Abstract
The parabrachial nucleus is important for thermoregulation because it relays skin temperature information from the spinal cord to the hypothalamus. Prior work in rats localized thermosensory relay neurons to its lateral subdivision (LPB), but the genetic and neurochemical identity of these neurons remains unknown. To determine the identity of LPB thermosensory neurons, we exposed mice to a warm (36°C) or cool (4°C) ambient temperature. Each condition activated neurons in distinct LPB subregions that receive input from the spinal cord. Most c-Fos+ neurons in these LPB subregions expressed the transcription factor marker FoxP2. Consistent with prior evidence that LPB thermosensory relay neurons are glutamatergic, all FoxP2+ neurons in these subregions colocalized with green fluorescent protein (GFP) in reporter mice for Vglut2, but not for Vgat. Prodynorphin (Pdyn)-expressing neurons were identified using a GFP reporter mouse and formed a caudal subset of LPB FoxP2+ neurons, primarily in the dorsal lateral subnucleus (PBdL). Warm exposure activated many FoxP2+ neurons within PBdL. Half of the c-Fos+ neurons in PBdL were Pdyn+, and most of these project into the preoptic area. Cool exposure activated a separate FoxP2+ cluster of neurons in the far-rostral LPB, which we named the rostral-to-external lateral subnucleus (PBreL). These findings improve our understanding of LPB organization and reveal that Pdyn-IRES-Cre mice provide genetic access to warm-activated, FoxP2+ glutamatergic neurons in PBdL, many of which project to the hypothalamus.
the parabrachial nucleus (PB) contains sensory relay neurons that facilitate a variety of homeostatic functions. Its many neuronal subpopulations intermingle between the rostral pons and the caudal midbrain. These subpopulations receive several distinct types of sensory information, ranging from pain and temperature via the spinal cord to vagal viscerosensory signals via the lower brain stem. Most PB neurons relay information to the forebrain. For example, gustatory and gastrointestinal signals from the nucleus of the solitary tract are relayed to the amygdala and thalamus to modulate ingestive behavior (7, 48, 54). Other PB-relayed signals influence blood pressure, extracellular fluid volume, thirst, sodium appetite, and autonomic tone (4, 11, 17). Some PB neurons relay nociceptive signals from the spinal cord to the amygdala and diencephalon, modulating emotional and autonomic responses to pain (1, 3, 10, 25).
A more recently discovered subpopulation of PB neurons relays thermal information from the skin, enabling physiological responses to changes in ambient temperature (37, 38). The PB is an obligate relay for afferent signals from the skin that promote thermogenesis in a cold environment and heat dissipation in a warm environment. In humans, damage in or near the PB can profoundly disrupt thermoregulation, often producing fatal hyperthermia (46).
Skin warming and cooling are detected by peripheral thermosensory nerves, which deliver this information to the spinal cord. Second-order thermosensory neurons in the spinal cord project to the PB, where third-order neurons relay this information to neurons in the preoptic area that govern thermoregulation, osmoregulation, and sleep-wake homeostasis.
Thermosensory relay neurons are located in the lateral subdivision of the PB (LPB). They are necessary for the autonomic reflexes triggered by changes in skin temperature, as demonstrated in anesthetized rats by Nakamura and Morrison (37, 38). These investigators mapped the thermosensory LPB neurons by combining cholera toxin, subunit b (CTb) retrograde labeling from the hypothalamus and c-Fos labeling after exposure to either a warm or cool ambient temperature. The neurons double-labeled for CTb and c-Fos formed a rostrocaudal continuum through subregions of the LPB known to receive input from the spinal cord (2, 5, 14, 24). Warm-activated neurons were clustered caudally, primarily in the PBdL, while cool-activated neurons were clustered rostrally, in a region labeled the external lateral subnucleus (see supplemental data in Ref. 38).
Localizing thermosensory relay neurons within the LPB was a significant advance, but the genetic identity of these neurons remains unknown. This represents a significant knowledge gap in a region like the LPB, which contains heterogeneous subpopulations with diverse patterns of gene expression and input-output connections. Lacking information about the genetic identity of these cells limits our ability to harness genetic techniques like Cre-lox and Flp-FRT to manipulate specific subpopulations of LPB neurons in physiological and behavioral experiments.
Here, we determine the genetic identity and subnuclear localization of LPB thermosensory neurons in the mouse. We began this study by observing that in the rat, LPB warm-activated neurons (5, 38) appear in a distribution similar to that of neurons expressing the gene Prodynorphin (Pdyn) (25). We also observed that the combined distributions of warm- and cool-activated neurons coincides with a larger subpopulation of neurons in the LPB that express FoxP2 (36). This transcription factor demarcates roughly half of all PB glutamatergic neurons (19). On the basis of their position within the LPB, these FoxP2+ neurons likely relay spinal information to the hypothalamus, unlike neurons in nearby subnuclei like PBeL, which relays vagal viscerosensory information to the amygdala (18, 19, 36, 45). We hypothesized that thermosensory neurons in the LPB are FoxP2+ and that caudal, warm-relay neurons express Pdyn and project to the preoptic hypothalamus.
To test these hypotheses, we exposed GFP reporter mice for Pdyn-IRES-Cre to warm and cool ambient temperatures and then immunolabeled FoxP2 and c-Fos to identify and characterize activated neurons across the LPB. We also examined FoxP2 expression in reporter mice for glutamatergic and GABAergic genetic markers, and mapped c-Fos expression after retrograde tracer injection in the preoptic area followed by exposure to a warm environment.
MATERIALS AND METHODS
Mice.
We used adult, male Pdyn-IRES-Cre; R26-loxSTOPlox-L10-GFP mice (abbreviated Pdyn-GFP) to visualize putative dynorphin neurons (31). In these “dynorphin reporter” mice, cells and cell lineages that express the Prodynorphin (Pdyn) gene, or that did so earlier in development, produce Cre-recombinase, which induces GFP expression by deleting a floxed STOP sequence. In this reporter line, GFP is conjugated to the ribosomal L10 subunit (23), thus restricting fluorescent labeling to the soma and proximal dendrites of neurons, resulting in excellent cell body visualization and no axonal labeling. The distribution of GFP throughout the brain and the colocalization between GFP and Pdyn mRNA in the paraventricular hypothalamic nucleus of a Pdyn-GFP mouse neurons are shown in supplemental Fig. 5 of Ref. 31.
Fig. 5.
FoxP2+ neurons in most LPB subnuclei are glutamatergic. A: GFP cytoplasmic labeling (green) colocalizes with FoxP2 nuclear immunoreactivity (magenta) in the caudal LPB in a Vglut2-GFP reporter mouse [dorsal-lateral (dL) PB; bregma −5.3 mm]. B: GFP and FoxP2 colocalization is also apparent in the rostral to external lateral (PBreL; bregma −4.9 mm). Scale bars: 50 μm.
Michael Krashes and David Olsen of the Lowell laboratory at BIDMC produced the Pdyn-IRES-Cre and R26-loxSTOPlox-L10-GFP mice, respectively. We bred these mice on a mixed background and confirmed the genotype of each mouse via separate PCR assays for Cre and L10-GFP.
For our initial c-Fos experiments, we used 12 adult, male Pdyn-GFP mice (8–10 wk). All mice were F1 progeny of the same breeding pair and were heterozygous for both Pdyn-IRES-Cre and R26-loxSTOPlox-L10-GFP. One additional F1 Pdyn-GFP mouse was used for fluorescence in situ hybridization followed by immunofluorescence labeling for GFP (below), and another was used for sagittal brain stem template images to generate the summary figure in this paper.
We immunolabeled FoxP2 in brain stem sections from “Vglut2-reporter” (Vglut2-IRES-Cre;R26-loxSTOPlox-L10-GFP) and “Vgat-reporter” mice (Vgat-IRES-Cre; R26-loxSTOPlox-L10-GFP) to determine whether thermosensory relay neurons in the LPB are likely glutamatergic or GABA/glycinergic. The Vglut2- and Vgat-IRES-Cre mice were generated in the Lowell laboratory (reported initially in Ref. 51). We used four additional Pdyn-GFP mice for retrograde tracing combined with warm exposure, as described below.
All mice were housed in our animal facility on a 12: 12-h light-dark cycle in cages with corncob bedding, nesting material, a hut, water, and standard rodent chow available ad libitum. Littermates were weaned at 21 days and group-housed five or fewer per cage in ventilated racks in a barrier facility room with stable air temperature (21–23°C) and humidity (30–40%). The Institutional Animal Care and Use Committee at Beth Israel Deaconess Medical Center approved all animal husbandry and experimental protocols.
Ambient temperature manipulations.
We manipulated ambient temperature using a thermostat-controlled environmental chamber (RIS52SD; Powers Scientific). This chamber is enclosed, insulated, and ventilated, with fluorescent lamps on each sidewall to maintain the internal light-dark cycle.
We placed mice in individual cages inside this chamber to habituate for 2–3 days at a set temperature of 24°C. Temperature oscillations of less than 0.5°C inside the chamber and within each cage were slow (period ∼90 min) and averaged ∼0.6°C warmer during lights-on, which may reflect warming from the fluorescent lamps.
We logged the ambient temperature inside each cage using a Thermochron iButton (DS1921H; Dallas Semiconductor; range +15–46°C; step 0.125°C; accuracy ± 1°C) secured above the food hopper wires in each cage. We also placed an iButton DS1921G (range −30 to +70; precision 0.5°C; accuracy ± 1°C) in every cool-exposed cage to track temperatures accurately below the DS1921H's minimum of 15°C. Additional DS1921H iButtons were placed outside the cages, one on each sidewall, to log the chamber air temperature in parallel with the chamber's digital thermostat and a mercury thermometer on the upper shelf. Across all experiments, all three measures of chamber temperature were concordant within 1°C.
