This paper clarifies intrinsic mechanisms of cardiac remodeling in Marfan syndrome. The mouse model of Marfan syndrome caused by fibrillin-1 haploinsufficiency develops an early onset of cardiac remodeling not associated with valvular abnormalities. With age, cardiac remodeling progresses into two distinct phenotypes, characterized either by concentric hypertrophy or dilatation.
Keywords: fibrillin-1, Marfan syndrome, myocardial hypertrophy, cardiac function
Abstract
Marfan syndrome (MFS) is a systemic disorder of connective tissue caused by mutations in fibrillin-1. Cardiac dysfunction in MFS has not been characterized halting the development of therapies of cardiac complication in MFS. We aimed to study the age-dependent cardiac remodeling in the mouse model of MFS FbnC1039G+/− mouse [Marfan heterozygous (HT) mouse] and its association with valvular regurgitation. Marfan HT mice of 2–4 mo demonstrated a mild hypertrophic cardiac remodeling with predominant decline of diastolic function and increased transforming growth factor-β canonical (p-SMAD2/3) and noncanonical (p-ERK1/2 and p-p38 MAPK) signaling and upregulation of hypertrophic markers natriuretic peptides atrium natriuretic peptide and brain natriuretic peptide. Among older HT mice (6–14 mo), cardiac remodeling was associated with two distinct phenotypes, manifesting either dilated or constricted left ventricular chamber. Dilatation of left ventricular chamber was accompanied by biochemical evidence of greater mechanical stress, including elevated ERK1/2 and p38 MAPK phosphorylation and higher brain natriuretic peptide expression. The aortic valve regurgitation was registered in 20% of the constricted group and 60% of the dilated group, whereas mitral insufficiency was observed in 40% of the constricted group and 100% of the dilated group. Cardiac dysfunction was not associated with the increase of interstitial fibrosis and nonmyocyte proliferation. In the mouse model fibrillin-1, haploinsufficiency results in the early onset of nonfibrotic hypertrophic cardiac remodeling and dysfunction, independently from valvular abnormalities. MFS heart is vulnerable to stress-induced cardiac dilatation in the face of valvular regurgitation, and stress-activated MAPK signals represent a potential target for cardiac management in MFS.
NEW & NOTEWORTHY
This paper clarifies intrinsic mechanisms of cardiac remodeling in Marfan syndrome. The mouse model of Marfan syndrome caused by fibrillin-1 haploinsufficiency develops an early onset of cardiac remodeling not associated with valvular abnormalities. With age, cardiac remodeling progresses into two distinct phenotypes, characterized either by concentric hypertrophy or dilatation.
marfan syndrome (mfs) is an autosomal-dominant systemic connective-tissue disorder affecting ∼1 in 5,000 to 1 in 10,000 people, caused by heterozygous (HT) mutations in the gene encoding fibrillin-1 (FBN-1) (26). FBN-1 is a 350-kDa calcium binding matrix glycoprotein that assembles to form 10- to 12-nm myofibrils in the extracellular matrix (ECM). This protein has two key physiological functions: participating in the assembly of specialized matrices that define structural properties of connective tissue, and providing extracellular control for transforming growth factor (TGF)-β and bone morphogenic growth factors signaling (8a). There are a number of skeletal and ocular symptoms characteristic of MFS, ranging from joint supermobility to abnormally flat cornea to scoliosis to bone overgrowth (4). The most important cardiovascular manifestation consists of aortic root dilatation and subsequent predisposition for aortic root dissection and/or rupture, the later being a leading cause of morbidity and mortality in patients with MFS. Other cardiovascular manifestations of MFS include mitral valve prolapse, calcification of the mitral valve annulus, dilatation of the main pulmonary artery, and dilatation or dissection of the descending aorta (3, 21, 25). Vascular manifestations of MFS stem from overall abnormality in the homeostasis of the ECM, in which reduced or mutated forms of FBN-1 lead to alterations in the mechanical properties of tissues, cell-matrix interactions, and impaired hemodynamic load sensing (24, 28, 34, 38). Affected tissues have a signature of increased TGF-β signaling with increased phosphorylation and nuclear translocation of the TGF-β receptor-activated SMAD proteins (SMAD2 and SMAD3), increased expression of TGF-β-responsive gene products, such as collagen, connective tissue growth factor, and plasminogen activator inhibitor-1 and/or increased activation of noncanonical TGF-β signaling cascades, including mitogen-activated protein kinase 42/44 [extracellular regulated kinase (ERK) 1/2] (2, 5, 10, 18, 23).
The abnormality of the extracellular connective tissue matrix and matrix-cardiomyocyte interaction associated with FBN-1 mutation may affect the cardiac muscle. FBN-1 fibers are oriented in the longitudinal axis of cardiomyocytes and play an important role in transmitting contractile forces from myocytes to the extracellular connective tissue framework (3). An impaired force transmission and consequent decreased tensile strength of the myocardium associated with FBN-1 mutation may lead to compensatory neurohumoral activation to support cardiac output and, eventually, to a hypertrophic remodeling. Left ventricular (LV) enlargement observed by echocardiography had been reported in a mouse model of MFS (30). However, cardiac manifestations were described in context of abnormal mitral valve morphology, significant mitral regurgitation, and/or aortic valve insufficiency, resulting in hemodynamic overload imposed on LV. Whether cardiac remodeling, adaptive or maladaptive, could develop due to FBN-1 mutation independently from hemodynamic overload remains unclear. We examined the age-dependent pathophysiological consequences of FBN-1 deficiency on LV remodeling and cardiac function in a mouse model of MFS. Analysis of systolic and diastolic LV functions, as well as assessment of cardiac remodeling were performed in FbnC1039G+/− mouse (Marfan HT mouse) harboring a cbEGF domain cysteine substitution (C1039G) in an endogenous allele (16, 29). The FbnC1039G+/− mouse model manifests the effects of HT missense mutations, analogous to the clinical manifestations in affected humans.
MATERIALS AND METHODS
Transgenic mice.
A male pair of Marfan mice, provided by Dr. Dietz, Johns Hopkins School of Medicine, had C57 background. Wild-type (WT) mice and mice HT for the C1039G mutation (FbnC1039G+/−, Marfan HT mice) were studied at two age groups: 2–4 mo and 6–14 mo of age. Both male and female mice in 50%/50% proportion were used. Experiments were conducted in accordance with the National Institutes of Health guidelines and with the National Institute on Aging Animal Care and Use Committee approval.
Echocardiography.
