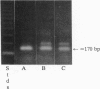
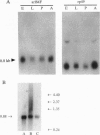
Abstract
The ADP-ribosylation factor (ARF) family is one of four subfamilies of the RAS superfamily of low molecular weight GTP-binding proteins (G proteins). Highly degenerate oligonucleotides encoding two conserved regions were used in a PCR reaction to amplify cDNAs encoding each of the known ARF proteins and eight additional cDNA fragments encoding previously unreported human members of the ARF family. Additional sequences were obtained from yeast or fly libraries by using this technique. These oligonucleotides specifically amplify members of the ARF family but not the structurally related G protein alpha subunits or members of the other three subfamilies of the RAS superfamily. Fragments obtained by PCR were used to obtain full-length sequences encoding highly homologous ARF-like (ARL) gene products from human and Drosophila melanogaster libraries, termed ARL2 and Ar184F, respectively. The encoded proteins are each 184 amino acids long and are 76% identical, with 40-45% identity to human ARF1 and Drosophila arf-like (arl) proteins. These genes appear to be generally expressed in human tissues and during Drosophila development. The purified human ARL2 protein differed in several biochemical properties from human ARF proteins, including the complete absence of ARF activity. Thus, the ARF family of low molecular weight GTP-binding proteins includes at least 15 distinct but structurally conserved members, including both the functionally conserved ARF proteins and the functionally disparate ARL proteins. The latter proteins currently comprise two distinct gene products in Drosophila (arl and ARL84F) and one in man (ARL2).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsip G. R., Konkel D. A. A processed chicken pseudogene (CPS1) related to the ras oncogene superfamily. Nucleic Acids Res. 1986 Mar 11;14(5):2123–2138. doi: 10.1093/nar/14.5.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Kahn R. A., Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992 Jun 25;267(18):13053–13061. [PubMed] [Google Scholar]
- Bobak D. A., Nightingale M. S., Murtagh J. J., Price S. R., Moss J., Vaughan M. Molecular cloning, characterization, and expression of human ADP-ribosylation factors: two guanine nucleotide-dependent activators of cholera toxin. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6101–6105. doi: 10.1073/pnas.86.16.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A. L., Taylor T. C., Melançon P., Wilson K. L. A role for ADP-ribosylation factor in nuclear vesicle dynamics. Nature. 1992 Aug 6;358(6386):512–514. doi: 10.1038/358512a0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Segev N., Stearns T., Hoyt M. A., Holden J., Kahn R. A. Diverse biological functions of small GTP-binding proteins in yeast. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):629–636. doi: 10.1101/sqb.1988.053.01.072. [DOI] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kahn R. A., Kern F. G., Clark J., Gelmann E. P., Rulka C. Human ADP-ribosylation factors. A functionally conserved family of GTP-binding proteins. J Biol Chem. 1991 Feb 5;266(4):2606–2614. [PubMed] [Google Scholar]
- Kahn R. A. Quantitation and purification of ADP-ribosylation factor. Methods Enzymol. 1991;195:233–242. doi: 10.1016/0076-6879(91)95169-k. [DOI] [PubMed] [Google Scholar]
- Kahn R. A., Randazzo P., Serafini T., Weiss O., Rulka C., Clark J., Amherdt M., Roller P., Orci L., Rothman J. E. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992 Jun 25;267(18):13039–13046. [PubMed] [Google Scholar]
- Lenhard J. M., Kahn R. A., Stahl P. D. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992 Jun 25;267(18):13047–13052. [PubMed] [Google Scholar]
- Monaco L., Murtagh J. J., Newman K. B., Tsai S. C., Moss J., Vaughan M. Selective amplification of an mRNA and related pseudogene for a human ADP-ribosylation factor, a guanine nucleotide-dependent protein activator of cholera toxin. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2206–2210. doi: 10.1073/pnas.87.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Nakańo A., Muramatsu M. A novel GTP-binding protein, Sar1p, is involved in transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1989 Dec;109(6 Pt 1):2677–2691. doi: 10.1083/jcb.109.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. G., Calvert I., Clark J., Helman L., Kahn R., Kung H. F. Molecular cloning, sequence analysis and mRNA expression of human ADP-ribosylation factor. Biofactors. 1989 Mar;2(1):45–49. [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Schleifer L. S., Kahn R. A., Hanski E., Northup J. K., Sternweis P. C., Gilman A. G. Requirements for cholera toxin-dependent ADP-ribosylation of the purified regulatory component of adenylate cyclase. J Biol Chem. 1982 Jan 10;257(1):20–23. [PubMed] [Google Scholar]
- Sewell J. L., Kahn R. A. Sequences of the bovine and yeast ADP-ribosylation factor and comparison to other GTP-binding proteins. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4620–4624. doi: 10.1073/pnas.85.13.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Kahn R. A., Botstein D., Hoyt M. A. ADP ribosylation factor is an essential protein in Saccharomyces cerevisiae and is encoded by two genes. Mol Cell Biol. 1990 Dec;10(12):6690–6699. doi: 10.1128/mcb.10.12.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Kahn R. A., Kissinger M., Brizuela B. J., Rulka C., Scott M. P., Kennison J. A. The arflike gene encodes an essential GTP-binding protein in Drosophila. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3120–3124. doi: 10.1073/pnas.88.8.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M., Price S. R., Tsai S. C., Moss J., Vaughan M. Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells. J Biol Chem. 1991 Feb 15;266(5):2772–2777. [PubMed] [Google Scholar]
- Weiss O., Holden J., Rulka C., Kahn R. A. Nucleotide binding and cofactor activities of purified bovine brain and bacterially expressed ADP-ribosylation factor. J Biol Chem. 1989 Dec 15;264(35):21066–21072. [PubMed] [Google Scholar]