At 7:30 AM [zeitgeber time (ZT) +1.5 h] on the day of the experiment, we removed the hut, food hopper, and water bottle from each cage to reduce confounds from ingestive and other behavioral responses to warm and cool temperature changes. Chamber temperature was then set to 36.0°C (warm), 4.0°C (cool), or left at 24.0°C (control) for 4 Pdyn-GFP mice per group. Four hours later, mice were removed from the chamber in pairs for perfusion (11:30 AM–12:00 PM; ZT +5.5–6.0 h).
Thermal imaging and core temperature recordings.
We brought a separate cohort of wild-type mice (C57BL/6J from Jackson ImmunoResearch) directly from their cage racks (room temperature) into the thermal chamber set at 36°C, and later 4°C, for thermal imaging using a FLIR E4 thermal camera (FLIR Systems). Immediately before imaging, mice were removed from the chamber and placed in a room-temperature cage, which provided a neutral thermal background for imaging contrast relative to warm or cool body surface temperatures. Images were captured in the first minute after cage change; in our experience, thermal changes caused by stress in mice are not evident in this short time. The resulting infrared, quantitative images demonstrate the vasomotor thermoregulatory changes that occurred in response to the ambient temperature changes in the environmental chamber, reflecting primarily redistribution of blood flow into or away from vascular beds in the skin of the limbs and tail. Tail blood flow is particularly sensitive to vasomotor thermoregulatory changes (41), so we measured the mid-tail temperature using FLIR Tools infrared image analysis software.
For abdominal-core body temperature measurements, we calibrated telemetric temperature transmitters (G2 E-Mitter, Starr Life Sciences) in a water bath before placing them into the peritoneal cavity of a separate group of mice. Later, we exposed these mice to a similar protocol of warm and cool ambient temperature for 4 h using the same environmental chamber. We gathered core temperature data separately from the mice we used for c-Fos analysis to minimize spurious c-Fos labeling in the LPB, where many neurons can be activated by noxious or inflammatory visceral stimuli (25). This cohort of comparison animals were a combination of adult male Vgat-(Ai9)tdtomato and Vglut2-(Ai9)tdtomato reporter mice (n = 9; 8–16 wk; 25–32 g; later used for separate anatomical experiments). The temperature protocol was identical to the Pdyn-GFP groups except that 27°C was used as a control ambient temperature instead of 24°C, and all mice were exposed to 36°C for 4 h, and then, after 3 days at the control temperature, they were exposed to 4°C for 4 h.
CTb injections into MnPO.
The distribution of warm-thermosensory neurons that project to the preoptic area overlaps that of dynorphin neurons in the caudal LPB (26, 35, 38). To determine whether any warm-activated dynorphin or FoxP2+ neurons project to the preoptic area, we injected the retrograde tracer CTb into the preoptic area of male, Pdyn-GFP mice (n = 4, age 18–22 wk; 31–36 g).
Under anesthesia with ketamine-xylazine (100 mg/kg and 10 mg/kg), we made stereotaxic microinjections (0.2 or 1.0% CTb in sterile 0.9% saline) via a fine-tipped micropipette. The injection was placed in the midline, 0.6 mm rostral to bregma. Using controlled puffs of compressed air, we injected 9–12 nl at each of three depths (4.8, 4.6, and 4.4 mm deep to bregma) at an average rate of 1 nl/min. Postoperatively, we administered 1.0 ml isotonic saline with meloxicam (5 mg/kg sc) and housed each CTb-injected mouse in the environmental chamber for 1 wk to allow for retrograde transport. After that, the chamber warmed to 36°C for 4 h, as above.
Perfusions and tissue preparation.
We anesthetized each mouse with ketamine-xylazine (150 mg/kg and 15 mg/kg ip) and perfused them through the ascending aorta with 50 ml of PBS at room temperature followed by 100 ml of 10% formalin-PBS (Fisher Scientific).
The brain was then removed and fixed overnight in 10% formalin-PBS at 4°C. After cryoprotection in 20% sucrose-PBS, we sectioned the rostral brain stem into 30-μm thick axial sections on a freezing microtome. We collected the sections in a 1-in-3 series for a rostrocaudal resolution of 30 μm in each 90-μm span through the PB (60 μm between sections). Sections were stored in PBS-azide at 4°C for less than one day before immunofluorescence labeling.
Immunofluorescence labeling.
After three washes in PBS, we incubated sections overnight at room temperature (RT) on a tissue shaker in netwells with 4 ml of primary antibody solution. This PBS-based solution contained 3% normal horse serum for blocking, 0.3% Triton X-100 detergent, and two primary antisera. We used a sheep polyclonal antiserum (diluted 1:5,000) raised against a recombinant peptide sequence corresponding to Ala-640–Glu-715 of human FoxP2 (lot no. CCUB0109061 of no. AF5647; R&D Systems) and a rabbit polyclonal antibody (diluted 1:10,000) raised against a synthetic peptide corresponding to amino acids 4–17 of human c-Fos (lot no. 000088552 of no. PC38/Ab-5; Calbiochem/Oncogene; no longer commercially available). GFP remained bright after immunostaining, so we imaged Pdyn-GFP+ neurons directly, without immunolabeling for GFP.
After three washes in PBS, sections were transferred to a secondary antibody solution for 90 min at RT. This solution contained Cy3-conjugated donkey anti-rabbit antiserum (lot no. 62692 of no. 711-165-152; Jackson ImmunoResearch) and biotin-conjugated donkey anti-sheep (lot no. 100270 of no. 713-065-147 from Jackson Immunoresearch), each diluted 1:500 in the same base solution (PBS with 0.3% Triton and 3% horse serum). After three additional washes in PBS, followed by a 1-h incubation in Cy5-streptavidin (Molecular Probes no. SA1011, lot no. 800372C1; diluted 1:1,000 in PBT), sections were washed again in PBS and then mounted on gelatin-coated slides. After drying, slides were coverslipped with fade-retardant mounting medium containing DAPI (Vectashield; Vector Laboratories), which aids in visualizing sections at low magnification for whole-slide fluorescence imaging.
We used similar double-immunofluorescence protocols to label CTb with FoxP2 or c-Fos. In a 1:3 series, CTb was labeled using a goat polyclonal antiserum (1:5,000; List Biological Laboratories, no. 703, lot no. 115037) in combination with rabbit anti-c-Fos. This was followed by a secondary antibody combination of biotinylated anti-goat (1:500; Jackson ImmunoResearch, no. 705-065-147, lot no. 111646) and Cy3-conjugated anti-rabbit (same as for c-Fos). In this series, we immunolabeled GFP using a chicken anti-GFP polyclonal antiserum (1:2,000; Invitrogen A10262, lot no. 1229709) followed by Alexa Fluor 488-conjugated donkey anti-chicken (1:500; Jackson ImmunoResearch no. 703-545-155, lot no. 115037); this immunofluorescence step did not alter the number or distribution of Pdyn-GFP neuronal labeling relative to native GFP fluorescence. In an adjacent 1:3 tissue series, we labeled CTb with a rabbit polyclonal antiserum (1:1,000; Biodesign, no. 65927R, lot no. 8E12914) combined with sheep anti-FoxP2 (as above), followed by Cy3-anti-rabbit and biotinylated anti-sheep and then streptavidin-Cy5 (each as above), and visualized GFP via native fluorescence.
In tissue from Vglut2-GFP and Vgat-GFP reporter mice, we immunolabeled FoxP2 using Cy3-conjugated donkey anti-sheep (1:500, Jackson ImmunoResearch) or biotin-conjugated donkey anti-sheep (1:500, Jackson ImmunoResearch) secondary antisera followed by Cy5-streptavidin. We imaged Cre-reporter-positive neurons in these tissues using their native GFP fluorescence.
To label neuroanatomical landmarks in the sagittal plane for Fig. 11, we immunolabeled sagittal sections (30 μm thick, 1:3 series from another male Pdyn-GFP mouse) for choline acetyl transferase (ChAT) and tyrosine hydroxylase (TH). Individual, free-floating sagittal sections were incubated with goat anti-ChAT (Millipore AB1542, diluted 1:1,000) and sheep anti-TH (Millipore AB144P, lot no. 2387396, diluted 1:1,000) in a 9-well glass plate overnight at 4°C. These primary antisera were followed by Alexa Fluor 555-conjugated donkey anti-sheep (Invitrogen A21436, lot no. 1371048; diluted 1:500) and biotinylated donkey anti-goat (as above), and then Cy5-streptavidin (as above).
Fig. 11.
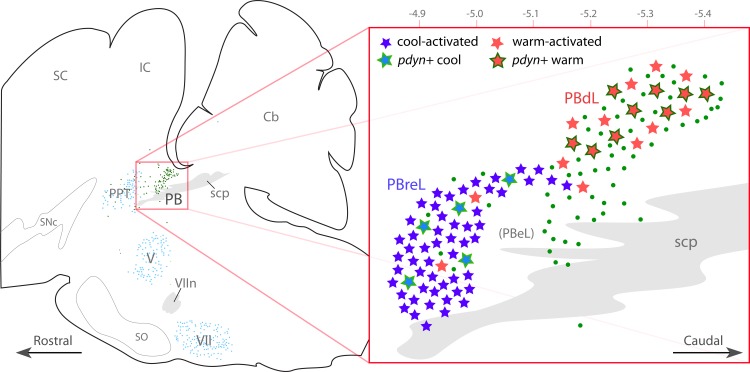
Neurons relaying warm and cool thermosensory information exist in largely separate clusters along the rostral to caudal axis of the LPB. At left, in a sagittal brain stem drawing (1.2–1.3 mm lateral to bregma), Pdyn neurons are shown as green dots; aqua dots represent cholinergic nuclei, as anatomic landmarks. The expanded inset at right shows the PB region. We observed two patterns of c-Fos expression: cool-activated neurons cluster in the far-rostral LPB and are predominantly FoxP2+ and nondynorphinergic. Warm-activated neurons are clustered caudally and include many FoxP2+ dynorphin neurons. Abbreviations: Cb, cerebellum; IC, inferior colliculus; M, medulla; SNc, substantia nigra, pars compacta; PB, parabrachial nucleus; PBdl, dorsolateral parabrachial nucleus; PBeL, external lateral PB; PBreL, rostral-to-external PB; PPT, pedunculopontine tegmental nucleus; SC, superior colliculus; scp, superior cerebellar peduncle; SO, superior olivary nuclear complex; V, trigeminal motor nucleus (cholinergic motor neurons in dark blue); VII, facial motor nucleus; VIIn, facial nerve fascicles (postgenu).
