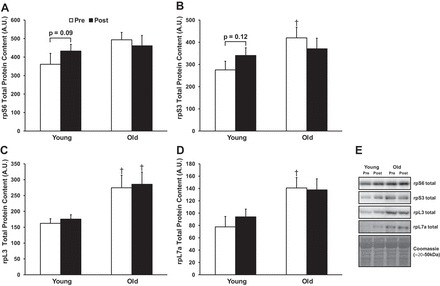
Effects of age and resistance exercise on abundance of select ribosomal proteins of the 40S (A and B) and 60S (C and D) subunits. E: representative immunoblots. †P < 0.05, significantly different from younger group at respective time point. A.U., arbitrary units.
Ribosome biogenesis signaling.
Levels of signaling proteins that regulate rRNA transcription and ribosomal protein mRNA translation are depicted in Fig. 3. Baseline levels of TIF-1a, a critical regulator of rRNA transcription, were higher in O (+79%; P < 0.05) vs. Y (Fig. 3A), as were baseline levels of p-p70S6K (Thr421/Ser424; Fig. 3B; +260%; P < 0.001). Nucleolin levels appeared almost twofold higher in O, although this was nonsignificant (P = 0.20). Interestingly, baseline levels of phosphorylated Rb (Ser780) tended to be higher in Y (+61%; P = 0.10), while total Rb protein content was no different between groups. RL increased phosphorylation of mTOR at one of its sites (Ser2448) in both Y and O to a similar extent from pre- to post-RL (+302% and + 241%, respectively; P < 0.01 for both). O decreased p70S6K phosphorylation (Thr421/Ser424) from pre- to post-RL (−62%; P < 0.001) to levels similar to those of Y. Only Y increased TIF-1a levels from pre- to post-RL (+75%; P < 0.05). There were no group or time differences observed for c-myc or UBF protein levels. An ancillary analysis was conducted to see if there were any effects of gender on ribosome biogenesis. There were no compelling gender × time interaction effects; however, several proteins involved in ribosome biogenesis appeared to be greater in females compared with males. For example, p-mTOR (Ser2448) was significantly higher in females vs. males (+96%; P < 0.05) and levels of TIF-1a and rpL7a tended to be higher in females vs. males (+44%; P < 0.08, and +38%, P < 0.11). Interestingly, this corresponded with significantly higher basal 45S pre-rRNA expression in females vs. males (≈85% higher; P < 0.05), irrespective of age.
Fig. 3.
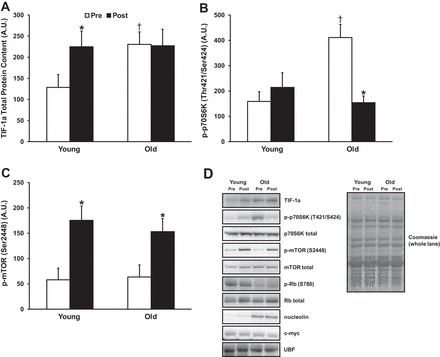
A–C: effects of age and resistance exercise on abundance of signaling proteins that regulate ribosome biogenesis. D: representative immunoblots. *P < 0.05, significant change from pre- to postresistance exercise. †P < 0.05, significantly different from younger group at respective time point.
DISCUSSION
While it is generally accepted that older adults have an attenuated hypertrophic response to long-term RT, the molecular mechanisms regulating this phenomenon remain unclear. The present findings suggest older adults appear to have dysregulated ribosome biogenesis at baseline as well as an impaired ability to activate muscle ribosome biogenesis following a bout of resistance exercise, which may partly explain the attenuated hypertrophic response to RT observed in some older adults.