Marfan HT mice and age-matched WT littermates of different age groups were assessed in vivo by transthoracic echocardiography using a probe with a center frequency of 30 MHz (Visualsonics, Toronto, Ontario, Canada). The mice were placed under anesthesia using isoflurane (2.5% in oxygen) and maintained in the supine position on the heated pad. Measurements of aortic root diameter were made at systole in parasternal long-axis view at sinus level. The heart was imaged in the two-dimensional mode (M-mode) in the parasternal long-axis view at the plane of the aorta and mitral valve with visualization of the LV apex. The M-mode tracings of the LV were obtained and analyzed from the parasternal long-axis view of the LV. The intraventricular septal wall thickness, LV posterior wall thickness, and LV internal diameters (LVIDs) were measured in end systole and end diastole. LV fractional shortening (FS) and LV mass were calculated using software provided by the manufacturer, as previously described (36).
The presence of aortic and mitral valve insufficiencies resulting in regurgitation flow was assessed by Doppler interrogation. Doppler recordings were performed on semilunar valve outflow in the parasternal long-axis view and on the atrioventricular valve (mitral valve) inflow in the apical four-chamber view. All measurements were obtained using a 45° angle of interrogation. Aortic regurgitation was assessed as reversal of outflow in diastole. Mitral valve regurgitation (reversal of inflow in systole) (11), was defined as valve incompetence, if regurgitation exceeded 1 SD of the average regurgitation for WT mouse of the same age.
Pressure-volume measurement.
In vivo LV function was assessed by pressure-volume (P-V) technique, as previously described (39, 41). Mice were anesthetized with isoflurane (2.5% in O2), intubated, ventilated at 120 breaths/min and a tidal volume of 200 μl, and maintained on a heated pad. A standard 1.4 French pressure-conductance catheter (SPR 839; Millar Instruments, Houston, TX) was volume calibrated for the conversion of relative volume units to absolute volume. The LV apex was exposed, and P-V catheter was advanced through apex. The catheter was positioned along the cardiac longitudinal axis with the distal electrode in the aortic root and the proximal electrode in the LV apex. P-V data were obtained at steady state and during transient reduction in venous return by occluding the inferior vena cava (40). All steady-state and caval occlusion P-V loops were acquired with the PVAN software (Conductance Technologies, San Antonio TX; and Millar, Houston, TX) acquisition system while the ventilation was momentarily turned off. P-V loops were analyzed with the PVAN 3.5 software package (Millar Instruments). The major load-dependent and load-independent parameters of contractility and stiffness of the LV included end-systolic volume and end-diastolic volume, arterial elastance (Ea), stroke work, preload recruitable stroke work (PRSW), arterio-ventricular coupling [Ea/end-systolic elastance (Ees)], the slope of end-systolic (Ees) and end-diastolic P-V relationship [end-diastolic elastance (Eed)], maximal rate of pressure rise (+dP/dt) and decline (−dP/dt), and relaxation time constant calculated by Weiss (τ) (39).
Histological assessment.
For histological assessment, mice were anesthetized with pentobarbital sodium (100 mg/kg body wt) intraperitoneally. The hearts were removed and weighed (wet weight), fixed with 10% formalin overnight, and embedded in paraffin. The hearts were further cut into two pieces through the long axis, measured, sectioned at 5-μm slices, and subjected to hematoxylin-eosin (HE) staining. Digital images of stained sections were obtained from light microscopy and analyzed using a digital imaging analysis system (MCID, InterFocus Imaging, Cambridge, UK). Myocyte diameter was measured as the shortest distance across the nucleus in transverse cell sections. Diameters of 100 myocytes from 5 randomly selected microscope fields (×200 magnification) from the LV posterior wall were averaged to represent the myocyte diameter. Myocyte density was calculated from the same area in the same fashion. Myocardial tissue fibrosis was measured in Masson's trichrome-stained sections and was expressed as a fraction of a microscopic field (×100 magnification) of the LV posterior wall. An average of five randomly selected fields represented results of a given specimen.
Western blot analysis.
Protein analysis was performed using standard techniques. LV tissues were homogenized with RIPA buffer with phosphatase inhibitors cocktails I and II (Calbiochem) and protease inhibitor cocktail (Cell Signaling). Samples were clarified by centrifugation at 14,000 rpm, with the proteins fractionated on 8–20% SDS-polyacrylamide gels. Immunoblotting was carried out with antibodies (from Cell Signaling) using the following titers: SMAD2/3 (1:500), phosphor-SMAD2/3 (1:250), ERK1/2 MAPK (1:1,000), phosphor-ERK1/2 MAPK (1:500), p38 MAPK (1:1,000), phosphor-p38 MAPK (1:500), atrium natriuretic peptide (ANP) (1:250, Santa Cruz), brain natriuretic peptide (BNP) (1:250, Abcam), α-smooth muscle actin (SMA) (1:1,000, Sigma), and GAPDH (Sigma, 1:1,000). Detection was by enhanced chemiluminescence (ECL Select Western Blotting Detection). Band intensity was quantified by Kodak image software. The ratio for protein examined was normalized against GADPH.
Statistical analysis.
All data are reported as means ± SE. Differences were analyzed by one- and two-way ANOVA with Bonferroni posttest correction. Statistical significance was accepted at P < 0.05.
RESULTS
Cardiac remodeling and its association with valvular function in MFS mice.
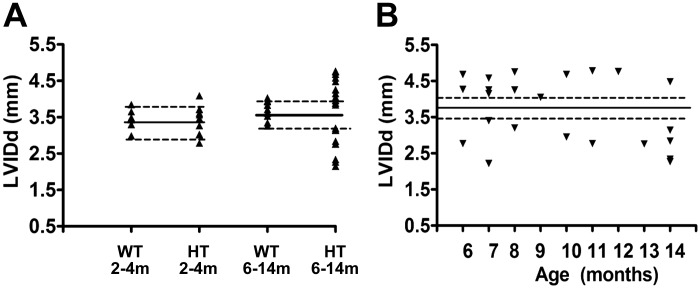
A scattered diagram (Fig. 1A) presents results of sonographic measurements of LV internal end-diastolic diameter (LVIDd) in young and older adult WT and HT mice. Average LVIDd in young WT mice was 3.43 ± 0.35 mm. Variability of this index among young HT was slightly higher than in young WT mice (SD ±0.39 mm); however, only in two HT mice was it outside of 1 SD of mean for WT (in one mouse it was higher than the average for WT, in another it was lower). In contrast, among older animals, the distribution of the LVIDs in HT mice was much wider than in WT (SD ±0.24 in WT vs. ±0.84 in HT); LVIDd of only one older HT mouse was within 1 SD of mean for the older WT. Distribution of LVIDd in older HT mice clearly indicated two different phenotypes of remodeling with either dilated or reduced (constricted) LV chamber. Such a distribution strongly suggests that separation into two phenotypes, dilated and nondilated (constricted), was not age dependent.