Fluorescence in situ hybridization with immunofluorescence.
Cre-dependent reporter lines produce GFP or other marker proteins permanently in cell lineages that express Cre at any point in development. The expression of Pdyn changes over development (44), and even in adult neurons, it is altered by various factors (13, 27, 47). Thus, to compare the adult pattern of Pdyn mRNA expression with the distribution of GFP, we performed fluorescence in situ hybridization (FISH) for Pdyn in an adult Pdyn-GFP mouse, followed by immunofluorescence labeling for GFP.
On day 1, we perfused a female Pdyn-GFP mouse (18 wk) with 50 ml of PBS with 0.1% diethyl pyrocarbonate (DEPC; Sigma D5758) followed by 10% formalin in PBS. The brain was removed immediately and post-fixed for 4 h in 10% formalin-PBS, and then cryoprotected at 4°C overnight using 20% sucrose in formalin-PBS.
On day 2, after cleaning a freezing microtome with RNAaseZap (Ambion/Life Technologies), we cut 30-μm sections into a 1:4 series and placed them in 1% formalin in DEPC-PBS. After 1 h on a shaker at room temperature, sections were stored in a cryoprotectant solution, RNAlater (Ambion), overnight at −20°C.
On day 3, we divided a whole-brain 1:4 series of sections into four tissue wells, washed them 4 times in DEPC-PBS, and then incubated them in hybridization buffer (50% formamide, Fisher; 5 × SSC, Promega; 0.5 mg/ml t-RNA, Roche; 5% dextran, Sigma; 1 × Denhardt's solution, Sigma; 0.1% Tween-20, Sigma; DEPC-water) for 1 h at 54°C. We transcribed a digoxigenin-tagged RNA probe for the entire Pdyn coding sequence in mouse using a plasmid generously provided by D. Strochlic (initial RNA transcription described in Ref. 31). We linearized this TOPO plasmid with NOT1 (New England Biolabs) and then transcribed riboprobes using T3 polymerase (Roche) and dNTPs containing digoxigenin-conjugated UTP (Roche). The anti-sense riboprobe (300 ng/μl) was added to each well and incubated at 54°C overnight.
On day 4, we washed sections in 2 × SSC in 50% formamide for 45 min at 54°C, followed by two 30-min washes in 2 × SSC, and removed nonhybridized riboprobes using RNAase. Sections were washed twice (5 min) in RNase buffer (0.5 M NaCl and 10 mM Tris·HCl in ddH2O) before and after 30-min incubation in RNase A (50 μg/ml) for 30 min at 37°C. Sections were then washed in 2 × SSC (5 min at RT), then 2 × SSC-50% formamide (5 min at 54°C) followed by two washes in 1 × SSC-50% formamide in DEPC-H2O (5 min at RT), and then three washes in TBS (5 min at RT). Next, we incubated sections in the Boehringer blocking reagent (Roche; diluted to 5% wt/vol in TBS with 0.1% Tween, dissolved × 5 min at 54°C) for 30 min, and then added anti-digoxigenin-POD Fab fragments (Roche no. 11-207-733-910; lot no. 14299300) at a 1:200 dilution for overnight incubation at RT.
On day 5, sections were removed from the anti-dig-POD solution and washed three times in TBS (10 min each) before fluorescence tyramide signal amplification. Sections were incubated for 30 min at RT in biotinylated tyramide (Perkin-Elmer, no. FP1019, lot no. 1673734) diluted 1:50 in P-E solution no. FP1050 (lot no. 1734999). Sections were then washed in TBS (5 min), PBS (brief rinse), PBS (10 min), and PBT (2 h), followed by incubation in streptavidin-Alexa Fluor 555 (Invitrogen no. A32355, lot no. 1094426; diluted 1:1,000 in PBT) for 2 h at RT. After three 5-min washes in PBS, sections were checked for successful Pdyn FISH labeling and then immunolabeled for GFP as the FISH protocol eliminated the native fluorescence. We used a chicken polyclonal antiserum to label GFP (Invitrogen no. A10262, lot no. 1229709; diluted 1:3,000 in the same solution described in our immunofluorescence protocol above). Sections were incubated in this solution overnight at RT.
Finally, on day 6, sections were washed four times in PBS, incubated for 2 h in Alexa Fluor 488-conjugated donkey anti-chicken antiserum (Molecular Probes no. A11039, lot no. 148638; diluted 1:500 in PBT-NHS), washed four times in PBS, and then mounted and coverslipped.
Imaging and data analysis.
Temperature logger data were downloaded from iButtons using the one-wire USB adapter and OneWire software (www.maximintegrated.com). E-Mitter abdominal-core temperature data were acquired with Spike2 (Cambridge Electronic Design).
We used a slide scanner microscope (Olympus VS120) for whole-slide imaging. All four epifluorescence channels—DAPI (blue), GFP (green), Cy3 (red), and Cy5 (far-red)—were acquired at ×100 magnification. Far-red light (Cy5, for FoxP2-immunoreactivity) was pseudocolored magenta using OlyVIA software (Olympus). After 100 × whole-slide imaging, we acquired high-magnification (×200) image stacks bilaterally in every section containing the LPB. We acquired images in 1.5 μm z-steps through the full thickness of each section. 200 × z-stacks were tiled across a broad region containing the entire parabrachial region and part of the adjacent brain stem nuclei and tracts.
We reviewed every section containing the parabrachial region of interest bilaterally for landmarks in the dorsolateral brain stem, described in Table 1, and assigned each section to a rostrocaudal level best approximating the bregma-level schema in a stereotaxic atlas of the mouse brain (39).
Table 1.
Approximate bregma levels for mouse lateral parabrachial nucleus analysis
| Bregma | Neuroanatomic Landmarks |
|---|---|
| −4.9 mm | Far-rostral mesencephalic parabrachial nucleus (PB). PBreL (FoxP2+) is prominent; PBeL (FoxP2−) is not evident. The nucleus of the lateral lemniscus (NLL) is prominent as a large population of FoxP2+ neurons dorsolateral to the lateral parabrachial nucleus (LPB), intermingled with the auditory projections of the LL. The superior cerebellar peduncle is diffuse, turning rostro-medially toward its decussation. |
| −5.0 mm | Rostral mesencephalic PB. PB rostral-to-external lateral (PBreL) is prominent as an extensive population of FoxP2+ neurons surrounding the rostral tip of pB external lateral (PBeL), which is FoxP2−. NLL present. Pdyn-GFP+ neurons are scattered and few in number. |
| −5.1 mm | Middle mesencephalic PB. PBeL (FoxP2−) is prominent. PB dorsolateral (PBdl) (FoxP2+) is absent, but the rostral part of the central lateral subnucleus (FoxP2+ neurons; PB central lateral (PBcL) becomes prominent, as it merges rostrolaterally into PBreL. |
| −5.2 mm | Caudal mesencephalic PB. A thin isthmus of tissue at the pons-midbrain junction usually connects the caudal-ventral inferior colliculus to the LPB and pons. PBdL (FoxP2+) is present, merging into PBcL (also FoxP2+). PBeL is prominent (FoxP2−). |
| −5.3 mm | Rostral pontine PB. PBdL (FoxP2+) is prominent, along with a few cells in the caudal-most PBcL (FoxP2+). Caudal PBeL (FoxP2−) is present. |
| −5.4 mm | Middle pontine PB. Caudal PBdL (FoxP2+) is prominent. Caudal PBeL (FoxP2−) is thin or absent. FoxP2+ neurons are present at the extreme ventrolateral border of the LPB (lateral crescent and Kolliker-Fuse nuclei). |
| −5.5 mm | Far-caudal pontine PB. LPB is vanishingly thin, with very few neurons occupying the space between the superior cerebellar peduncle (scp) and ventral spinocerebellar tract, as they approach one another caudally (where the scp exits the cerebellum). |
Next, for each fluorescence channel, we placed a tiled ×200 image from every focal plane into an individual layer in Photoshop for every region of interest at every bregma level. Double-immunolabeled cell nuclei (FoxP2 plus c-Fos) as well as Pdyn-GFP+ neurons with c-Fos+ or FoxP2+ nuclei were identified only when they were definitively colocalized, defined as present and in-focus in the same cell at the same z-axis focal depth. Double-labeled cells were plotted throughout the LPB at every level. Ambiguous objects or out-of-focus objects were not plotted; this resulted in under-counting of less-well defined FoxP2+ nuclei in some sections, as our counting criteria were conservative and designed to prevent false-positive data. We counted neurons throughout the LPB. LPB boundaries were defined medially, by the superior cerebellar peduncle (scp) at all levels; caudally, by the approximation of the scp with the ventral spinocerebellar tract (vsct); dorsolaterally, by the vsct (in the pons), cuneiform nucleus, and nucleus of the lateral lemniscus (in the midbrain). The rostral border of the LPB with the pedunculopontine nucleus and midbrain tegmentum is less well defined. We did not count levels rostral to bregma −4.9, where the lateral margins of the scp become indistinct. Plots of single- and double-labeled neurons were counted using Photoshop's counting tool. Triple-labeled data from 36°C warm-exposed mice with preoptic CTb injections were quantified similarly, but only at caudal LPB levels containing PBdL (bregma −5.3 mm to −5.4 mm). The resulting data were analyzed and charted in Microsoft Excel.
