Abstract
Chronic musculoskeletal pain is debilitating and affects ∼20% of adults. Tissue acidosis is present in painful musculoskeletal diseases like rheumatoid arthritis. ASICs are located on skeletal muscle and joint nociceptors as well as on nonneuronal cells in the muscles and joints, where they mediate nociception. This review discusses the properties of different types of ASICs, factors affecting their pH sensitivity, and their role in musculoskeletal hyperalgesia and inflammation.
Overview of Musculoskeletal Pain
About 20% of the adult population suffers from chronic musculoskeletal pain (74, 83), a debilitating condition that is globally recognized as the second leading cause for disability (113). In the U.S., chronic musculoskeletal pain is estimated to cost over $950 billion annually, half of which is due to lost wages (3). Musculoskeletal pain conditions are complex in nature and can arise from traumatic and/or non-tramatic injury to any part of the musculoskeletal system, including muscles, joints, and bones (73, 99). Pain from the musculoskeletal system is often classified as inflammatory or non-inflammatory. Inflammatory musculoskeletal pain conditions are either localized to one site or joint, like osteoarthritis (11, 31), or they are widespread, like rheumatoid arthritis (70). In parallel, pain either can be localized to the site of injury/inflammation (primary hyperalgesia) or can spread to non-injured sites (referred pain or secondary hyperalgesia) depending on the site and nature of the inflammation (34, 99). Non-inflammatory musculoskeletal pain conditions are more complex and much more difficult to characterize or diagnose because of the lack of observable tissue damage or inflammation. Similar to inflammatory conditions, non-inflammatory conditions can be localized to a specific site, like back or neck pain, or they can be widespread, like fibromyalgia (24, 33, 33a, 99).
Musculoskeletal pain conditions are associated with a decrease in local tissue pH (20, 30, 41, 53, 93). For instance, the pH of synovial fluid extracted from joints of rheumatoid arthritis patients is lower than that of normal individuals (20, 30, 41). Similarly, trapezius muscles of myofascial pain patients, with myofascial trigger points, have higher proton concentrations than in individuals with no pain (93). Moreover, fatigued and inflamed muscles both have higher concentrations of protons and lactic acid than non-fatigued muscles (38, 43, 92). Furthermore, when acidic solutions are infused into a muscle in healthy human subjects, it produces both primary and secondary (referred) pain (34, 104, 106), as well as primary and secondary hyperalgesia (i.e., decreases in pressure pain thresholds) (34). Acid-sensing ion channels (ASICs) are the primary sensors of decreased tissue pH (118), are located on muscle and joint nociceptors as well as in nonneuronal cells in local tissue, and are implicated in musculoskeletal pain (2, 98). This review will provide an overview of the properties of different types of ASICs and their role in musculoskeletal nociception.
Biophysical Properties of ASICs
Fluctuations in extracellular neuronal pH occur as a result of multiple pathological and non-pathological conditions that include ischemia (60, 84), inflammation (30, 105), fatiguing exercise (107), and neuronal activity (29). Accordingly, neurons express specific ASICs to detect and respond to these pH fluctuations (63). ASICs are voltage-independent proton-gated sodium channels that belong to the degenerin-epithelial Na+ channel (DEG-ENaC) family of ion channels (118). They are activated by a drop in extracellular pH (up to pH 5.0), as well as an increase in extracellular pH (up to pH 8.0) (23). Under acidic conditions, these receptors are activated to produce an amiloride-sensitive, fast-rising, and rapidly desensitizing inward current (117), whereas, under basic conditions, they produce a sustained outward current (23). In addition to pH fluctuations, ASICs are also activated by mechanical stimulation and are suggested to act as mechanoreceptors (14, 82, 85, 86). For example, genetically altering ASIC3 (formerly known as DRASIC or dorsal root ganglia ASIC) enhances the sensitivity of mechanoreceptors that detect light touch but also diminishes the sensitivity of mechanonociceptors that detect noxious pinch (86). On the other hand, genetically altering ASIC2 (formerly known as BNC1 or brain sodium channel-1) enhances the sensitivity of mechanoreceptors to light touch (85). In addition, ASIC2 is an important regulator of blood pressure and parasympathetic control of circulation by acting through baroreceptors on arterial sensory neurons (69, 75). Moreover, ASICs can be regulated by treatment with compounds like MitTx, amiloride, GMQ, FMRFamide, dynorphin A, big dynorphin, and Mambalgin (reviewed in Ref. 128).
There are six different ASIC subunits discovered to date; these include ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (1, 46, 65, 68, 87, 116, 128). These subunits are located throughout the central and peripheral nervous systems (103, 128) where they function as homomeric (identical subunits) or heteromeric (different subunits) trimers to sense changes in extracellular pH (63, 64, 118, 128). All ASIC subunits have the ability to assemble and form functional homomeric and heteromeric channels, except for ASIC2b and ASIC4, which only assemble to form heteromeric channels with other ASIC subunits and modulate channel activity (5, 46, 68).