Fig. 1.
Left ventricular internal diameter in end-diastole (LVIDd) in wild-type (WT) and heterozygous (HT) mice of different ages. A: scattered plot of LVIDd for WT and HT in young (left) and older (right) age groups. B: scattered plot of LVIDd among older HT mice at different ages. Solid horizontal line is the average LVIDd for a WT of corresponding age. Dashed horizontal lines reflect ±1 SD of average LVIDd for WT of corresponding age.
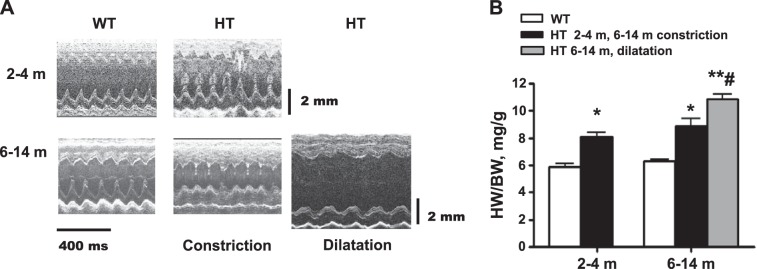
Representative echocardiographic images of young and older adult WT and HT mice in M-mode are presented in Fig. 2A and numerical results of echocardiographic assessment in Table 1. Based on two phenotypes shown in Fig. 1, Table 1 presents two groups of older adult HT mice, mice with either dilated or constricted LV chamber. At 2–4 mo of age, HT mice have exhibited some characteristics of LV remodeling: thickness of LV posterior wall and intraventricular septum were noticeably higher in HT than in WT (P < 0.05). At the same time, diameter of LV chamber, both in systole and diastole, in young adult mice did not differ between WT and HT, and FS was not affected by genotype (Table 1).
Fig. 2.
Left ventricular chamber remodeling in Marfan HT mice. A: representative sonographic (M-mode long-axis) images of the heart in Marfan HT mice and WT age-matched controls. Marfan HT mice display a mild cardiac hypertrophy at age 2–4 mo and cardiac hypertrophy with constricted (Con) or dilated (Dil) left ventricle at age 6–14 mo. B: quantification of ratio of heart weight (HW) to body weight (BW). Values are means ± SE; n = 6 (WT, 2–4 m), n = 7 (Marfan HT, 2–4 m), n = 13 (WT 6–14 m), n = 6 (Marfan HT, 6–14 m concentric hypertrophy), and n = 7 (Marfan HT, 6–14 m, dilatation). *P < 0.05 and **P < 0.01, WT vs. HT constriction. #P < 0.05, Dil vs. Con in 6- to 14-m age group.
Table 1.
Echocardiographic indexes for WT and HT mice
| 2–4 m, Young |
6–14 m, Older adult |
||||
|---|---|---|---|---|---|
| Parameters | WT | HT | WT | HT constricted | HT dilated |
| n | 7 | 7 | 11 | 11 | 12 |
| IVSd, mm | 0.87 ± 0.038 | 1.19 ± 0.1* | 1.06 ± 0.04 | 1.49 ± 0.10** | 1.31 ± 0.09* |
| LVIDd, mm | 3.43 ± 0.14 | 3.17 ± 0.18 | 3.70 ± 0.07 | 2.80 ± 0.12** | 4.47 ± 0.07*## |
| LVIDs, mm | 2.15 ± 0.10 | 2.06 ± 0.20 | 2.44 ± 0.11 | 1.34 ± 0.14* | 3.25 ± 0.11**## |
| LVPWd, mm | 0.93 ± 0.05 | 1.18 ± 0.08* | 1.01 ± 0.03 | 1.34 ± 0.08** | 1.21 ± 0.07*# |
| FS, % | 37.9 ± 2.14 | 35.5 ± 4.3 | 37.8 ± 1.4 | 44.4 ± 2.49 | 26.8 ± 1.7*# |
| Calculated LV mass, mg | 130 ± 8 | 177 ± 20* | 154 ± 6 | 203 ± 9** | 275 ± 8*# |
| Aortic regurgitation frequency, % | 0 (n = 5) | 0 (n = 8) | 0 (n = 9) | 20 (n = 5) | 60 (n = 10) |
| Aortic diameter, mm | 1.56 ± 0.08 (n = 6) | 1.71 ± 0.08 (n = 5) | 1.547 ± 0.07 (n = 7) | 2.04 ± 0.15* (n = 5) | 2.50 ± 0.15**# (n = 7) |
| Mitral regurgitation frequency, % | 0 (n = 5) | 13 (n = 8) | 0 (n = 5) | 40 (n = 5) | 100 (n = 7) |
Values are means ± SE; n, no. of mice. WT, wild type; HT, heterozygous; IVSd, intraventricular septum in diastole; LVIDd, left ventricular (LV) internal diameter in diastole; LVIDs, LV internal diameter in systole; LVPWd, LV posterior wall in diastole; FS, fractional shortening.
P < 0.05 and
P < 0.01: WT vs. HT in corresponding age groups.
P < 0.05 and
P < 0.01: 6–14 m HT constricted vs. dilated.
On the contrary, among older groups, noticeable thickening of septum and LV posterior wall in HT vs. WT (P < 0.05) was accompanied by significant changes in LV chamber dimension: the LV diameters (both systolic and diastolic) were significantly reduced in the constricted HT group and increased in dilated HT (P < 0.05 vs. WT). Consequently, LV diameters in the constricted and dilated HT groups were significantly different from each other (P < 0.05). Thickness of septum and posterior wall were also higher in the HT groups vs. WT (P < 0.05), but only in posterior wall was the thickness significantly less expressed in the HT dilated group than in the constricted. LV remodeling in the older HT dilated group was also accompanied by a functional decline: FS in dilated HT was significantly smaller than in older WT (P < 0.05). The small increase of FS in the constricted HT group did not reach statistical significance compared with WT.
Compared with WT, aortic root in HT mice was dilated in both age groups (Table 1); however, in the young group, the increase of aortic diameter did not reach statistical significance. At the older age (6- to 14-mo age group), the aortic diameter was markedly increased in HT mice, exceeding that of WT by 29% for the constricted group (P < 0.05) and by 61% in the dilated group (P < 0.05); the increase of diameter of aortic root in the dilated HT group significantly exceeded that of the constricted group (P < 0.05).