OlyVIA 100 × images were captured and transferred to Adobe Photoshop for brightness and contrast adjustments. Images were then placed in Adobe Illustrator to outline anatomical landmarks and create final figure layouts. To visualize neurons double-labeled with Pdyn-FISH and Pdyn-GFP, stacks of 10 images at ×200 magnification (spanning 15 μm in the z-axis) were aligned and layered in Photoshop, and in-focus light from each image was combined into a single two-dimensional image.
Data points and error bars represent means ± SE. We performed statistical comparisons between the three groups with one-way ANOVA followed by Bonferroni's multiple comparison tests using GraphPad Prism.
We performed this study to reveal qualitative patterns of neuronal labeling in the LPB and to describe large differences between conditions, not to estimate or determine the exact numbers of neurons. Thus, we did not use a stereologic procedure or apply a counting correction. Because of our conservative criteria, by which we kept false-positive counts to a minimum, these data likely represent an underestimate of the true degree of colocalization, particularly for FoxP2+ nuclei, which exhibited the lowest signal-to-background labeling among the three markers.
RESULTS
Changes in ambient temperature and thermoregulatory responses.
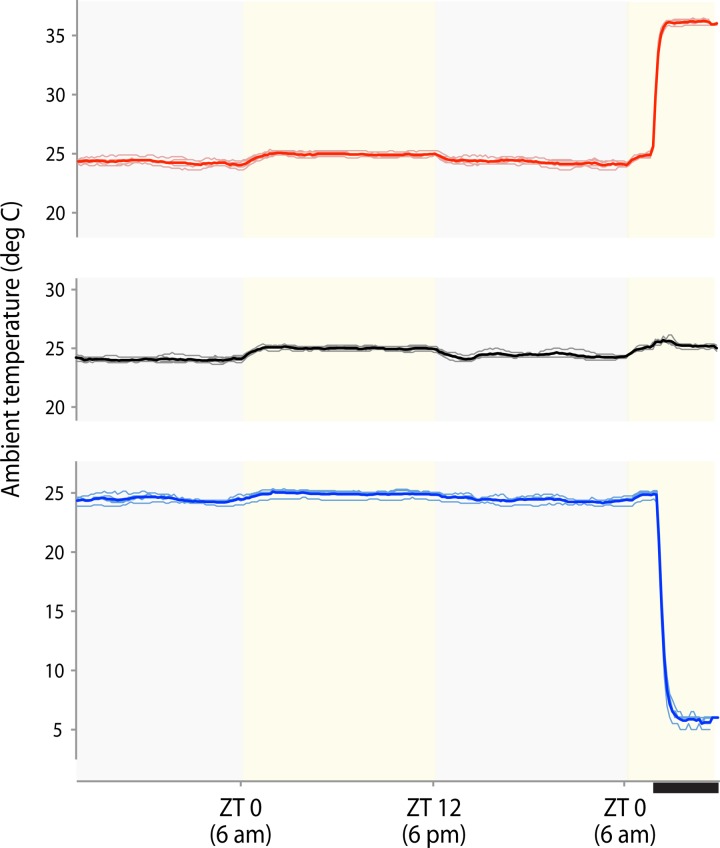
During warm or cool exposure, cage temperature reached the target temperature within 15–30 min (Fig. 1). For the remainder of the 4-h period, the temperature inside each cage stabilized within 0.5°C of the chamber set temperature in the warm group (range 35.9–36.4°C) and within 1.0–2.5°C in the cool group (range 5.0–6.5°C).
Fig. 1.
Ambient temperature trends inside individual cages from each group (n = 4 each). Thin lines denote temperatures from individual cages in each group: warm-exposed, control, and cool-exposed; thick lines represent group averages. At baseline, ambient cage temperatures remain stable near 24°C during the dark period and approach 25°C during the light period, possibly due to heat generation by fluorescent lamps along the chamber walls. Warm-exposed cage temperatures rapidly increase to 36°C for the last 4 h of the experiment. In the control group, ambient temperatures remain between 24 and 25°C. Cool-exposed cage temperatures fall quickly to 5–6°C for the final 4 h of the experiment. ZT is zeitgeber time (ZT 0 is the time when lights were turned on). The thick black bar beneath the x-axis indicates the 4-h period of ambient temperature change before perfusion.
In a group of comparison mice, these ambient temperatures produced small changes in core temperature. Over the last 30 min of the 4-h experimental period, control mice (average cage temperature 27.4°C) had an average core temperature of 35.6 ± 0.1°C, cool-exposed mice (average cage temperature 5.0°C) had a reduced core temperature of 34.7 ± 1.0°C (P < 0.01), and warm-exposed mice (average cage temperature 35.4°C) had an elevated core temperature of 37.6 ± 0.3°C (P < 0.001).
When removed for perfusion, thermal imaging revealed marked changes in distal skin temperature. Warm-exposed mice had high surface temperatures in the tail and paws, whereas cool-exposed mice had low tail and paw temperatures (Fig. 2). Surface temperatures in the head and trunk showed relatively little change across conditions.
Fig. 2.
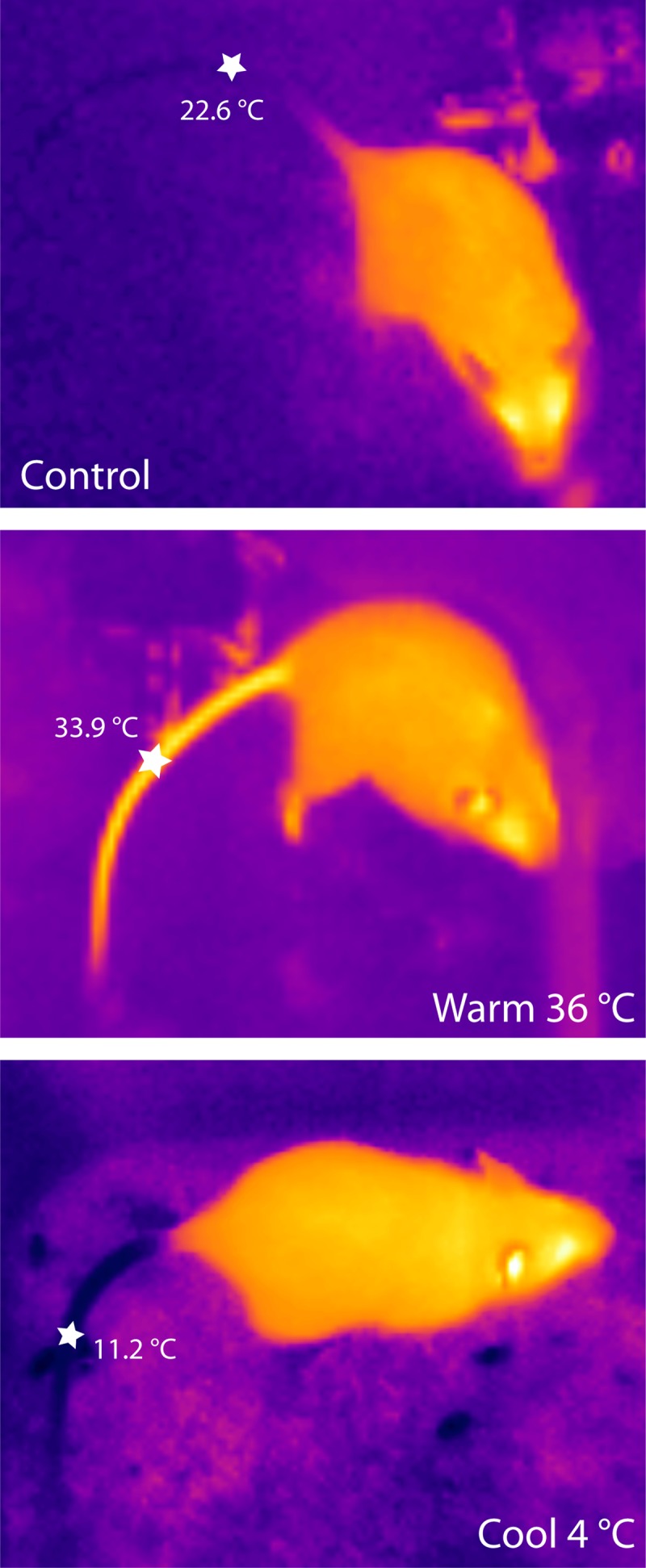
Thermal images show mice immediately after removal from a warm (36°C) or cool (4°C) ambient temperature. Images were taken at the same ambient temperature (22°C). The mid-tail temperature, a proxy for thermoregulatory vasodilation (41), is indicated by white star. Tail temperature is near room temperature in control mice, high after warm exposure, and very low after cool exposure.
At the end of the experiment, warm-exposed mice exhibited grossly normal behavior, moving about their cages and attempting to evade the experimenter like control mice. Cool-exposed mice lay awake with eyes open, curled in nesting material in the back corner of their cages, without visible shivering.
LPB anatomy: FoxP2 and Pdyn-GFP.
The distribution of neuronal nuclei with FoxP2 immunofluorescence in the LPB (Fig. 3) was similar to that in rats (18, 19, 36, 45) and similar to publicly available in situ hybridization images for foxp2 in mouse (33). In brief, a continuous population of FoxP2+ neurons stretched from a rostral cluster, which we designated the rostral-to-external lateral subnucleus (PBreL), then back through the central lateral subnucleus (PBcL), and clustered caudally in the PBdL. FoxP2 expression also was found in adjacent nuclei, including the nucleus of the lateral lemniscus and the Kolliker-Fuse nucleus (KF).
Fig. 3.