The subunit composition of ASICs dictates the channel's properties, including acid sensitivity, ion selectivity, and desensitization kinetics (10, 48). Activation of homomeric ASIC1a, ASIC1b, and ASIC2a receptors, expressed in heterologous CHO (Chinese hamster ovary cells), by rapid application of pH4 produces a transient activation current (48). On the other hand, activation of homomeric ASIC3 produces two different activation profiles. In some cells, it produces a transient activation current similar to that observed in other ASIC homomers (10, 48, 68), whereas in other cells it produces a biphasic inward current composed of a transient activating current followed by slower current (FIGURE 1, A–E) (7, 48). Furthermore, desensitization (τ desensitization) of homomeric ASIC1a, ASIC1b, and ASIC2a is dependent on pH where greater acidic pH results in greater desensitization. This is in contrast to ASIC3, where the desensitization rate is independent of pH (FIGURE 1, G–H) (48). Similarly, pH sensitivity is also dependent on ASIC subunit. Homomeric ASIC2a is the least sensitive, requiring the lowest activation pH (pH50 of ∼4.5) (10, 12, 48, 128), and ASIC3 is the most sensitive, responding to pH within physiological ranges (pH50 of ∼6.6) (10, 27, 108, 128). ASIC1a and ASIC1b have similar proton affinities (pH50 of ∼6.1-5.8) (10, 48, 128); however, ASIC1a is activated across a wider pH range (Hill Slope = 0.75) than ASIC1b (Hill Slope = 4.8) (FIGURE 1F) (48).
FIGURE 1.
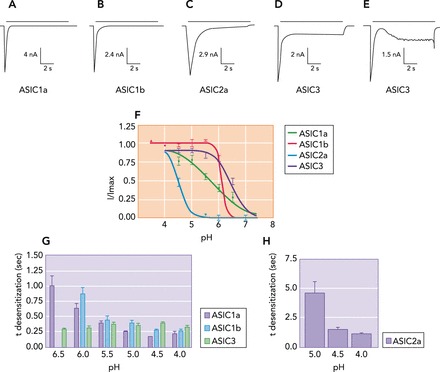
Acid-evoked currents and biophysical properties of homomeric ASICs
A–E: CHO-K1 cells transfected with homomeric ASICs were bathed in ringer solution and activated by rapid application of a pH 4 solution. F: proton affinities (pH sensitivity) for homomeric ASICs were measured by lowering the extracellular pH from 7.4 to 4. G and H: the rate of desensitization (τ desensitization) of ASICs was measured at different extracellular pH values and demonstrated that desensitization of all ASIC subunits except ASIC3 is increased when pH is lowered. Figure was adapted from Ref. 48, with permission from The Journal of Biological Chemistry.
Studying the functional features of homomeric ASIC subunits in heterologous cells helps explain some, but not all, of the observed acid-evoked currents produced by peripheral sensory neurons. For example, homomeric ASIC3, expressed in COS-7 cells, has similar pH sensitivity (activated at pH 7) and mimics the acid-evoked currents produced by cardiac sensory afferents (108, 124). However, the biophysical properties of H+-gated currents observed in medium to large dorsal root ganglia (DRG) neurons and skeletal muscle afferents differ from those observed in any homomeric receptor combination (10, 36), indicating that these currents are produced by heteromeric ASICs. Thus, in DRG, ASICs form heteromeric channels that have distinct properties and desensitization kinetics that differ from those formed by their homomeric subunits (5, 9, 10, 48, 95, 122). In addition, ASIC subunits also alter receptor trafficking and localization. For instance, ASIC1a localization on dendritic spines of hippocampal neurons is dependent on the presence of ASIC2 and their binding to postsynaptic density protein 95 (PSD-95) (47, 129).
In addition to their pH-dependent activation, ASICs are also activated and modulated by non-proton ligands at normal physiological pH (7.4) (119) that bind to a non-proton ligand-sensing domain (127). For instance, GMQ (2-guanidine-4-methylquinazoline) activates ASIC3 at normal pH and produces pain-related behaviors when injected into the hind paw in an ASIC3-dependent manner (127). Another ligand for this non-proton ligand-sensing domain is agmatine (AGM), a GMQ metabolite. Similar to GMQ, AGM produced hyperalgesia when injected intraplantarly, and inflammatory mediators (arachidonic acid and lactate) potentiate AGM-induced ASIC3 current and hyperalgesia (66). Furthermore, intraplantar injection of MitTx, the active component in Texas coral snake venom, activates both ASIC1 and ASIC3 at normal physiological pH and produces hyperalgesia (12). These data demonstrate that, in addition to protons, ASICs can be activated by non-proton natural ligands to produce pain.