Ribosome biogenesis is a critical regulator of cellular growth and proliferation and is tightly controlled at multiple levels. Production and assembly of the four rRNAs and ≈80 ribosomal proteins that make up the mature 80S eukaryotic ribosome require activation of all three classes of RNA polymerases as well as synthesis of hundreds of molecules involved in ribosome processing, assembly, and nuclear import/export (20). Synthesis of rRNA is a major rate-limiting step in ribosome biogenesis, with rRNA comprising >85% of total cellular RNA (38). Three of the four rRNAs (28S, 18S, and 5.8S rRNAs) are transcribed from a single gene (ribosomal DNA; rDNA) that exists in hundreds of tandem repeats throughout the genome. Transcription of rDNA via RNA polymerase 1 (Pol1) results in production of a precursor rRNA (i.e., 45S pre-rRNA), which is processed to form the 28S, 18S, and 5.8S rRNAs. This rate-limiting step of pre-rRNA production has been shown to occur early during load-induced muscle growth in mice (36) and humans (28), with significant increases in 45S pre-rRNA expression occurring as soon as 4 h following an acute bout of RL in humans. It is unknown whether an increase in rRNA synthesis and subsequent ribosome biogenesis is required for load-induced skeletal muscle hypertrophy, but it is a reasonable hypothesis that ribosome biogenesis would be advantageous for sustaining elevated rates of protein synthesis during long-term RT. We have previously shown that only individuals with an extreme hypertrophic response to 16 wk of RT significantly increase muscle RNA content (indicative of increased rRNA production) 24 h after the first full exercise bout, while individuals with only a moderate hypertrophic response or no response at all do not increase muscle RNA content at this time (16). These data suggest that an increase in ribosome biogenesis early in a RT program may be beneficial for maximizing RT-induced hypertrophy, and we speculate that this is a critical process that regulates load-induced muscle growth.
Here, we attempted to understand the specific pathways regulating resistance exercise-induced ribosome biogenesis and how they may be dysregulated in older adults. We show that several markers of ribosome biogenesis are upregulated 24 h after an acute bout of resistance exercise in younger adults. For example, at this time point, 45S pre-rRNA expression was increased almost +35% above baseline, likely driven by a concomitant increase in protein levels of TIF-1a (+75%), an initiation factor essential for Pol1-induced transcription of rDNA (33). Additionally, younger adults tended to increase rpS3 and rpS6 levels (approximately equal to +20–25%), which are necessary components of the 40S ribosomal subunit. On the other hand, older adults did not significantly increase 45S pre-rRNA expression or increase ribosomal protein production 24 h after the acute bout of RL, indicating either a delayed or blunted ribosome biogenesis response to loading. Our results are in agreement with those from Kirby et al. (17), which show that aged mice have blunted increases in total RNA, 47S pre-rRNA, and 28S rRNA content following synergist ablation compared with younger mice. These data implicate that ribosome biogenesis may be a key cellular process that regulates the attenuated RT-induced hypertrophic response seen in older adults.
Perhaps one of the most well-studied pathways involved in skeletal muscle growth is the mTOR pathway. Activation of mTOR is required for load-induced muscle hypertrophy, and inhibition of mTOR via rapamycin abolishes load-induced hypertrophy (7). Aside from enhancing translation initiation, activation of the mTOR pathway can promote muscle growth by increasing ribosome biogenesis. Increased ribosome biogenesis driven by mTOR activation occurs via enhanced rRNA production and translation of ribosomal protein mRNAs (reviewed in Ref. 21). In the current study, RL substantially increased mTOR phosphorylation on Ser 2448 in both younger and older adults. Using a model similar to the current study (fasted state, 8 sets × 10 repetitions of knee extension at 70% 1RM), Fry et al. (13) reported increased S2448 phosphorylation at 3, 4, and 24 h postexercise in young only. The discrepancy between the two studies cannot be explained, particularly given similar balanced gender composition and age ranges among the older adults in the two studies (i.e., 76 Y here vs. 70 Y in Fry et al.). However, since mTOR is phosphorylated at multiple residues by multiple mechanisms (1, 9, 34), S2448 phosphorylation is not necessarily indicative of mTOR activation. mTOR activity as measured by downstream phosphorylation of p70S6K (Thr389) and 4EBP1 has in fact been shown to be blunted in old within the first hour after RE (19).
One mechanism by which mTOR augments ribosome biogenesis in muscle cells is by phosphorylating Rb, thus releasing UBF to activate rDNA transcription (27). In the current study, neither Rb phosphorylation nor total levels of UBF protein were changed 24 h following the bout of RL. However, younger adults tended to have ≈60% higher levels of phosphorylated Rb (Ser780) in the resting state compared with older adults, suggesting that, before exercise, there may have been more unbound UBF to initiate rDNA transcription when provided a stimulus (e.g., resistance exercise). Additionally, only younger adults increased protein levels of the Pol1 cofactor, TIF-1a (+75%), which likely helped drive their enhanced 45S pre-rRNA expression following RL.