Distributions of FoxP2+ and dynorphin neurons in the lateral parabrachial nucleus (LPB). Sections span the caudal midbrain (A and B) through the rostral pons (C). Magenta nuclear labeling marks neurons that express FoxP2. Green somatic labeling (Pdyn-GFP) marks cells with Prodynorphin gene expression. These neurons constitute a small, caudal subset of the larger population of FoxP2+ neurons in the LPB. Scale bar: 200 μm. Abbreviations: Cb, cerebellum; Cun, cuneiform nucleus; KF, Kolliker-Fuse nucleus; LDT, laterodorsal tegmental nucleus; Me5, mesencephalic nucleus of the trigeminal nerve; NLL, nucleus of the lateral lemniscus; PB, parabrachial nucleus; PBcL, central lateral subnucleus; PBdL, dorsal lateral subnucleus; PBeL, external lateral subnucleus; PBlc, lateral crescent subnucleus; PBm, medial subnucleus; PBreL, rostral-to-external lateral subnucleus; scp, superior cerebellar peduncle.
In Pdyn-GFP mice, the pattern of GFP reporting (Fig. 3) was similar to publicly available in situ hybridization images for mouse Pdyn mRNA (33) and to the distribution of Pdyn mRNA in rats (35). At every level of the LPB in every reporter mouse, 90–100% of Pdyn-GFP+ neurons were also FoxP2+. Using FISH, we found Pdyn mRNA labeling in the same subnuclear distribution as GFP+ neurons, most prominently in PBdL. GFP and Pdyn mRNA colocalized in many neurons (Fig. 4). We did not find Pdyn-GFP+ neurons in brain regions that do not express Pdyn in adults or embryos, nor was Pdyn FISH signal present in any brain regions without GFP+ neurons.
Fig. 4.
Green fluorescent protein (GFP)-immunoreactivity in Pdyn-IRES-Cre reporter mice (Pdyn-GFP, green) colocalizes with Pdyn mRNA in the lateral parabrachial nucleus (LPB) (Pdyn-FISH, in red). These examples are in the caudal dorsal-lateral parabrachial nucleus (approximate bregma −5.4 mm). Arrowheads indicate neurons with prominent colocalization. Scale bar is 50 μm.
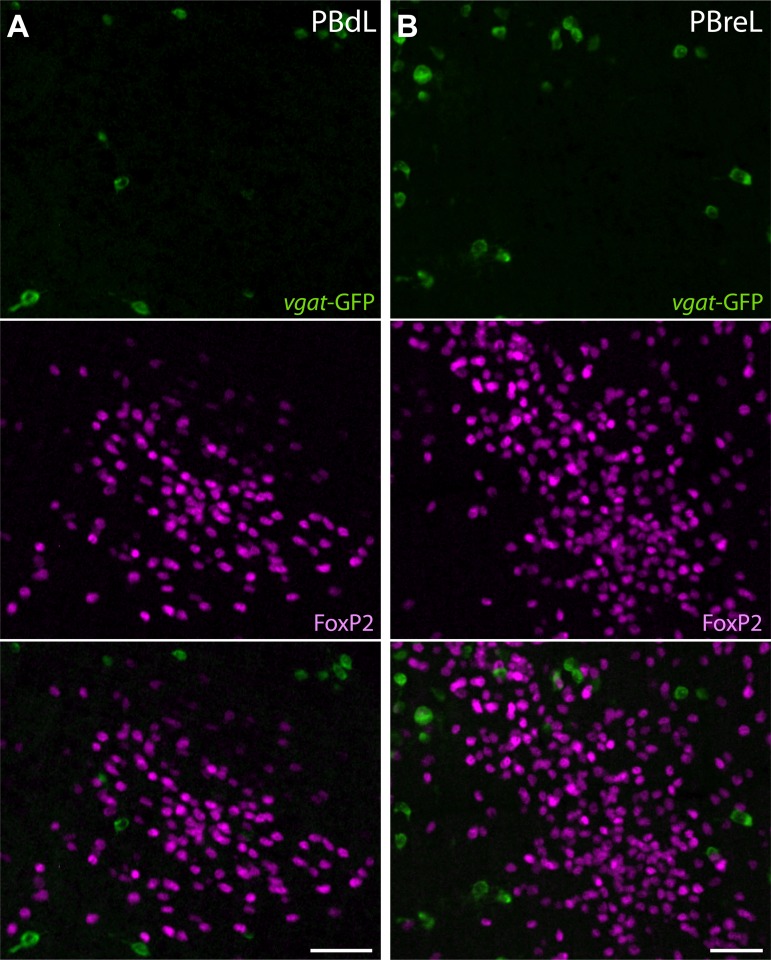
Most FoxP2+ neurons in the LPB are derived from Math1/Atoh1-expressing progenitor cells in the rhombic lip (19, 52). These neurons are probably glutamatergic because Math1/Atoh1-derived neurons here express Vglut2 (40). Likewise, in adult rats, most FoxP2+ neurons in the LPB express Vglut2 (36). In a reporter mouse for Vglut2, we found that GFP colocalized ubiquitously with FoxP2 in the LPB (Fig. 5). Conversely, in a Vgat reporter with abundant GFP in nearby neurons, FoxP2 did not colocalize with GFP in any thermosensory subregions of the LPB (Fig. 6), indicating that throughout development, thermosensory LPB neurons are never GABAergic.
Fig. 6.
FoxP2+ neurons in PBdL and PBreL are not GABAergic. A: in a Vgat-GFP reporter mouse, GFP does not colocalize with FoxP2 immunoreactivity in PBdL (bregma −5.3 mm). B: likewise, FoxP2 and GFP do not colocalize in any neurons of PBreL (bregma −5.0 mm). Scale bars: 50 μm.
The ventrolateral margin of the caudal LPB contains a small subpopulation of FoxP2+ neurons that may produce GABA (Vgat-GFP+; Vglut2-GFP-; FoxP2+; not shown). These atypical FoxP2+ neurons at the ventrolateral margin of the LPB (labeled in Fig. 3C as “PBlc-KF”) are probably Ptf1a-derived similar to cerebellar Purkinje cells (see Fig. 4A in Ref. 19). These neurons lie outside the bounds of LPB subnuclei that receive thermosensory information from the spinal cord and project to the hypothalamus, so they are not relevant to the present study.
c-Fos expression after warm or cool exposure.
Over the entire LPB, the total numbers of FoxP2+ and Pdyn-GFP+ neurons were similar between control, warm-exposed, and cool-exposed mice (P = 0.9 for Pdyn-GFP; P = 0.6 for FoxP2). There were no significant differences in these cell counts at any rostrocaudal (bregma) level (not shown). Their patterns of c-Fos expression, however, were distinct.
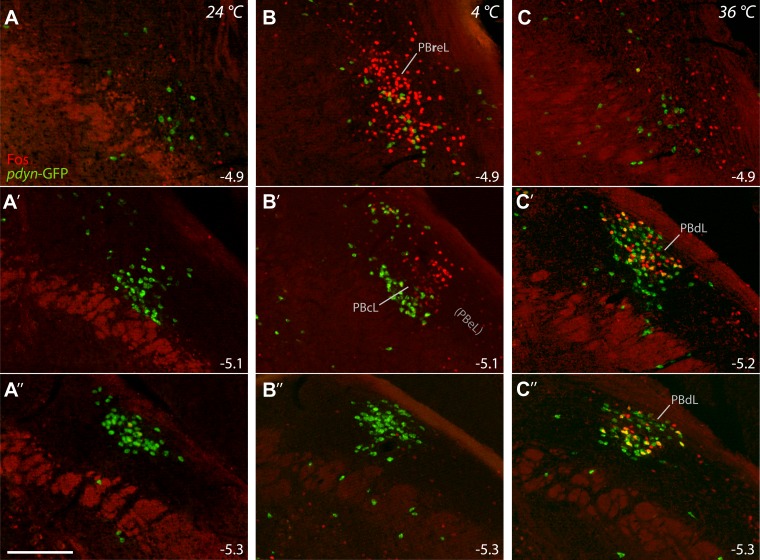
24°C controls.
Control mice had few c-Fos+ neurons in the LPB (Fig. 7A). At caudal levels (bregma −5.3 and −5.4), roughly half of the c-Fos+ neurons were Pdyn-GFP+, but they accounted for just 5 ± 1% of Pdyn-GFP+ neurons at these levels.
Fig. 7.
LPB neurons express c-Fos in distinct patterns after exposure to cool or warm ambient temperature. c-Fos immunofluorescence (red) and Pdyn-GFP fluorescence (green) are shown at three rostrocaudal levels of the LPB. A: control mice (24°C) have minimal c-Fos throughout the LPB. B: cool-exposed mice (4°C) express c-Fos in the far-rostral LPB, clustering in PBreL. Cool-activated, Fos+ neurons extend caudally into a rostral, lateral portion of the PBcL, as shown in B′, but are absent at caudal levels. C: Warm-exposed mice (36°C) express c-Fos primarily at caudal levels of the LPB. c-Fos labeling in warm-exposed mice colocalizes with and overlaps the dense cluster of Pdyn-GFP+ neurons in PBdL. Approximate bregma level (in mm) is indicated in each panel. Scale bar: 200 μm.
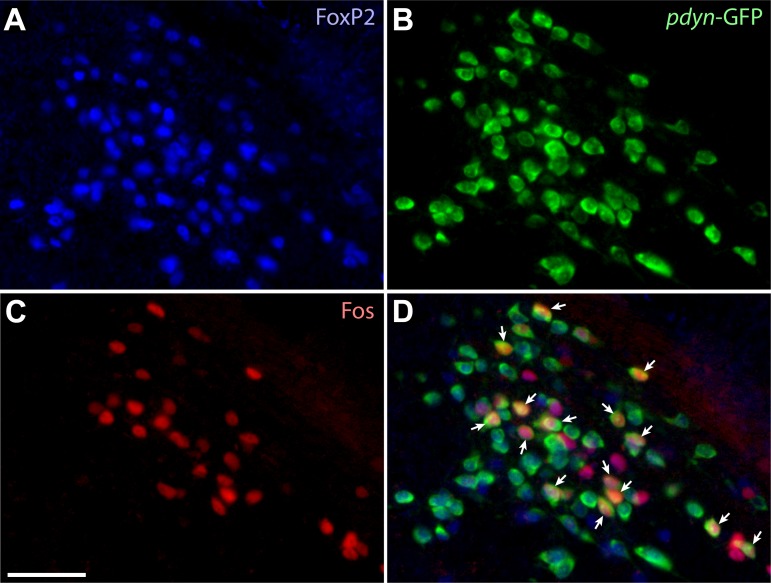
4°C cool exposure.