Distribution of ASICs
The expression pattern and distribution of ASICs on sensory neurons shows they are ideally localized to play a role in musculoskeletal pain (78, 112, 116, 117). Acid-evoked currents in skeletal muscle sensory neurons are mediated by heteromeric ASICs comprised of ASIC1a, ASIC2, and ASIC3 subunits (2, 10, 36). In unidentified DRGs, ASIC1 is located on small- (<25 μm), medium-, and large-diameter cells (>40 μm), whereas ASIC2 and ASIC3 are mostly located on medium- and large-diameter cells (2). In small sensory DRG neurons, ASIC1a is expressed in 62% of substance P-positive cells and 41% of isolectin B4 (IB4)-positive cells (112), whereas ASIC3 is expressed in 50% of substance P-positive cells, 43% of IB4-positive cells (81, 112), 68% of TrkA-positive cells, 25% of TrkC-positive cells, and 12% of TRPV1-positive neurons (78). Moreover, small sensory neurons innervating skeletal muscles express more ASIC3 than those innervating the skin, and 80% of these ASIC3-positive skeletal muscle afferents co-express CGRP, affirming the role of these receptors in detecting muscle acidosis and pain (78). Similarly, in DRG neurons innervating the knee joint, ASIC3 is located on 31% of joint afferents and colocalizes with 20% of CGRP-positive cells (52).
In the CNS, ASIC subunits are also localized in regions that are involved in nociceptive transmission and plasticity (32, 117). For instance, ASIC1a, ASIC2a, and ASIC2b are all expressed in dorsal horn neurons of the spinal cord, a region where nociceptive signals are received from DRGs and relayed to the brain (8, 28, 121). ASIC1a is expressed in the peri-aqueductal grey a primary brain region responsible for descending pain modulation (19). ASICs are also localized to nonneuronal cells like muscles (FIGURE 2A), synoviocytes (FIGURE 3, D AND E), macrophages, and dendritic cells, where they act as pH sensors that participate in nociception and inflammation (42, 61, 62, 111, 114).
FIGURE 2.
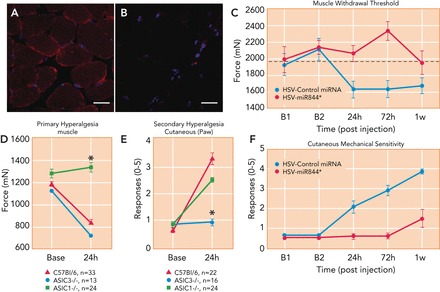
ASICs are expressed on muscle tissue and play a role in carrageenan-induced inflammatory muscle pain
A and B: representative muscle tissue sections from wild-type (A) and ASIC3−/− (B) mice, showing ASIC3 (red) and DAPI (nuclear stain, blue) staining. Figure reproduced from Ref. 114 with permission. C: genetic knockdown of ASIC3 in primary afferent fibers innervating muscle, using HSV-miRNA to ASIC3 (red, HSV-miR844), prevent the development of primary muscle hyperalgesia induced by intramuscular carrageenan injection compared with animals treated with control HSV-miRNA (blue). *Significantly greater than controls (P < 0.05). Reproduced from Ref. 114 with permission. D: muscle withdrawal thresholds (primary hyperalgesia) before and after induction of muscle inflammation in WT (red), ASIC3−/− (blue), and ASIC1−/− (green) mice. ASIC1−/− do not develop muscle hyperalgesia 24 h after inflammation with carrageenan compared with WT and ASIC3−/− mice. *Significantly greater than WT and ASIC3−/− (P < 0.05). Reproduced from Ref. 115 with permission. E: responses to repeated mechanical stimulation of the paw (secondary hyperalgesia) before and after induction of muscle inflammation in WT (red), ASIC3−/− (blue), and ASIC1−/− (green) mice. ASIC3−/− do not develop secondary hyperalgesia 24 h after inflammation with carrageenan compared with WT and ASIC1−/− mice. Reproduced from Ref. 115 with permission. F: genetic knockdown of ASIC3 in primary afferent fibers innervating muscle, using HSV-miRNA to ASIC3 (red, HSV-miR844), prevents development of secondary hyperalgesia induced by intramuscular carrageenan compared with animals treated with control HSV-miRNA (blue). *Significantly greater than controls (P < 0.05). Reproduced from Ref. 114 with permission.
FIGURE 3.