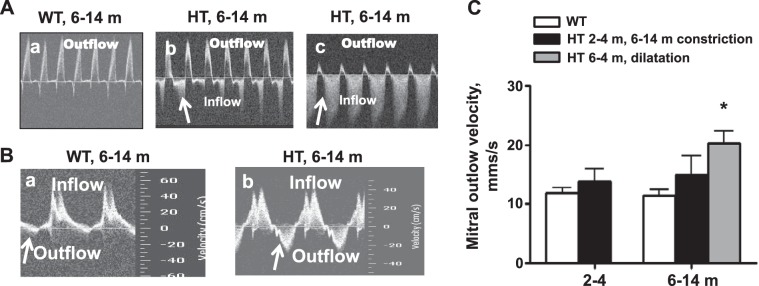
Representative Doppler sonograms reflecting aortic and mitral valve function are shown on Fig. 3. No aortic insufficiency was recorded among young WT or HT groups or among older adult WT mice (Table 1). Among older HT mice, the aortic valve regurgitation was observed in 20% of the constricted group and 60% of dilated. Mitral valvular insufficiency was defined as velocity of backflow exceeding the average backflow velocity of WT of corresponding age group by >1 SD. Mitral valve insufficiency was not observed in young or older WT mice. It was recorded, however, in 13% of young HT mice. Statistical analysis of echo parameters presented in Table 1 was performed for young HT, excluding animals with mitral regurgitation. Resultant statistics demonstrated statistically significant changes in the thickness of LV posterior wall and ventricular septum, indicating mild cardiac hypertrophy. Among older HT mice, mitral valve insufficiency was observed in 40% of the concentric group and in all (100%) of the dilated (Table 1). On average, the increase of severity of mitral valve insufficiency (average velocity of regurgitation) among older mice in the HT concentric group was not statistically different from WT (Fig. 3C), while average outflow velocity was significantly higher in the dilated HT group vs. WT.
Fig. 3.
Representative Doppler profiles for aortic valve (AoV) outflow and mitral valve inflow velocity. A: at the 6- to 14-mo age group, Doppler interrogation demonstrates normal left ventricular outflow (AoV) tract without regurgitation (a), mild aortic regurgitation (b), and severe aortic regurgitation (c). B: mitral valve outflow (a) and normal mitral valve outflow (b). Significant outflow (regurgitation) patterns are observed (arrows) in HT mice with chamber dilatation. C: quantification of mitral valve outflow velocity (regurgitation). Outflow velocity was significantly increased in 6- to 14-mo-old Marfan HT mice with cardiac dilatation. Values are means ± SE; n = 5–8 animals per group. *P < 0.05, WT vs. HT mice.
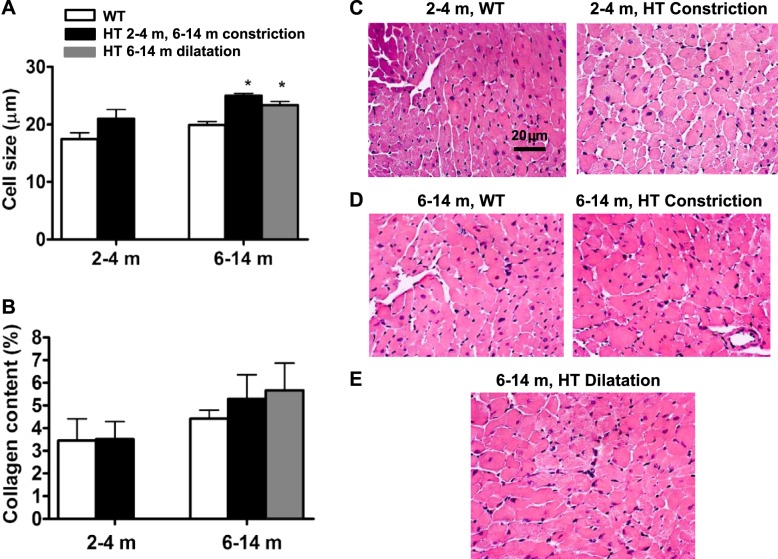
Myocardial hypertrophy in young and older adult HT mice revealed by echo measurements was confirmed by higher heart weight-to-body weight ratio in HT mice compared with WT counterparts measured postmortem (Fig. 2B) and by higher cardiomyocyte thickness measured histologically (Fig. 4, A, C–E); however, in young HT mice, the increase of cardiomyocyte short diameter drawn through a nucleus did not reach statistical significance. Traditional histochemical HE staining can impose substantial technical limitations on studies used for cell size measurement. It is possible that relatively modest changes in cardiomyocyte size in young HT mice were not detected with HE staining.
Fig. 4.
Cardiomyocyte hypertrophy in Marfan HT mice of different age groups. A: quantification of ventricular myocytes transverse-section size in WT and Marfan HT mice. Data represent the short diameter of cardiomyocyte drawn through a nucleus. B: quantification of interstitial fibrosis in WT and Marfan HT mice. Values are means ± SE; n = 5–10 per group. *P < 0.05, WT vs. Marfan HT mice. C–E: representative histological sections of the myocardium from WT and Marfan HT mice: 2–4 m (C) and 6–14 m WT and HT constriction (D) and 6–14 m HT dilation (E).
Note that myocardial hypertrophy in HT mice was not accompanied by the increase in collagen content (Fig. 4B).
MFS mice demonstrate complex systolic and diastolic dysfunction.
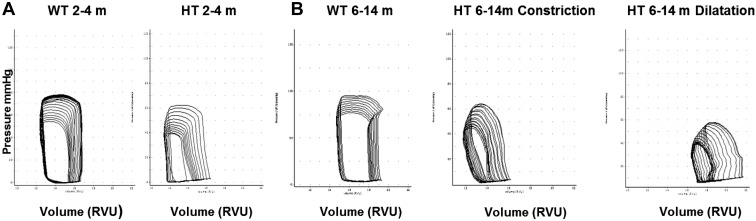
Representative P-V loops for each studied group are shown in Fig. 5. Results of measurements are presented in the Table 2. The LV chamber volumes were compatible with LV dimensions obtained by echo measurements: in young HT mice, LV chamber did not differ from WT either in diastole or in systole; among older groups in dilated HT, both end-diastolic volume and end-systolic volume were significantly larger (P < 0.05) than in WT mice; but in the constricted HT group, the end-diastolic LV chamber was significantly smaller than in WT.
Fig. 5.
Representative pressure-volume loops during inferior vena cava occlusion in HT and WT littermates at different ages. A: representative pressure-volume loops at 2 mo of age. B: representative pressure-volume loops at 6–14 mo of age demonstrating two distinct hypertrophic phenotypes (Con and Dil) among HT. RVU, relative volume units.