Cool exposure greatly increased the number of c-Fos+ neurons in the far-rostral LPB. Specifically, every cool-exposed mouse had a cluster of intense c-Fos labeling just rostral to the rostral tip of PBeL (Fig. 7C; Fig. 8). 68 ± 2% of c-Fos+ neurons in the rostral LPB expressed FoxP2 in these cases. We named this population of FoxP2+ cool-activated neurons the PBreL to distinguish it from the FoxP2-negative neurons located caudally in the external lateral subnucleus (PBeL). Cool exposure also induced bright c-Fos expression in a small number of neurons trailing one or two levels behind this cluster, where it wraps around PBeL caudally and merges into the rostral extent of PBcL (Fig. 7C′).
Fig. 8.
Cool-exposed (4°C) mice express c-Fos in many FoxP2+ neurons in the far-rostral LPB, primarily in the PBreL. Roughly half of the FoxP2+ neurons in PBreL are cool-activated (Fos+). This rostral region of the LPB contains relatively few Pdyn-GFP+ neurons, but some were c-Fos+ (arrowheads). Approximate bregma level is −4.9 mm. Scale bar: 50 μm.
In the rostral LPB overall (bregma levels −4.9 to −5.1), cool-exposed mice had significantly more c-Fos+ neurons (695 ± 76) than controls (208 ± 111; P < 0.01). The total c-Fos+ neurons at these rostral LPB levels did not differ significantly from warm-exposed mice (441 ± 47; P > 0.05). However, specifically among FoxP2+ neurons, which are clustered mainly in PBreL at these levels, substantially more were c-Fos+ in cool-exposed (476 ± 60) than in warm-exposed (148 ± 24; P = 0.001) or control mice (53 ± 18; P < 0.001).
Most cool-activated neurons were not Pdyn-GFP+ (see Fig. 7C). Among the Pdyn-GFP+ neurons scattered at rostral LPB levels (bregma levels −4.9 to −5.1), significantly more were c-Fos+ in cool-exposed (33 ± 6%) than in warm-exposed (10 ± 2%, P < 0.01) or control mice (6 ± 3%, P < 0.001 vs. cool-exposed). At these rostral levels, Pdyn-GFP+ neurons constituted just 15% of c-Fos+ neurons. At caudal levels containing the densely clustered Pdyn-GFP+ neurons in PBdL, only 6 ± 3% of Pdyn-GFP+ neurons were c-Fos+ in cool-exposed mice, similar to controls (7 ± 2%; P > 0.05).
36°C warm exposure.
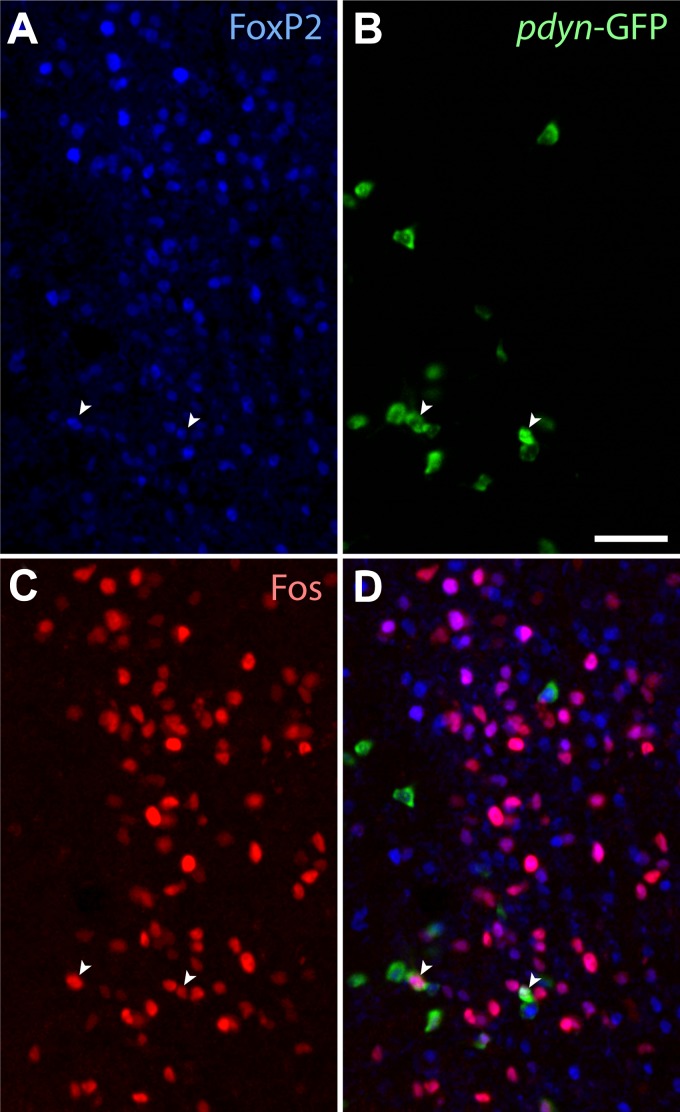
Warm exposure prominently increased c-Fos in the caudal LPB, producing a dense cluster of intense c-Fos+ neurons in PBdL (Fig. 7B′–B′′). At caudal bregma levels (−5.3 and −5.4 mm), most c-Fos+ neurons were FoxP2+ (67 ± 4%). These c-Fos+ neurons overlapped the cluster of Pdyn-GFP+ neurons in PBdL, and half of all c-Fos+ neurons at these levels were Pdyn-GFP+ (49 ± 11%). The percentage of Pdyn-GFP+ neurons containing c-Fos in the caudal LPB was higher in warm-exposed (27 ± 2%) than control (7 ± 2%, P < 0.001) and cool-exposed mice (6 ± 3%, P < 0.001). Many PBdL neurons were triple-labeled for Pdyn-GFP, FoxP2, and c-Fos (Fig. 9).
Fig. 9.
Warm-exposed (36°C) mice express c-Fos in FoxP2+, Pdyn-GFP+ neurons in PBdL. Triple-labeled, warm-activated neurons are indicated by white arrows. Most c-Fos+ neurons lacking Pdyn-GFP still express FoxP2 (magenta nuclei). Bregma level is approximately −5.4 mm. Scale bar: 50 μm.
36°C exposure after preoptic CTb injection.
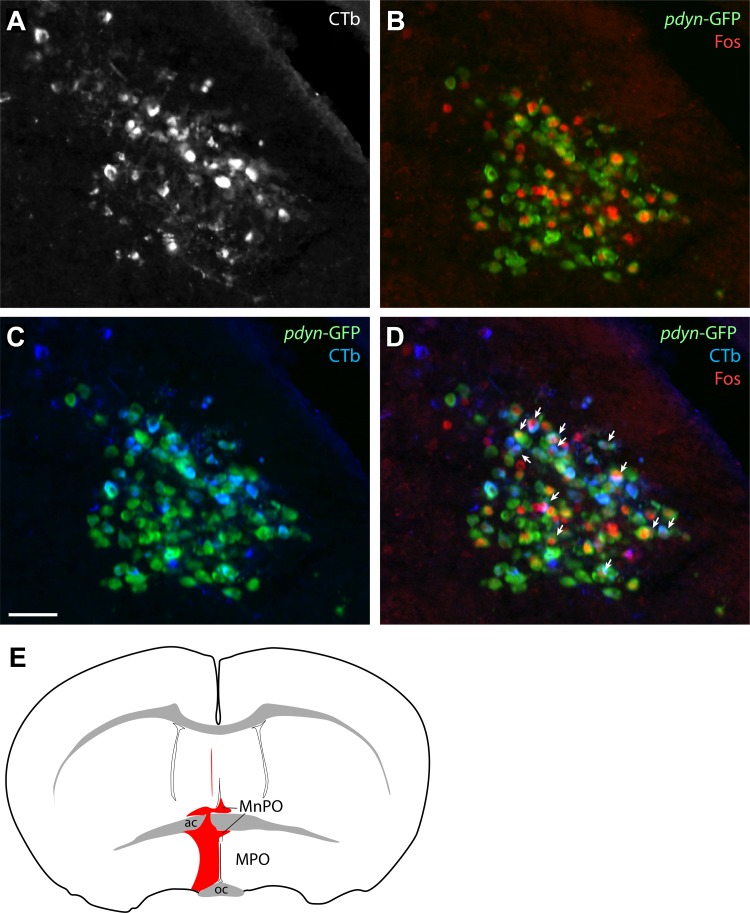
To determine whether warm-activated, Pdyn-GFP+ neurons in PBdL project to the preoptic area, we injected CTb into the preoptic area and examined the pattern of retrograde labeling in LPB in warm-exposed mice. Overall, retrogradely labeled neurons were located in an LPB distribution similar to that described in rats (8, 26, 37, 38, 42) in primarily FoxP2+ subnuclei (PBdL, PBcL, and PBreL). Every Pdyn-GFP+ neuron labeled with CTb was FoxP2+. After warm exposure, in the case shown in Fig. 10, 31% of PBdL Pdyn-GFP+ neurons were c-Fos+, similar to the warm-exposed cases described above. More than half of these c-Fos+, Pdyn-GFP+ neurons were retrogradely labeled with CTb (55% triple-labeled). Conversely, among all the CTb-labeled neurons in PBdL, 49% were c-Fos+, and 83% of those (CTb+, c-Fos+) were also Pdyn-GFP+ (triple-labeled).