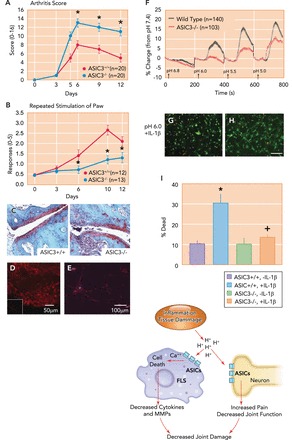
ASIC3 mediates the nociception produced by collagen-antibody-induced arthritis, while protecting the joint from inflammation and destruction
A: ASIC3−/− mice (blue) show enhanced inflammation after induction of collagen antibody-induced arthritis (CAIA) compared with WT mice (red). *Significantly greater than controls (P < 0.05). Reproduced from Ref. 102 with permission. B: CAIA-induced secondary hyperalgesia of the paw (number of responses to repeated mechanical stimuli) was attenuated in the ASIC3−/− mice (blue) compared with wild-type mice (red). *Significantly lower than wild-type mice (P < 0.05). Reproduced from Ref. 102 with permission. C: representative sections from the ankle joint of animals 12 days after induction of CAIA stained with Safronin-0 from wild-type (ASIC3+/+) and ASIC3−/− mice. Notice enhanced inflammation and damage in tissue section from an ASIC3−/− mouse. Reproduced from Ref. 102 with permission. D: fluorescent micrograph from the synovium of the knee joint immunohistochemically stained for ASIC3 in wild-type mice. Inset shows ASIC3 immunohistochemistry in synovium from ASIC3−/− mice. Reprinted from Ref. 61 with permission. E: fluorescent immunohistochemical staining for ASIC3 (red) in cultured fibroblast-like synovocytes (FLS). Nuclei are stained with TO-PRO3 (blue). Reprinted from Ref. 61 with permission. F: intracellular calcium concentration ([Ca]2+) was calculated in wild-type (black) and ASIC3−/− (red) FLS, loaded with [Ca]2+-sensitive fluorescent indicator Oregon Green BAPTA-1 AM (OGB-1), in response to exposure to decreasing pH values from 6.8 to 5 (150-s incubation). The response was normalized as percentage change from pH 7.4. Reproduced from Ref. 42 with permission. G and H: live/dead assay of cells of cultured FLS extracted from wild-type (G) and ASIC3−/− (H) mice. FLS were treated with pH 6 after prior incubation with IL-1β. Green staining represents live cells, whereas red staining represents dead cells. Reproduced and modified with permission from Ref. 102. I: the average % dead cells after treating FLS cells with pH 6 and IL-1β in wild-type (red) and ASIC3−/− (blue) FLS. Notice a significant increase in the number of dead cells in wild-type mice treated with pH 6 and IL-1b (*) that did not occur in ASIC3−/− mice treated with pH 6 and IL-1b (+). Reproduced and modified with permission from Ref. 102. J: schematic presentation for the protective role of ASIC3 in collagen-induced arthritis model (CAIA). In this model, the acidic enviroment assocaited with inflammation activates ASIC3 on FLS. Activation of ASIC3 enhances intracellular Ca2+ release, leading to cell death, which limits the secretion of inflammatory cytokines and metalloproteases (MMPs) by FLS and limits joint damage (42). ASIC3 located on neurons is also activated by decreases in pH associated with inflammation, which increases pain limiting joint function, to ultimately decrease joint damage. The red arrows show the proposed pathways for activation of ASICs in FLS and in neurons, and how they ultimately lead to reduced joint damage.
ASICs and Nociception
ASICs and Non-inflammatory Muscle Pain
Skeletal muscle pain is associated with tissue acidosis (53, 93), and infusion of acid into muscle produces pain (34, 104, 106). In humans, intramuscular infusion of an acidic solution (pH 5.2) into the tibialis anterior muscle of the leg produces local muscle pain at the site of infusion and referred pain at the ankle (34). The acid infusion also results in decreased pressure pain thresholds at the site of infusion and in the referred pain area at the ankle (34), demonstrating that human muscle nociceptors are activated by acidic pH to produce both primary and secondary hyperalgesia. The pH that activates ASICs on muscle afferents is affected by the presence of muscle metabolites (67). For instance, <4% of group III and IV DRG muscle afferents respond to pH values of >6. However, when these DRG neurons are treated with a combination of ATP and lactate at pH 7.4, the percentage of responding neurons increases to 44% (56, 67). This effect is blocked by the ASICs antagonist A-317567 (67), indicating that ASICs located on muscle nociceptors are activated at neutral physiological pH.
In mice, chronic widespread mechanical hyperalgesia develops after two injections of pH 4 saline, given 5 days apart, into the gastrocnemius muscle (16, 94, 100). The hyperalgesia develops at the site of injection in the muscle, in a referred area in the paw, contralaterally in the muscle and paw, as well as in the viscera (77, 94, 100, 126). This hyperalgesia is not accompanied by observable tissue damage of the muscle or nerve (37, 100). This model mimics chronic widespread pain conditions like fibromyalgia and is sensitive to similar pharmacological and nonpharmacological treatments (4, 25). The first acid injection produces a transient hyperalgesia and primes muscle nociceptors for the chronic hyperalgesia (30 days) produced by the second injection (16, 100). Co-injection of the ASIC3 antagonist APETx2 with the first acid injection attenuates the initial transient hyperalgesia and abolishes the priming activity, such that the second injection of acid only produces a transient hyperalgesia instead of the long-lasting hyperalgesia (15, 16). Moreover, when APETx2 is co-injected with the second injection of acid, it also prevents the chronic hyperalgesia (59), indicating that ASIC3 contributes to the priming of nociceptors as well as development of the chronic hyperalgesia. However, when an ASIC antagonist is given after the development of hyperalgesia (i.e., after the second acid injection) it does not reverse the hyperalgesia, suggesting that ASIC3 is not involved in the maintenance of non-inflammatory hyperalgesia (37, 59).