Table 2.
Hemodynamic indexes of WT and HT groups in young and older adult mice
| 2–4 m, Young |
6–14 m, Older adult |
||||
|---|---|---|---|---|---|
| Pressure-Volume Parameters | WT | HT | WT | HT constricted | HT dilated |
| n | 7 | 6 | 12 | 5 | 7 |
| HR, beats/min | 561 ± 35 | 531 ± 29 | 547 ± 23 | 445 ± 31 | 463 ± 55 |
| ESV, μl | 21.3 ± 2.1 | 18.0 ± 2.1 | 19.7 ± 2.0 | 16.0 ± 1.7 | 38.7 ± 1.4**## |
| EDV, μl | 34.1 ± 1.9 | 30.6 ± 2.8 | 36.6 ± 1.1 | 26.2 ± 1.5* | 46.3 ± 1.7**## |
| SV, μl | 14.4 ± 1.7 | 11.6 ± 2.3 | 13.9 ± 1.4 | 5.4 ± 0.8** | 7.2 ± 0.9* |
| Ea, mmHg/μl | 6.1 ± 0.7 | 7.1 ± 0.9 | 7.2 ± 0.5 | 10.1 ± 1.3** | 4.3 ± 0.3*## |
| +dP/dt, mmHg/s | 8,566 ± 348 | 5,122 ± 313* | 8,507 ± 849 | 4,998 ± 606* | 3,647 ± 525** |
| −dP/dt, mmHg/s | 6,713 ± 654 | 4,713 ± 611* | 6,744 ± 807 | 3,928 ± 387* | 2,844 ± 434** |
| τW, ms | 7.9 ± 0.2 | 13.2 ± 1.1* | 7.5 ± 0.6 | 13.9 ± 1.8* | 9.5 ± 1.6 |
| PRSW, mmHg·μl−1·μl−1 | 69 ± 6 | 45 ± 4* | 67 ± 7 | 43 ± 5* | 36 ± 4** |
| Ees, mmHg/μl | 8.9 ± 1.2 | 6.9 ± 0.7 | 8.5 ± 1.1 | 3.1 ± 0.6** | 2.7 ± 0.5** |
| Eed, 10−3 × mmHg/μl | 0.22 ± 0.02 | 0.39 ± 0.03* | 0.23 ± 0.03 | 0.44 ± 0.05** | 0.42 ± 0.04** |
| Ea/Ees | 0.87 ± 0.08 | 0.94 ± 0.01 | 0.68 ± 0.05 | 2.7 ± 0.06** | 1.6 ± 0.07*## |
Values are means ± SE; n, no. of mice. HR, heart rate; ESL, end-systolic volume; EDV, end-diastolic volume; SV, stroke volume; Ea, aortic elastance; +dP/dt, peak rate of pressure rise; −dP/dt, peak of pressure decline, τW, time rate of relaxation (Weiss); PRSW, preload recruitable stroke work; Ees, end-systolic elastance; Eed, end-diastolic elastance; Ea/Ees, arterioventricular coupling.
P < 0.05 and
P < 0 0.01: WT vs. HT (in respective age groups).
P < 0.01: HT constricted vs. dilated (Bonferroni post hoc comparison).
In the younger group, P-V loop analyses revealed significant diastolic dysfunction in HT shown by load-dependent indexes (reduction of −dP/dt and increase of τ, P < 0.05). Diastolic dysfunction in young HT was confirmed by significant increase of load-independent index, Eed (P < 0.05 vs. WT). Arterioventricular coupling (Ea/Ees) among young HT mice was maintained at WT level, because a small decline of the index of intrinsic contractility (Ees) was balanced a by small elevation of Ea. Nevertheless, systolic function was also affected, as indicated by a significant reduction of +dP/dt and by significant reduction of the index of systolic reserve, PRSW (P < 0.05).
Among older mice, the diastolic dysfunction in HT was clearly presented in both dilated and constricted groups: while τ was significantly elevated in constricted HT only, −dP/dt was significantly reduced in both HT groups. Moreover, the load-independent diastolic index (Eed) was doubled in both HT groups compared with WT. Decline of systolic function in older HT groups was obvious, not only through a significant reduction of PRSW, but also through substantial reduction of Ees, an index of intrinsic contractility: Ees in the constricted HT group was less than one-half of that in WT and even further reduced in dilated HT (P < 0.05). Massive loss of intrinsic myocardial contractility in HT mice resulted in a significant arterioventricular uncoupling, indicated by an elevation of Ea/Ees, which was only slightly less expressed in the dilated HT group due to reduction of Ea.
ANP and BNP hypertrophic markers and TGF-β-dependent signaling in Marfan HT mice.
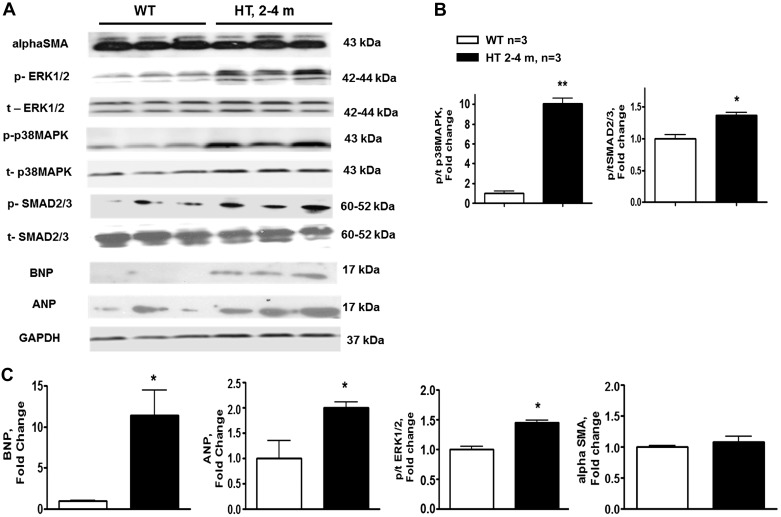
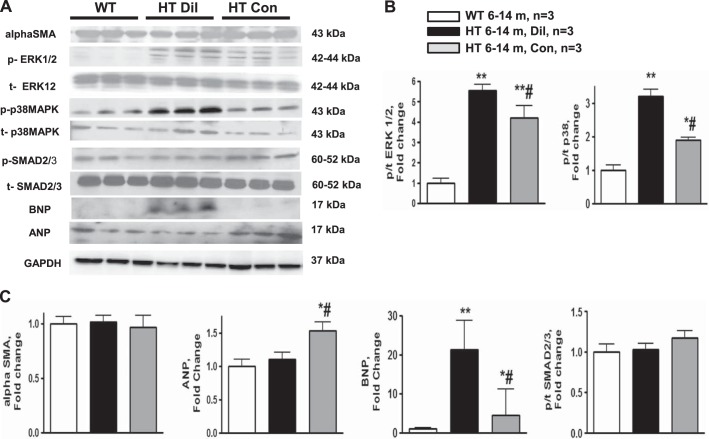
Western blots analysis (Fig. 6) of downstream targets of both canonical and noncanonical TGF-β signaling pathways showed a mild TGF-β activation in 2- to 4-mo-old HT mice, as reflected in phosphorylation of SMAD2/3 transcriptional factor; in whole heart homogenates of HT mice phosphorylated-to-total (p/t) SMAD2/3 ratio was significantly higher than in WT. We observed an increase of p/t ratios of ERK1/2 and p38 MAPK in 2- to 4-mo-old HT mice. To address the role of TGF-β signaling in dilated and constricted hearts, p/t ratio of SMAD2/3, ERK1/2, and p38 MAPK was compared between WT and HT mice with constricted and dilated hearts (Fig. 7). Among mice of the older age group, the ratios of p/t ERK1/2 and p/t p38 MAPK were increased in HT mice over WT mice and in the dilated group over the constricted group. p/t SMAD2/3 levels were similar between WT and HT mice in constricted and dilated groups at the older age group.