Fig. 10.
Warm-activated dynorphin neurons in PBdL project to the preoptic area. A: after cholera toxin subunit B (CTb) injection into the medial preoptic area (MPO), many PBdL neurons are retrogradely labeled (white). B: 36°C warm exposure produces abundant c-Fos (red) in Pdyn-GFP+ and intermingled neurons in PBdL. C: CTb (blue) labels many Pdyn-GFP+ neurons projecting to the preoptic area. D: many PBdL neurons are triple-labeled with CTb, Pdyn-GFP, and c-Fos, indicating that they are dynorphinergic, warm-activated, and project to the preoptic area. Arrows indicate triple-labeled neurons. E: CTb injection site involved both the MPO and the median preoptic nucleus (MnPO). Abbreviations: ac, anterior commissure; oc, optic chiasm.
DISCUSSION
This study provides several insights into the genetic and functional organization of the parabrachial nucleus. First, most thermosensory relay neurons in the LPB, including those projecting to the preoptic area, are FoxP2+. This indicates that they are Math1/Atoh1-derived glutamatergic neurons with a developmental origin, connections, and functions distinct from other neurons in this region. Their subnuclear distribution in the mouse is similar to that in rat, with cool-activated neurons clustered rostrally and warm-activated neurons caudally (Fig. 11). Second, cool-thermosensory neurons are located mainly in PBreL, which is distinct from PBeL. Third, many warm-activated PBdL neurons may release dynorphin in addition to glutamate in their efferent target sites, including the preoptic area.
Limitations.
Cre-reporter mice allow us to visualize cells that we cannot label reliably using antibodies (e.g., Vglut2- or Vgat-expressing somata) or for which immunolabeling would require toxic pretreatment with colchicine (e.g., Pdyn-expressing somata in the LPB). Cre-reporting is also a practical assay for learning which cells we can access and manipulate for physiological and behavioral experiments in a given Cre-driver mouse. However, Cre-reporters can “over-report” adult gene expression by permanently labeling cell lineages that expressed the Cre driver gene during development, yet no longer express it in the adult. For example, Pdyn-IRES-Cre reporter mice have many GFP+ neurons in the anteromedial thalamic nucleus (see supplemental Fig. 5 in Ref. 31), but neurons in this nucleus do not express Pdyn mRNA in adult mice (our unpublished observations and see Ref. 35). Conversely, Cre-reporters can sometimes “under-report” gene expression due to inefficiencies in Cre-lox recombination (34, 43). The following observations mitigate these concerns in this study: 1) Pdyn-GFP+ neurons in PBdL express Pdyn mRNA in adult mice; 2) Vglut2-GFP ubiquitously colocalizes with FoxP2 in our region of interest (arguing against underreporting for Vglut2); and 3) Vgat-GFP did not colocalize with FoxP2 in this region (arguing against overreporting of Vgat).
Another potential limitation is our use of c-Fos as a marker for neuronal activity. In most neurons, c-Fos is a robust indicator, with well-established kinetics (29). However, the absence of c-Fos does not imply inactivity, so it remains possible that additional neurons relay thermosensory information to the forebrain. However, warm- and cool-induced c-Fos in the LPB appears in a reliable pattern across laboratories and across rodent species and, more importantly, these are the same two LPB subregions that contain experimentally proven neurons signaling warming and cooling of the skin (5, 37, 38).
Next, although we confirmed that the LPB contains warm- and cool-activated neurons, in this study we did not confirm the source of signals leading to their activation. Warming and cooling the skin—without changing core body temperature—activate neurons in these same LPB subnuclei (37, 38), which receive ascending signals directly from the spinal cord (14), arguing that skin thermosensors, relaying through the spino-parabrachio-thalamic tract, drive c-Fos activity in LPB neurons. However, we cannot exclude the possibility that ascending vagal or descending hypothalamic projections deliver additional information representing core (visceral) or brain temperature or visceral signals related to osmotic or cardiovascular 'changes.
Genetic markers for LPB thermosensory neurons.
The PB contains many intermixed subnuclei, each connecting to separate brain regions that regulate distinct homeostatic functions. Because of their overlapping distributions, classic techniques for stimulating or inhibiting PB neurons alter the activity of multiple, functionally distinct brain circuits. New techniques like chemogenetics (49) and optogenetics (12) offer the possibility of studying an individual population of genetically distinct neurons without disturbing intermixed neurons having unrelated connections and functions.
Tapping the full potential of these techniques requires that we identify genetic markers for each subpopulation of PB neurons. Most PB neurons are glutamatergic, so a Cre mouse for a broadly expressed gene like Vglut2 offers little specificity over nonselective approaches. Neuropeptide markers like dynorphin, CGRP (6), and CCK (16) demarcate small subpopulations in the LPB, but these genes, even in combination, leave the majority of PB neurons inaccessible for study.
Another option beyond neuropeptide markers is to decipher the developmental-genetic hierarchy of PB neurons. For example, the transcription factors FoxP2 and Lmx1b subdivide the PB into three large, genetically distinct subpopulations (18, 19, 36). FoxP2 and Lmx1b are mutually exclusive in all subnuclei except the KF and lateral crescent nuclei, which express both (36). Many neuropeptide markers map onto one population or the other, as confirmed here for dynorphin (FoxP2+) and shown previously for CGRP (Lmx1b+) in the rat (36).
Most thermosensory relay neurons belong to the Math1/Atoh1, rhombic lip-derived subpopulation of FoxP2+ LPB neurons. That cool-activated neurons express FoxP2 is significant because these neurons were previously labeled as “LPBeL” (37, 38), yet FoxP2 is not expressed in PBel. Rather, PBel contains Lmx1b+ neurons, which receive viscerosensory input from the medulla and project to the extended amygdala. In contrast, the FoxP2+ neurons rostral to PBeL receive input from the spinal cord and project primarily to the diencephalon (45).
We have not yet identified a marker gene that distinguishes these cool-activated neurons in PBreL from the FoxP2+ neurons trailing it caudally in PBcL. At this time, PBreL neurons are distinguished from more caudal FoxP2 neurons only by their more rostral location and their phasic c-Fos expression during cool exposure. Genetic targeting techniques based on neuronal activity, such as fos-cre (21), might allow selective access to cool-relay neurons, but techniques based on connectivity (e.g., retrograde AAV-Cre delivery from an efferent target site) are unlikely to work because cool- and warm-thermosensory LPB neurons both project to the same region within the preoptic hypothalamus (38). Identifying a constitutively expressed genetic marker to distinguish PBreL from other FoxP2 LPB neurons would enable more selective experiments in the future.
Similarly, additional markers for warm-activated LPB neurons, beyond FoxP2 and Pdyn, could be helpful because intermingled neurons may relay other spinothalamic sensory modalities like pain and possibly itch. Many dynorphin neurons in PBdL appear to receive functional, warm-thermosensory input based on our c-Fos results, and unit recordings in PBdL confirmed that most warm-sensitive neurons in rats encode thermosensory information, not pain (38). However, many Pdyn-expressing PBdL neurons in rats also produced c-Fos after formalin injection into the hindpaw, a model of inflammatory pain (26). This finding was interpreted as evidence that dynorphin neurons relay pain, though an alternative possibility is that hindpaw vasodilation due to formalin-induced inflammation warmed the skin, activating warm-thermosensory input to PBdL dynorphin neurons. If PBdL dynorphin neurons relay signals related to nociception in addition to warmth, future studies could examine whether they integrate these sensory signals or transmit them in separate pathways.
Dynorphinergic, warm-thermosensory neurons in PBdL.
Our initial interest in PBdL stemmed from convergent patterns of Pdyn, c-Fos, and other findings here. First, dynorphin peptide (50) and Pdyn expression (35) revealed a prominent cluster of dynorphin neurons here in the rat. Second, retrograde tracing from the preoptic area revealed a similar distribution (8, 15, 38, 42), including many Pdyn+ neurons (Ref. 26 and present data). Third, c-Fos often appeared spontaneously in PBdL in control rats; this occurred most frequently when mice were perfused shortly after lights-on, when they normally fall asleep (see, for example, Fig. 15C in Ref. 17; see also Fig. 7A in Ref. 36). Fourth, injecting dynorphin directly into the preoptic area in rats produced a warm defense-like thermoregulatory response (core body temperature fell 1–2°C), and it increased sleep; both effects were mediated by the κ-opioid receptor (20, 55). PBdL is the main source of dynorphinergic input to this region of the hypothalamus (20), yet the modality of information it transmitted has remained unclear until now. It did not make sense that PBdL dynorphin neurons, relaying pain-related information (26), would be spontaneously active in sleeping animals, or that dynorphin release should decrease core body temperature or increase sleep. Thus, when warm-thermosensory relay neurons were identified in this location (38), we refocused our ideas about the function of PBdL dynorphin neurons.
We provide evidence that roughly one-half of PBdL neurons activated by warm ambient temperature express Pdyn, including 80% of those projecting to the preoptic area. These warm-activated, preoptic-projecting, dynorphin neurons in PBdL probably influence thermoregulation, although it remains possible that the other half of warm-activated PBdL neurons (c-Fos+; Pdyn-) subserves thermoregulation, and warm-activated dynorphin neurons serve some other purpose. Either way, these neurons probably promote other behaviors that are influenced by warm ambient temperature, including sleep.