The hyperalgesia induced by repeated acid injections does not develop in ASIC3−/− mice; however, ASIC1−/− mice still develop hyperalgesia after repeated acid injections (101). Twenty-four hours after the second injection, when animals are hyperalgesic, there are no changes in ASIC-like current properties in DRG neurons innervating the injected muscle (37). On the other hand, sensitization of neurons in the spinal cord parallels the widespread hyperalgesia in this model with expansion of receptive fields to include the contralateral hind limb and enhanced responsiveness to mechanical stimuli. These changes in dorsal horn neurons do not occur in ASIC3−/− mice (101). Once developed, this hyperalgesia is therefore independent of ASICs but appears to be mediated through changes in spinal and supraspinal mechanisms, including activation of the PKA-cAMP-pCREB pathway in the spinal dorsal horn (49), activation of the PKC-ERK pathway in the capsular central amygdaloid nucleus (18), and activation of NMDA and non-NMDA glutamate receptors in the spinal cord and brain stem (21, 90, 97).
Another model of non-inflammatory muscle nociception is induced by subcutaneous injection of reserpine (79, 80, 109). This model shares common aspects of chronic widespread pain conditions, including the long-lasting tactile and deep tissue hyperalgesia, dysfunction of descending pain inhibitory systems, and depression with the absence of observable damage (79, 80, 109). Subcutaneous injections of reserpine for 3 days depletes biogenic amines (dopamine, norepinephrine, and 5-hydroxytryptamine) in the spinal cord, thalamus, and prefrontal cortex. The reserpine-model increases expression of ASIC3 in DRG and produces widespread muscle and tactile hyperalgesia that is reversed by subcutaneous injection of APETx2 (79, 80, 109). Thus ASIC3 plays a role in the development of chronic widespread pain.
ASICs and Inflammatory Muscle Pain
The microenvironment of inflamed tissue is usually more acidic than surrounding normal tissue (53, 105). Both ASIC1 and ASIC3 are expressed on innate immune cells like macrophages and dendritic cells (62, 111), suggesting a possible role for ASICs in the inflammation process. In support, extracellular acidosis increases expression of maturation markers like CD80, CD86, and MHCII on the surface of macrophages and dendritic cells through activation of ASICs (62, 111). Acidosis also stimulates human monocytes to produce IL-1β, a proinflammatory cytokine involved in pain and inflammation (55, 91). In neurons, inflammatory mediators enhance ASIC signaling and expression. For example, proinflammatory mediators like NGF, serotonin, interleukin-1, and bradykinin increase the number of ASIC-expressing neurons and enhance the ASIC-like current intensity in sensory and nociceptive DRG neurons (71, 72, 112). Intramuscular injection of carrageenan results in infiltration of proinflammatory immune cells to the site of injection (22, 35), extravasation of red blood cells, rhabdomyolysis, and vasculitis (125), and enhanced expression of ASIC3 on nerve afferents surrounding blood vessels (125). Both the extravasation of red blood cells and vasculitis are attenuated in ASIC3−/− mice (125), indicating that ASIC3 plays a role in inflammatory pathogenesis. Thus ASICs may alter immune cell function to enhance release of inflammatory mediators that can subsequently activate nociceptors.
Carrageenan-induced muscle inflammation is a commonly used animal model of inflammatory muscle pain and is accompanied by primary hyperalgesia measured directly by squeezing the muscle with tweezers, and secondary hyperalgesia measured by applying noxious stimuli to the paw (35, 88). In animals with muscle inflammation, primary hyperalgesia does not develop in the ASIC1−/− mice, whereas secondary hyperalgesia does not occur in ASIC3−/− mice (FIGURE 2, D AND E) (115, 125), suggesting primary hyperalgesia is mediated by ASIC1 and secondary hyperalgesia is mediated by ASIC3. Reexpressing ASIC3 into primary afferents innervating muscle (using an HSV-1 vector), but not the skin, of ASIC3−/− mice reestablishes the hyperalgesia normally observed in wild-type mice with muscle inflammation. In contrast, selective knockdown of ASIC3 in DRG innervating the inflamed muscle, using miRNA (HSV-miR844), prevents development of both primary and secondary hyperalgesia (FIGURE 2, C AND F) (114). Interestingly, in CHO cells cotransfected with ASIC1 and ASIC3, knockdown of ASIC3 reduces the amplitude of pH-evoked currents, suggesting an overall inhibition of the surface expression of heteromeric channels that contain ASIC3 (i.e., ASIC3 was required for trafficking to the membrane) (114). In parallel, nonselective pharmacological blockade of ASICs using A-317567 reverses both the primary and secondary inflammatory muscle hyperalgesia produced by carrageenan, demonstrating the importance of ASICs in the maintenance of inflammatory muscle pain (115). Furthermore, DRG innervating the inflamed muscle shows increased pH-evoked current amplitudes and a lower rate of recovery from desensitization compared with DRG from control uninflamed mice (38), and carrageenan-induced muscle inflammation increases mRNA expression of ASIC2 and ASIC3 in DRG (115). Thus alteration in ASIC expression and function mediates the hyperalgesia associated with carrageenan-induced inflammation.