Fig. 6.
A: representative Western blots of natriuretic peptides atrium (ANP) and brain (BNP), α-smooth muscle actin (SMA), and phosphorylated (p) and total (t) SMAD2/3, ERK1/2, and p38 MAPK using cardiac tissue lysates from WT and Marfan HT mice at 2–4 mo of age. B and C: summary data for immunoblots: phosphorylated-to-total (p/t) ratio of p38 MAPK and SMAD2/3 (B); and BNP, ANP, p/t ratio of ERK1/2, and α-SMA (C). Values are means ± SE; n = 3 per group. *P < 0.05 and **P < 0.01, WT vs. HT.
Fig. 7.
A: representative Western blots of natriuretic peptides ANP and BNP, α-SMA, and p- and t-SMAD2/3, ERK1/2, and p38 MAPK from WT and Dil and Con group Marfan HT mice at 6–14 mo of age. B and C: summary data for immunoblots: p/t ratio of ERK1/2 (B); and α-SMA, ANP, BNP, and p/t ratio of SMAD2/3 (C). Values are means ± SE; n = 3 per group. *P < 0.05 and **P < 0.01, WT vs. HT. #P < 0.05, Dil vs. Con.
Expression of cardiac hypertrophic markers, ANP and BNP, was increased in HT vs. WT mice at 2- to 4-mo age group. Both dilated and constricted hearts were characterized by increased expression of BNP compared with WT. The increase in BNP expression was more pronounced in mice with cardiac dilatation. Expression of ANP was increased only in the constricted group of older HT mice (Fig. 7).
TGF-β-dependent differentiation of fibroblast into myofibroblasts is identified by their expression of α-SMA. Expression of myofibroblast marker α-SMA was similar between WT and HT mice at both age groups (Figs. 6 and 7).
DISCUSSION
Extensive characterization of cardiac function and remodeling in the FbnC1039G+/− mouse model of MFS revealed factors contributing to the variances in clinical evaluation of cardiac dysfunction in MFS patients (7, 19, 20, 32, 33, 40). In our study, early remodeling was expressed as myocardial hypertrophy, accompanied by upregulation of biochemical indicators of hypertrophic growth, such as natriuretic cardiac peptides ANP and BNP universally observed in young 2- to 4-mo-old HT mice. Histological examination with HE staining revealed a trend to increased diameter of cardiomyocytes in 2-to 4-mo-old HT mice compared with WT mice. Technical limitation of HE staining for plasma membrane delineation may be a possible explanation of why histomorphometric measurements did not correlate strongly with heart weight-to-body weight ratio and LV mass indexes of cardiac hypertrophy in young HT mice. Cardiomyocytes cross-sectional area evaluation using wheat germ agglutinin staining provide better delineation of the plasma membrane and detection of myocardial remodeling at the cellular level.
There were no indications for aortic valve insufficiency in this group: mitral valve insufficiency was observed only in 13% of young HT mice, and Ea was not significantly elevated. Therefore, this early manifestation of cardiac remodeling was not associated with hemodynamic overload, but likely was triggered by intrinsic mechanisms related to the altered myocardial matrix causing persistent mechanical stress.
Diastolic dysfunction in the young HT mice was easily proven by a significant elevation of τ and by increased end-diastolic stiffness. Systolic dysfunction in young HT was revealed only via P-V analyses of LV performance, which demonstrated a significantly lower PRSW, a load-independent index of systolic function reflecting a reduction of systolic reserve. These findings correspond to clinical observations: the Doppler-assisted measurements in children with MFS revealed a prolonged relaxation time, decreased deceleration velocity, and decreased peak velocity ratio, suggesting a selective impairment of LV relaxation. This early diastolic dysfunction was not accompanied by any changes in FS (33), i.e., systolic function appeared to be unaffected.
Stiffness of large arteries and associated elevation of pulse pressure is considered to be the main determinant of aortic dilatation in MFS patients. There is also a prevailing view linking hemodynamic afterload from increased pulse pressure to adverse cardiac remodeling and outcome (8, 15, 16, 37). A significant increase in aortic elastance was not detected in younger group of HT mice, while they already demonstrated a hypertrophic remodeling and tendency for aortic enlargement, suggesting that intrinsic cardiac mechanisms were likely involved in initiating a chamber remodeling process. In older mice, an increased aortic elastance was observed in constricted phenotype, where it could be a contributing hemodynamic factor in sustaining the hypertrophic remodeling process.
One very important findings of the present study is that older MFS HT mice manifest distinct degrees in severity of cardiac remodeling that were not sex dependent. Deficient ECM makes the heart vulnerable to severe cardiac dysfunction in the presence of valvular regurgitation or possibly other etiologies of hemodynamic overload. In addition to persistent mechanical stress from deficient ECM, dilated hearts are subjected to increased preload through deficient valves. In some older animals, severe vascular manifestations of MFS (aortic dilatation and valvular insufficiencies) lead to increased hemodynamic stress and, combined with altered cardiac mechanobiology, resulted in cardiac dilatation. Our findings in the mouse model support clinical observations of cardiomyopathies in MFS patients, where cardiac dilatation is observed with various frequencies, depending on age and sex of patients population, and different imaging techniques. Marfan patients often demonstrate rapid evolution of heart failure in the presence of hemodynamic overload well tolerated by the general population (7, 32, 40).