Warm ambient temperature and warm baths promote sleep (22, 30). At sleep onset, the skin warms to several degrees above waking temperatures due to cutaneous vascular dilation. This robust phenomenon predicts sleep onset better than any other autonomic parameter and persists until waking (32). In humans, the foot exhibits the greatest sleep-wake variation in skin temperature (32), so it may be important that PBdL neurons in rodents receive input primarily from the lumbar spinal cord (14). We hypothesize that prior to and throughout sleep, skin warming in the hindlimbs activates peripheral thermosensors, which, via lumbar spino-parabrachial projections, activate warm-relay neurons in PBdL. We have seen robust c-Fos expression in Pdyn-GFP+ PBdL neurons at ambient temperatures as low as 31°C (not shown), which is well within the range of distal skin temperatures recorded during sleep. This distal skin warming during sleep may explain the previously mysterious, spontaneous c-Fos activity reported in PBdL neurons in sleeping control rats (17).
Further, in line with their “warm-defense” autonomic role (38), we predict that dynorphin neurons in PBdL may also play a permissive role in stabilizing sleep. During sleep, the skin radiates heat from the core to the environment, causing core body temperature to fall 1°C or more. PBdL dynorphin neurons might help promote sleep only so long as distal skin temperature remains above a safe threshold (and cool-activated PBreL neurons may promote arousal from sleep if it falls below this threshold), guarding against excessive heat loss that would cause a dangerous drop in core body temperature. A warm skin-driven increase in PBdL dynorphin neuron activity and the known hypnotic effect of dynorphin in the preoptic area (20) might explain the potent, sleep-promoting effect of warm ambient temperature (22) in humans.
Whatever their physiological effects, further experiments are needed to understand the information conveyed by PBdL dynorphin neurons to target sites in the hypothalamus. Many of these sites contain κ-opioid receptor (KOR), a Gi-coupled inhibitory receptor that responds selectively to dynorphin and mediates the effects of dynorphin on thermoregulation and sleep (20, 55). Depending on the location of its receptor, dynorphin can produce presynaptic or postsynaptic inhibition (28). Further, PBdL neuron release of dynorphin probably occurs in combination with a fast-excitatory transmitter (glutamate), so the net effect of PBdL neuron activity in vivo may be complex and could vary from site to site, depending on KOR presence and location (presynaptic or postsynaptic). It may seem paradoxical that neurons would release both a fast-excitatory transmitter and a slow-inhibitory neuropeptide, but this is not unprecedented (53). For example, glutamatergic orexin/hypocretin neurons in the hypothalamus also produce dynorphin (9). Further experiments should determine whether PBdL neurons corelease glutamate and dynorphin and what effects this produces in each target region, beginning with the preoptic area.
Perspectives and Significance
The mouse lateral parabrachial nucleus contains two clusters of neurons that relay skin-temperature information from the spinal cord to the hypothalamus. Most of these neurons express the transcription factor FoxP2 and are glutamatergic. Cool-relay neurons are located rostrally, in PBreL, and most do not produce dynorphin. In contrast, warm-relay neurons are located caudally in PBdL; roughly half of them produces dynorphin, and many project to the preoptic area. This information advances our understanding of the functional and genetic organization of LPB neurons and provides a foundation for using Pdyn-IRES-Cre mice to access and manipulate warm-thermosensory relay neurons in PBdL.
GRANTS
This study was supported by National Institutes of Health Grants: National Institute of Neurological Disorders and Stroke R25 NS070682 (to J. C. Geerling), National Heart, Lung, and Blood Institute P01 HL095491, National Institute of Dental and Craniofacial Research R01 DE022912 (T. E. Scammell), and NIH-T32 AG000222 (to C. E. Mahoney).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.G. conception and design of research; J.C.G., C.E.M., S.B.A., L.J.A., and A.S.G. performed experiments; J.C.G. and M.Y.K. analyzed data; J.C.G. interpreted results of experiments; J.C.G. prepared figures; J.C.G. drafted manuscript; J.C.G., C.E.M., S.B.A., L.J.A., and T.E.S. edited and revised manuscript; J.C.G., M.Y.K., C.E.M., S.B.A., L.J.A., A.S.G., M.J.K., B.B.L., and T.E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Martin Fisher for sharing time in the Maratos-Flier lab environmental chamber at BIDMC, as well as the Lowell lab for generously sharing time on Dr. Lowell's slide-scanner microscope. We thank David Olsen for generating L10-GFP mice and Linh Vong for generating Vglut2- and Vgat-IRES-Cre mice. Dave Strochlic generated and provided the riboprobe plasmid for Pdyn. Intellectual input and feedback from Kazuhiro Nakamura, Paul Gray, Arthur Loewy, Clifford Saper, and Nancy Chamberlain were helpful in guiding our understanding of PB anatomy and physiology.
REFERENCES
- 1.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63: 473–490, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol 353: 480–505, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bester H, Chapman V, Besson JM, Bernard JF. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J Neurophysiol 83: 2239–2259, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Blair ML, Mickelsen D. Activation of lateral parabrachial nucleus neurons restores blood pressure and sympathetic vasomotor drive after hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol 291: R742–R750, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bratincsák A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience 127: 385–397, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Carter ME, Han S, Palmiter RD. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci 35: 4582–4586, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci 22: 977–990, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21: RC168, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci 26: 303–307, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Davern PJ. A role for the lateral parabrachial nucleus in cardiovascular function and fluid homeostasis. Front Physiol 5: 436, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deisseroth K. Optogenetics. Nat. Methods 8: 26–29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci 17: 2212–2218, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol 353: 506–528, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res 319: 229–259, 1984. [DOI] [PubMed] [Google Scholar]
- 16.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab 20: 1030–1037, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geerling JC, Loewy AD. Sodium deprivation and salt intake activate separate neuronal subpopulations in the nucleus of the solitary tract and the parabrachial complex. J Comp Neurol 504: 379–403, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Geerling JC, Stein MK, Miller RL, Shin JW, Gray PA, Loewy AD. FoxP2 expression defines dorsolateral pontine neurons activated by sodium deprivation. Brain Res 1375: 19–27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J Appl Physiol 104: 1513–1521, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Greco MA, Fuller PM, Jhou TC, Martin-Schild S, Zadina JE, Hu Z, Shiromani P, Lu J. Opioidergic projections to sleep-active neurons in the ventrolateral preoptic nucleus. Brain Res 1245: 96–107, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guenthner CJ, Miyamichi K, Yang HH, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78: 773–784, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskell EH, Palca JW, Walker JM, Berger RJ, Heller HC. The effects of high and low ambient temperatures on human sleep stages. Electroencephalogr Clin Neurophysiol 51: 494–501, 1981. [DOI] [PubMed] [Google Scholar]
- 23.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135: 738–748, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Hermanson O, Blomqvist A. Subnuclear localization of FOS-like immunoreactivity in the rat parabrachial nucleus after nociceptive stimulation. J Comp Neurol 368: 45–56, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Hermanson O, Telkov M, Geijer T, Hallbeck M, Blomqvist A. Preprodynorphin mRNA-expressing neurones in the rat parabrachial nucleus: subnuclear localization, hypothalamic projections and colocalization with noxious-evoked fos-like immunoreactivity. Eur J Neurosci 10: 358–367, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Héron A, Traiffort E, Allix M, Dimitriadou V, Schwartz JC. Prodynorphin mRNA expression in the rat dentate gyrus after cerebral ischemia. Neuropeptides 30: 355–358, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol 85: 1153–1158, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14: 173–213, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Horne JA, Reid AJ. Night-time sleep EEG changes following body heating in a warm bath. Electroencephalogr Clin Neurophysiol 60: 154–157, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature 401: 36–37, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Willet SG, Bankaitis ED, Xu Y, Wright CVE, Gu G. Non-parallel recombination limits Cre-LoxP-based reporters as precise indicators of conditional genetic manipulation. Genesis 51: 436–442, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merchenthaler I, Maderdrut JL, Cianchetta P, Shughrue P, Bronstein D. In situ hybridization histochemical localization of prodynorphin messenger RNA in the central nervous system of the rat. J Comp Neurol 384: 211–232, 1997. [PubMed] [Google Scholar]
- 36.Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD. Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience 218: 110–125, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107: 8848–8853, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2008. [Google Scholar]
- 40.Rose MF, Ahmad KA, Thaller C, Zoghbi HY. Excitatory neurons of the proprioceptive, interoceptive, and arousal hindbrain networks share a developmental requirement for Math1. Proc Natl Acad Sci USA 106: 22,462–22,467, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289: R1244–R1252, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res 288: 21–31, 1983. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol 8: 665–668, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Sharifi N, Ament M, Brennan MB, Hochgeschwender U. Isolation and characterization of the mouse homolog of the preprodynorphin (Pdyn) gene. Neuropeptides 33: 236–238, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat 42: 1–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverstein A. Pontine infarction (chap. 2) and Primary pontine hemorrhage (chap. 3). In: Vascular Diseases of the Nervous System, Part II, edited by Vinken PJ, Bruyn GW. Handbook of Clinical Neurology. Amsterdam: North-Holland Publishing, 1972, p. 13–53. [Google Scholar]
- 47.Simerly RB. Prodynorphin and proenkephalin gene expression in the anteroventral periventricular nucleus of the rat: Sexual differentiation and hormonal regulation. Mol Cell Neurosci 2: 473–484, 1991. [DOI] [PubMed] [Google Scholar]
- 48.Spector AC, Norgren R, Grill HJ. Parabrachial gustatory lesions impair taste aversion learning in rats. Behav Neurosci 106: 147–161, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37: 387–407, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Vincent SR, Hökfelt T, Christensson I, Terenius L. Dynorphin-immunoreactive neurons in the central nervous system of the rat. Neurosci Lett 33: 185–190, 1982. [DOI] [PubMed] [Google Scholar]
- 51.Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71: 142–154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48: 31–43, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature 362: 423–427, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483: 594–597, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xin L, Geller EB, Adler MW. Body temperature and analgesic effects of selective mu and kappa opioid receptor agonists microdialyzed into rat brain. J Pharmacol Exp Ther 281: 499–507, 1997. [PubMed] [Google Scholar]