ASICs and Inflammatory Joint Pain
Rheumatoid arthritis is a debilitating disease characterized by inflammation, stiffness, and pain of joints (57, 58, 73, 83, 96, 99). Joint inflammation is usually associated with a decrease in local synovial fluid pH to as low as pH 6 (20, 30, 39–41), within the physiological pH range for activation of ASIC3 (10, 48). ASIC3 is present on primary afferents innervating the knee joint, articular cartilage, growth plate, meniscus, and Type B synoviocytes lining the knee joint (FIGURE 3, D AND E) (51, 52, 61), suggesting that ASIC3 could be involved in both arthritic pain and inflammation.
Localized inflammatory hyperalgesia is modeled by injection of carrageenan into a single joint (45, 89). This results in infiltration of inflammatory cells that persists for months and is accompanied by primary and secondary hyperalgesia (45, 51, 89). Carrageenan-induced joint inflammation increases expression of ASIC3 and colocalization of ASIC3 with CGRP on primary afferent fibers innervating the synovium. In DRG innervating the knee joint, ASIC3 expression increases from 31% to 50%, CGRP expression increases from 30% to 50%, and colocalization of ASIC3 and CGRP increases from 19% to 31% (51, 52). Interestingly, ASIC3−/− mice with knee joint inflammation only develop primary and not secondary hyperalgesia, indicating that ASIC3 mediates the development of secondary hyperalgesia in this model (51). Thus, like muscle inflammation with carrageenan, there is an upregulation of ASIC3 in primary afferents innervating the inflamed tissue, and ASIC3 mediates secondary hyperalgesia.
Another model used to study arthritic pain is the collagen-induced arthritis model (CAIA), which mimics widespread inflammatory arthritis (e.g., rheumatoid arthritis) (45). Injecting mice with an antibody to collagen Type II results in widespread distal joint inflammation, joint destruction, and enhanced joint expression of inflammatory markers [e.g., IL-6, MMP-3 (metalloprotease-3) and MMP-13] (102). In parallel, there is primary hyperalgesia at ankle joint, secondary hyperalgesia of the skin on the paw, and accompanying decreased activity levels that lasts for days after induction of CAIA (102). Similar to carrageenan models, ASIC3−/− mice with CAIA do not develop secondary hyperalgesia of the paw but still develop primary hyperalgesia (102). Furthermore, ASIC3−/− mice with CAIA have higher activity levels than wild-type mice with CAIA.
Interestingly, despite the decreased hyperalgesia, these ASIC3−/− mice show greater inflammation, more joint destruction, and increased inflammatory mediator mRNA than their wild-type littermates (FIGURE 3, A–C) (42, 102). In addition, cultured fibroblast-like synoviocytes (FLS) from wild-type mice show increased release of hyaluronan, increased intracellular calcium concentrations, and reduced phosphorylation of ERK (p-ERK; extracellular signal-regulated kinase) to acidic pH. After treatment of FLS with the inflammatory mediator IL-1β, there is increased expression of ASIC3, and acidic pH enhances intracellular calcium concentrations and p-ERK, and produces cell death (FIGURE 3, F–I) (42, 61, 102). The increases in hyaluronan and intracellular calcium, and decreases in p-ERK to acidic pH do not occur in ASIC3−/− mice. Furthermore, the cell death to the combination of IL-1β and acidic pH also does not occur in ASIC3−/− FLS (42, 61, 102) and is prevented by blockade of intracellular calcium and ERK. Together, these data suggest that ASIC3 plays a protective role in inflammatory arthritis by reducing synovitis and the accompanying inflammation and joint destruction through facilitation of synoviocyte cell death, and thus ASIC3 activation may reduce disease severity (FIGURE 3J). In addition, ASIC3 may provide joint protection by increasing the secretion of hyaluronan (61), an important component of articular cartilage that protects it from damage due to compression and inflammation (17). Lastly, ASIC3 activation on nociceptors results in pain and reduced mobility during inflammation that may serve to protect the joint from excessive damage (FIGURE 3J).