Myocyte-specific signaling mechanisms caused by deficient FBN microenvironments in MFS HT mice may be similar to vascular molecular signals, leading to aortic aneurism initiation and progression, but may be different. Cardiac hypertrophy in FbnC1039G+/− mice may result from primary contractile dysfunction of the heart due to the underlying alterations in the mechanical features of the cardiac muscle. Both depressed contractility and relaxation may initiate signaling for cardiac remodeling, reflecting complexity of calcium-dependent intracellular progrowth pathways (9, 13).
Similar to aortic growth and remodeling, the hypertrophic response in the heart with FBN-1 deficiency is likely to involve TGF-β signaling network. Activation of TGF-β signaling, as a result of impaired sequestration of latent complexes in the ECM, is likely to initiate an entire program of TGF-β disease-predisposing signals (12, 14, 27, 29, 31). This signaling includes noncanonical MAPK cascades, such as ERK1/2, and network of kinases, including p38 MAPK, as well as ras-MEK, Rho GTPase, and phosphoinositide-3-kinase. If canonical SMAD-dependent signaling pathway plays a major role in cardiac fibrosis, noncanonical myocyte-targeted TGF-β signaling have a central role in the maladaptive cardiac response to sustained pressure overload (21, 25, 35). In young Marfan HT mice, ECM abnormalities and phosphorylation of SMAD2/3 was not associated with the increase of interstitial fibrosis, and, therefore, selectivity of TGF-β/SMAD2/3 axis for fibroblasts, and resulting net fibrosis, is not a detrimental contributor to aberrant cardiac function in early onset cardiac remodeling in Marfan HT mice. Transformation of fibroblast to myofibroblasts characterized by increased expression of α-SMA is associated with increased production of ECM components (35). The signaling axis of TGF-β-dependent fibroblast transdifferentiation and upregulation of myofibroblast marker SMA was not involved in the cardiac remodeling in our model of MFS. In contrast to other MFS manifestations, profibrotic canonical TGF-β/SMAD2/3 signaling and nonmyocyte proliferation do not play a prominent role in age-dependent cardiac remodeling in MFS.
With age, a structurally deficient matrix component compromises the cardiac function as well as adaptation to increased workload, resulting in the development of two distinct phenotypes of cardiac remodeling. These two distinct cardiac phenotypes have differences in cellular signaling associated with severity of cardiac remodeling. Cellular pool of natriuretic peptides ANP and BNP was different in the dilated and constricted group. The ANP expression was increased in the constricted group only, suggesting that this important adaptive mechanism was inactivated in the dilated group. Increased BNP expression in the dilatation group may indicate severity of cardiac remodeling, as BNP is the strongest biomarker in terms of its consistent activation with adverse cardiovascular disease outcome and heart failure (1).
The roles of ERK1/2 and p38 MAPK are well established in cardiac progrowth signaling network and may be a myocyte-targeted signaling pathway in MFS-related cardiac pathology. Cardiac dilatation was associated with biochemical evidence of greater hemodynamic stress associated with elevated ERK1/2 and p38 MAPK activity, and this hyperactivity paralleled the severity of cardiac remodeling in older HT mice. The degree of MAPK hyperactivity may depend on hemodynamic load and represent an informative biomarker of cardiac remodeling severity, with the stress response ERK1/2 and p38 kinases serving as molecular readabouts of biomechanical stress. In hearts with FBN-1 deficiency, abnormal ERK1/2 signaling is likely to be independent from angiotensin II-mediated TGF-β activation and may be activated through other upstream signaling pathways responsive to persistent mechanical stress, leading to increased angiotensin receptor 1 signaling and conformational changes in β-arrestin-2 pathway (6). Undoubtedly, mechanistic exploration and understanding of the specificity of TGF-β-dependent and -independent pathways transmitting signals from ECM with deficient fibrillin-1 to cardiomyocytes will offer opportunities to clarify the mechanism of cardiac disease initiation and optimize treatment for cardiac rescue.
In the present study, we found that FBN-1 haploinsufficiency results in the display of early onset of cardiac hypertrophy and dysfunction. With age, cardiac remodeling progresses into two distinct phenotypes, characterized either by concentric hypertrophy or dilatation of the LV. Dilatation of the LV manifests into progressive systolic and diastolic dysfunction in the face of valvular regurgitation.
GRANTS
This research was fully supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.-J.T., N.N.P., and M.T. conception and design of research; H.-J.T., N.N.P., S.M., and M.K. performed experiments; H.-J.T., N.N.P., S.M., M.K., and M.T. analyzed data; H.-J.T., N.N.P., and M.T. interpreted results of experiments; H.-J.T. and N.N.P. prepared figures; H.-J.T., N.N.P., S.M., M.K., and M.T. approved final version of manuscript; M.T. drafted manuscript; M.T. edited and revised manuscript.
ACKNOWLEDGEMENTS
Mice, heterozygous for the C1039G mutation (FbnC1039G+/−) were kindly provided by Dr. Dietz.
Present address of H.-J. Tae: Dept. of Biomedical Science and Research, Institute for Bioscience and Biotechnology, Hallym University, Chunchon, South Korea.