While the data presented here demonstrate that only younger adults increase markers of ribosome biogenesis 24 h after RL, it should be noted many of these same markers were elevated in the skeletal muscle of older adults in the basal, resting state. For example, muscle from older adults had significantly higher total RNA content (+28%; Fig. 2) and ribosomal protein content (approximately equal to +50–80%; Fig. 3) and tended to have higher 45S pre-rRNA expression (Fig. 1) compared with younger adults. Additionally, levels of signaling proteins that are known to regulate ribosome biogenesis were significantly higher or tended to be higher in muscle from older adults, including: TIF-1a (+79%), p-p70S6K (Thr421/Ser424; +260%), and nucleolin (+188%; Fig. 3). These findings are in agreement with a previous study showing that total muscle RNA content is higher in older rodents (15) at rest, indicating that aging may induce ribosome accumulation in skeletal muscle. Additionally, Zahn et al. (39) have shown that the expression of several ribosomal genes are significantly increased during human aging. To our knowledge only one published human study contradicts these aging differences (32). It is unknown why skeletal muscle from older adults appears to have a higher proportion of ribosomes compared with younger adults. This may be an attempt to compensate for the blunting of stimulus-induced translational efficiency that has been observed and may also be what is limiting any further increases in ribosome biogenesis following RL (i.e., a ceiling effect on skeletal muscle ribosome content). It is important to point out that an accumulation of ribosomes in resting older muscle is not necessarily indicative of enhanced translational capacity because we do not know the functional status of these ribosomes (e.g., ability to form polysomes). Future research should examine why skeletal muscle from older adults appears to have an abnormal accumulation of ribosomes in the resting state and how this purportedly large ribosomal pool functions during long-term RT.
The data from the present study clearly demonstrate an age-related deficit in ribosomal biogenesis following resistance exercise; however, our study is not without limitations. As with most studies involving muscle biopsies, the time point at which the biopsy is taken postexercise is a major factor. While we were able to detect significant increases in 45S pre-rRNA expression 24 h following the RL bout in the younger group, total muscle RNA content did not change at this point. Using a similar resistance exercise protocol, Roberts et al. (32) did not observe an increase in total muscle RNA content 24 h postexercise in a cohort of younger adults either. This suggests that within the first 24 h following an acute resistance exercise bout ribosome biogenesis is enhanced in younger adults (assessed by increased 45S pre-rRNA expression) but not enough rRNA accumulates within this time period to cause major changes to the total RNA pool. However, Kim et al. (16) reported that total RNA content was increased in individuals who had an extreme hypertrophic response to RT just 24 h after an acute resistance exercise bout, suggesting that ribosome biogenesis was sufficiently increased in this extreme responder cohort to be measurable as an accumulation in the total RNA pool.
Regarding protein signaling, we and others have found altered phosphorylation states as late as 24 h after resistance exercise (13, 22, 23); however, early, short-lived signaling events are missed in this model (e.g., Ref. 19). For example, protein levels of c-myc, an oncoprotein that regulates rDNA transcription (3), was unchanged 24 h following resistance exercise in the current study, while protein levels of c-myc have been found to be increased to a greater extent in young vs. old quail 1 h following stretch (2). We previously found an induction of p70S6K Ser421/Thr424 phosphorylation (autoinhibitory domain) that persisted 24 h after acute RL in a group that experienced extreme myofiber hypertrophy during a subsequent 16-wk period of RT (22). We were therefore surprised by the lack of induction at 24 h in the present study, and more surprised by the decrease in p70S6K (Ser421/Thr424) phosphorylation found among old (with no change in total p70S6K). We have no explanation for this result, but clearly this and other studies would benefit from multiple tissue sampling time points postexercise. Such multiple time point studies would help elucidate the time course of RL-induced ribosome biogenesis in both younger and older adults and whether alterations to ribosome biogenesis modulate long-term RT outcomes.
In conclusion, the data presented here demonstrate that although older adults appear to have a large pool of ribosomes in their resting skeletal muscle, they are unable to increase markers of ribosome biogenesis to the same extent as younger adults following an acute bout of resistance exercise. We suggest that this blunted ribosome biogenesis response to acute resistance exercise contributes to the attenuated RL-induced hypertrophy seen in older adults. Very few studies examining the RL-induced regulation of muscle translational capacity exist to date, and we believe that this is an important, yet understudied, mechanism regulating skeletal muscle hypertrophy in both younger and older adults. Future research examining the basic mechanisms controlling RL-induced ribosome biogenesis and how these mechanisms are altered in aging muscle may help to further elucidate the cause of impaired RT-induced hypertrophy in older adults.