Injection of mono-iodoacetate into a single joint is a model of degenerative knee osteoarthritis that results in destructive histological changes that include cartilage degeneration, loss of chondrocytes, thickening of subchondral bone, and osteophyte formation in the knee joint (45, 54). This model induces nociceptive behaviors as measured by reduced weight bearing on the injured limb and enhanced sensitivity to noxious mechanical stimuli of the paw (secondary hyperalgesia) (54). Intra-articular injection of the ASIC3 antagonist APETx2 in this model attenuates cartilage damage and hyperalgesia, indicating that, in this model, ASIC3 contributes to joint damage rather than to joint protection (54).
Fatigue-Induced Muscle Pain
Muscle fatigue is the failure of a muscle to produce force as a result of excessive exercise (110) and can be associated with pain (76, 120). During muscle fatigue, tissue pH drops to ∼6.5 (43, 92), within the physiological pH range for activation of ASIC3 (10, 48). Moreover, fatigue is characterized by accumulation of muscle metabolites like lactic acid that can sensitize ASICs on skeletal muscle nociceptors to normal pH values of 7.3 (67, 76, 120). Our laboratory developed a mouse model of fatigue-induced hyperalgesia by combining low-intensity muscle insults (pH 5.0 saline) with 6 min of fatiguing contractions of the gastrocnemius muscle (126). The fatiguing contraction decreases pH, and the combination of muscle insult with fatigue produces muscle hyperalgesia that lasts for 2–4 wk (44). The fatigue-induced hyperalgesia is abrogated by pharmacological blockade of ASIC3 with APETx2 and genetic deletion of ASIC3 using ASIC3−/− mice. Interestingly, downregulating ASIC3 in primary afferents innervating the muscle (using HSV-miR844) has no effect on this hyperalgesia. Instead, there is an upregulation of macrophages after the fatiguing stimulation, and deletion of muscle macrophages using clodronate liposomes attenuates the hyperalgesia (43). Similarly, eccentric exercise produces fatigue and results in hyperalgesia that lasts for days (referred to as delayed-onset muscle soreness); this hyperalgesia is reversed by the nonselective ASIC antagonist amiloride (35). Together, these data suggest that hyperalgesia develops after decreases in pH produced by fatigue combined with a muscle insult and that ASIC3, potentially on local muscle macrophages, mediates the development of hyperalgesia in response to fatigue.
Other Models of Muscle Pain
Postoperative muscle pain.
Brennan and colleagues developed a rat model of postoperative pain that produces hyperalgesia after incision of the skin and muscle (13). In this model, the proportion of group III and group IV muscle afferent fibers sensitive to lactic acid (pH 6.0) stimulation increases from 20.8% in sham muscles to 55.4% in incised muscles (123). Incision also increases the expression of ASIC3 on muscle afferent fibers, most of which are small diameter, by 13% (26). In addition, thermal and mechanical hyperalgesia produced by incision are attenuated in ASIC3−/− mice and by blockade of ASIC3 with APETx2 (26). Thus ASIC3 appears to play a role in mediating postoperative pain associated with muscle incision.
Chemotherapy-induced muscle pain.
ASIC3 also plays a role in muscle pain induced by the anticancer drug cisplatin. Systemic administration of cisplatin, once a week for 5 wk, produces muscle hyperalgesia that is attenuated by the nonselective ASIC antagonist amiloride. In addition, cisplatin increases ASIC3 expression on muscle primary afferent fibers, indicating that cisplatin-induced muscle pain is mediated by ASIC3 activation (50).
Summary
Tissue acidosis occurs in most musculoskeletal pain conditions, including inflammatory, non-inflammatory, and fatigue-induced musculoskeletal pain. Consistently, injecting or infusing acid into muscle produces both primary and secondary (referred) musculoskeletal pain, and primary and secondary hyperalgesia. ASICs are not only localized in primary afferent fibers innervating muscle and joint tissue but also in nonneuronal cells that can communicate with nociceptors including muscles, cartilage synovium, and immune cells. ASIC expression on skeletal muscle and joint afferents colocalizes with other pain-producing peptides (substance P and CGRP) and receptors (TrkA and TRPV1), and are modifiable by extracellular mediators including cytokines and fatigue metabolites.
ASICs clearly play a role in multiple models of musculoskeletal pain as well as in the inflammatory process. Activation of ASICs is clearly pro-nociceptive in musculoskeletal models of hyperalgesia, including non-inflammatory and inflammatory models. Of all the ASICs, ASIC1 and ASIC3 are the most implicated in musculoskeletal hyperalgesia. In non-inflammatory pain models, ASIC3 is required for induction of both primary and secondary hyperalgesia. In inflammation models, ASIC1 is responsible for primary hyperalgesia, whereas ASIC3 is responsible for secondary hyperalgesia. Inflammatory models are associated with increases in expression of ASIC3 in nociceptors and are involved in both the induction and the maintenance of the hyperalgesia.