REFERENCES
- 1.Ahluwalia N, Blacher J, de Edelenyi FS, Faure P, Chantal J, Hercberg S, Galan P. Prognostic value of multiple emerging biomarkers in cardiovascular risk prediction in patients with stable cardiovascular disease. Atherosclerosis 228: 478–484, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Boileau C, Guo DC, Hanna N, Regalado ES, Detaint D, Gong L, Varret M, Prakash SK, Li AH, d′Indy H, Braverman AC, Grandchamp B, Kwartler CS, Gouya L, Santos-Cortez RL, Abifadel M, Leal SM, Muti C, Shendure J, Gross MS, Rieder MJ, Vahanian A, Nickerson DA, Michel JB; National Heart, Lung, and Blood Institute (NHLBI) Go Exome Sequencing Project , Jondeau G, Milewicz DM. TGFβ2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 44: 916–921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouzeghrane F, Reinhardt DP, Reudelhuber TL, Thibault G. Enhanced expression of fibrillin-1, a constituent of the myocardial extracellular matrix in fibrosis. Am J Physiol Heart Circ Physiol 289: H982–H991, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Canadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. 1. Pathophysiology and diagnosis. Nat Rev Cardiol 7: 256–265, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Cañadas V, Vilacosta I, Bruna I, Fuster V. Marfan syndrome. 2. Treatment and management of patients. Nat Rev Cardiol 7: 266–276, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Cook JR, Carta L, Bénard L, Chemaly ER, Chiu E, Rao SK, Hampton TG, Yurchenco P; GenTAC Registry Consortium , Costa KD, Hajjar RJ, Ramirez F. Abnormal muscle mechanosensing triggers cardiomyopathy in mice with Marfan syndrome. J Clin Invest 124: 1329–1339, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Backer JF, Devos D, Segers P, Matthys D, François K, Gillebert TC, De Paepe AM, De Sutter J. Primary impairment of left ventricular function in Marfan syndrome. Int J Cardiol 112: 353–358, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Dietz HC, Mecham RP. Mouse models of genetic diseases resulting from mutations in elastic fiber proteins. Matrix Biol 19: 481–488, 2000. [DOI] [PubMed] [Google Scholar]
- 8a.Gao LG, Luo F, Hui RT, Zhou XL. Recent molecular biological progress in Marfan syndrome and Marfan-associated disorders. Ageing Res Rev 9: 363–368, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, Reiken S, Elrod JW, Correll RN, York AJ, Sargent MA, Hofmann F, Moosmang S, Marks AR, Houser SR, Bers DM, Molkentin JD. Decreased cardiac L-type Ca2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest 122: 280–290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurism in a mouse model of Marfan syndrome. Science 312: 117–121, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinton RB Jr, Alfieri CM, Witt SA, Glascock BJ, Khoury PR, Benson DW, Yutzey KE. Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol 294: H2480–H2488, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGF-β signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332: 358–361, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houser S, Molkentin J. Does contractile Ca2+ control calcineurin-NFAT signaling and pathologic hypertrophy in cardiac myocytes? Sci Signal 24: 31, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor-beta binding protein 1 interacts with fibrillin and is microfibril-associated protein. J Biol Chem 278: 2750–2757, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Jeremy RW, Huang H, Hwa J, McCarron H, Hughes CF, Richards JG. Relation between age, arterial distensibility and aortic dilatation in the Marfan syndrome. Am J Cardiol 74: 369–374, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Jondeau G, Boutouyrie P, Lacolley P, Laloux B, Dubourg O, Bourdarias JP, Laurent S. Central pulse pressure is a major determinant of ascending aorta dilation in Marfan syndrome. Circulation 99: 2677–2681, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114: 172–181, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judge DP, Dietz HC. Marfan syndrome. Lancet 366: 1965–1976, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiotsekoglou A, Bajpai A, Bijnens BH, Kapetanakis V, Athanassopoulos G, Moggridge JC, Mullen MJ, Nassiri DK, Camm J, Sutherland GR, Child AH. Early impairment of left ventricular long-axis systolic function demonstrated by reduced atrioventricular plane displacement in patients with Marfan syndrome. Eur J Echocardiogr 9: 605–613, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Kiotsekoglou A, Saha SK, Moggridge JC, Kapetanakis V, Bijnens BH, Mullen MJ, Camm J, Sutherland GR, Wilkinson IB, Child AH. Effect of aortic stiffness on left-ventricular long-axis systolic function in adults with Marfan syndrome. Hellenic J Cardiol 51: 501–511, 2010. [PubMed] [Google Scholar]
- 21.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest 121: 2301–2312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay ME, Shepers D, Ajit Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, Bjeda D, Oswald G, Elias AF, Levy HP, Anderlid BM, Yang MH, Bongers EM, Timmermans J, Braverman AC, Canham N, Mortier GR, Brunner HG, Byers PH, Van Yek J, Van Laer LJ, Dietz HC, Loyes BL. Loss-of-function mutations in TGF-β2 cause a syndromic presentation of thoracic aortic aneurism. Nat Genet 44: 922–927, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariko B, Ghandour Z, Raveau S. Microfibrills and fibrillin-1 induce integrin-mediated signaling, proliferation and migration in human endothelial cells. Am J Physiol Cell Physiol 299: C977–C987, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto-Ida M, Takimoto Y, Aoyama T, Akao M, Takeda T, Kita T. Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol 290: H709–H715, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Mizuguchi T, Matsumoto N. Recent progress in genetics of Marfan syndrome and Marfan associated disorders. J Hum Genet 52: 1–12, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Mudd JO, Kass DA. Taking the heart failure in the twenty-first century. Nature 451: 919–928, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Nataatmadja M, West J, West M. Overexpression of transforming growth factor beta is associated with increased hyaluronan content and impairment of repair in Marfan syndrome aortic aneurism. Circulation 114: 1371–13775, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114: 1586–1592, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez F, Dietz HC. Fibrillin-rich microfibrills: structural determinants of morphogenetic and homeostatic events. J Cell Physiol 213: 326–330, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Rybczynski M, Koschyk DH, Aydin MA, Robinson PN, Brinken T, Franzen O, Berger J, Hofmann T, Meinertz T, von Kodolitsch Y. Tissue Doppler imaging identifies myocardial dysfunction in adults with Marfan syndrome. Clin Cardiol 30: 19–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savolainen A, Nisula L, Keto P, Hekali P, Viitasalo M, Kaitila I, Kupari M. Left ventricular function in children with the Marfan syndrome. Eur Heart J 15: 625–630, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Shores J, Berger KR, Murphy EA, Pyeritz RE. Progression of aortic dilatation and the benefit of long-term beta-adrenergic blockade in Marfan syndrome. N Engl J Med 330: 1335–1341, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collage synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A 102: 437–442, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syed F, Diwan A, Hahn HS. Murine echocardiography: a practical approach for phenotyping genetically manipulated and surgically modeled mice. J Am Soc Echocardiogr 18: 982–990, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Vitarelli A, Conde Y, Cimino E, D'Angeli I, D'Orazio S, Stellato S, Padella V, Caranci F. Aortic wall mechanics in the Marfan syndrome assessed by transesophageal tissue Doppler echocardiography. Am J Cardiol 97: 571–577, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Wilson DG, Bellamy MF, Ramsey MW, Goodfellow J, Brownlee M, Davies S, Wilson JF, Lewis MJ, Stuart AG. Endothelial function in Marfan syndrome: selective impairment of flow-mediated vasodilation. Circulation 99: 909–915, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationship. Am J Physiol Heart Circ Physiol 277: H1906–H1913, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Yetman AT, Bornemeier RA, McCrindle BW. Long-term outcome in patients with Marfan syndrome: is aortic dissection the only cause of sudden death? J Am Coll Cardiol 41: 329–332, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, Chuprun JK, Wang Y, Talan M, Dorn GW 2nd, Lakatta EG, Koch WJ, Feldman AM, Xiao RP. Gi-biased β2-AR signaling links GRK2 up-regulation to heart failure. Circ Res 110: 265–274, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]