GRANTS
This work was supported by a Veterans Affairs Merit Review Grant (to M. M. Bamman), National Institutes of Health Grants R01-AG-017896 (to M. M. Bamman) and F31-AG-044109 (to M. J. Stec), and the UAB Center for Clinical and Translational Science (UL1TR000165).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.S. and M.M.B. conception and design of research; M.J.S. performed experiments; M.J.S. analyzed data; M.J.S., D.L.M., and M.M.B. interpreted results of experiments; M.J.S. prepared figures; M.J.S., D.L.M., and M.M.B. drafted manuscript; M.J.S., D.L.M., and M.M.B. edited and revised manuscript; M.J.S. and D.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to the participants for the effort and dedication.
REFERENCES
- 1.Acosta-Jaquez HA, Keller JA, Foster KG, Ekim B, Soliman GA, Feener EP, Ballif BA, Fingar DC. Site-specific mTOR phosphorylation promotes mTORC1-mediated signaling and cell growth. Mol Cell Biol 29: 4308–4324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alway SE. Overload-induced C-Myc oncoprotein is reduced in aged skeletal muscle. J Gerontol A Biol Sci Med Sci 52: B203–211, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, Larsson LG, Wright AP. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol 7: 303–310, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem 42: 61–74, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 102: 2232–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol (1985) 97: 1329–1337, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang GG, Abraham RT. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J Biol Chem 280: 25485–25490, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14: 58–74, 2015. [DOI] [PubMed] [Google Scholar]
- 11.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (1985) 95: 1717–1727, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol (1985) 104: 1452–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat Cell Biol 7: 311–318, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Haddad F, Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol (1985) 100: 1188–1203, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol (1985) 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol (1985) 119: 321–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol (1985) 101: 531–544, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lempiainen H, Shore D. Growth control and ribosome biogenesis. Curr Opin Cell Biol 21: 855–863, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25: 6384–6391, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol (1985) 107: 1655–1662, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mero AA, Hulmi JJ, Salmijarvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpaa T, Hakkinen K, Kovanen V, Ahtiainen JP, Selanne H. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol 113: 641–650, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 115: 937–948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLoS One 9: e89431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Nader GA, von Walden F, Liu C, Lindvall J, Gutmann L, Pistilli EE, Gordon PM. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J Appl Physiol (1985) 116: 693–702, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol (1985) 98: 211–220, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Roberts MD, Kerksick CM, Dalbo VJ, Hassell SE, Tucker PS, Brown R. Molecular attributes of human skeletal muscle at rest and after unaccustomed exercise: an age comparison. J Strength Cond Res 24: 1161–1168, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Schnapp A, Pfleiderer C, Rosenbauer H, Grummt I. A growth-dependent transcription initiation factor (TIF-IA) interacting with RNA polymerase I regulates mouse ribosomal RNA synthesis. EMBO J 9: 2857–2863, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285: 7866–7879, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thalacker-Mercer AE, Dell′Italia LJ, Cui X, Cross JM, Bamman MM. Differential genomic responses in old vs. young humans despite similar levels of modest muscle damage after resistance loading. Physiol Genomics 40: 141–149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Walden F, Casagrande V, Ostlund Farrants AK, Nader GA. Mechanical loading induces the expression of a Pol I regulon at the onset of skeletal muscle hypertrophy. Am J Physiol Cell Physiol 302: C1523–C1530, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Welle S, Totterman S, Thornton C. Effect of age on muscle hypertrophy induced by resistance training. J Gerontol A Biol Sci Med Sci 51: M270–275, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Young V. The role of skeletal and cardiac muscle in the regulation of protein metabolism. Mammalian Protein Metab 4: 585–674, 1970. [Google Scholar]
- 39.Zahn JM, Sonu R, Vogel H, Crane E, Mazan-Mamczarz K, Rabkin R, Davis RW, Becker KG, Owen AB, Kim SK. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet 2: e115, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