Despite mediating nociception, ASICs can also modulate inflammation, and this modulation is dependent on tissue type, cell type, and animal model. On immune cells, protons can enhance release of inflammatory mediators and increase expression of maturation markers. In muscle inflammation, ASIC3−/− mice have less vasculitis and red blood cell extravasation, and, in a joint osteoarthritis model, blockade of ASIC3 prevents joint destruction. On the other hand, in an inflammatory arthritis model, there is enhanced inflammation and joint destruction in ASIC3−/− mice, and enhanced synoviocyte cell death in response to acidic pH combined with an inflammatory mediator.
Therefore, targeting of ASICs, with ASIC antagonists, for musculoskeletal pain therapy should be done with careful attention to potential effects on non-nociceptive processes, including inflammation and tissue damage. Furthermore, it may be possible to target ASICs with ASIC agonists to alter disease severity by limiting synovitis in those with inflammatory arthritic conditions like rheumatoid arthritis. Thus future experiments will need to be conducted to examine how to best target ASICs for the treatment of pain and inflammation in a disease-specific and tissue-specific manner.
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: R.E.A. and K.A.S. prepared figures; R.E.A. and K.A.S. drafted manuscript; R.E.A. and K.A.S. edited and revised manuscript; R.E.A. and K.A.S. approved final version of manuscript.
References
- 1.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport 11: 2217–2222, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 99: 2326–2331, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson G, and American Academy of Orthopaedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: American Acad. of Orthopaedic Surgeons, 2008. [Google Scholar]
- 4.Arendt-Nielsen L, Fernandez-de-Las-Penas C, Graven-Nielsen T. Basic aspects of musculoskeletal pain: from acute to chronic pain. J Man Manip Ther 19: 186–193, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem 279: 18296–18305, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem 72: 51–57, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Baron A, Voilley N, Lazdunski M, Lingueglia E. Acid sensing ion channels in dorsal spinal cord neurons. J Neurosci 28: 1498–1508, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassilana F, Champigny G, Waldmann R, de Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem 272: 28819–28822, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21: 16–21, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Bohlen CJ, Chesler AT, Sharif-Naeini R, Medzihradszky KF, Zhou S, King D, Sanchez EE, Burlingame AL, Basbaum AI, Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 479: 410–414, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain 64: 493–501, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Chen CC, Wong CW. Neurosensory mechanotransduction through acid-sensing ion channels. J Cell Mol Med 17: 337–349, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WN, Chen CC. Acid mediates a prolonged antinociception via substance P signaling in acid-induced chronic widespread pain. Mol Pain 10: 30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WN, Lee CH, Lin SH, Wong CW, Sun WH, Wood JN, Chen CC. Roles of ASIC3, TRPV1, NaV1.8 in the transition from acute to chronic pain in a mouse model of fibromyalgia. Mol Pain 10: 40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen 7: 79–89, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Cheng SJ, Chen CC, Yang HW, Chang YT, Bai SW, Chen CC, Yen CT, Min MY. Role of extracellular signal-regulated kinase in synaptic transmission and plasticity of a nociceptive input on capsular central amygdaloid neurons in normal and acid-induced muscle pain mice. J Neurosci 31: 2258–2270, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coryell MW, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie JA. Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit. Biol Psychiatry 62: 1140–1148, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Cummings NA, Nordby GL. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum 9: 47–56, 1966. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva LF, Desantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. J Pain 11: 378–387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol 51: 19–31, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delaunay A, Gasull X, Salinas M, Noel J, Friend V, Lingueglia E, Deval E. Human ASIC3 channel dynamically adapts its activity to sense the extracellular pH in both acidic and alkaline directions. Proc Natl Acad Sci USA 109: 13124–13129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng G, Zhang Y, Cai H, Gu W, Cai Y, Xie L, Liu B, Li J, Li S, Cheng D, Zhao Q. [Effects of physical factors on neck or shoulder pain and low back pain of adolescents]. Zhonghua yi xue za zhi 94: 3411–3415, 2014. [PubMed] [Google Scholar]
- 25.DeSantana JM, da Cruz KM, Sluka KA. Animal models of fibromyalgia. Arthritis Res Ther 15: 222, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deval E, Noel J, Gasull X, Delaunay A, Alloui A, Friend V, Eschalier A, Lazdunski M, Lingueglia E. Acid-sensing ion channels in postoperative pain. J Neurosci 31: 6059–6066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 23: 1516–1525, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duan B, Wu LJ, Yu YQ, Ding Y, Jing L, Xu L, Chen J, Xu TL. Upregulation of acid-sensing ion channel ASIC1a in spinal dorsal horn neurons contributes to inflammatory pain hypersensitivity. J Neurosci 27: 11139–11148, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endres W, Grafe P, Bostock H, ten Bruggencate G. Changes in extracellular pH during electrical stimulation of isolated rat vagus nerve. Neurosci Lett 64: 201–205, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Farr M, Garvey K, Bold AM, Kendall MJ, Bacon PA. Significance of the hydrogen ion concentration in synovial fluid in rheumatoid arthritis. Clin Exp Rheumatol 3: 99–104, 1985. [PubMed] [Google Scholar]
- 31.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology 39: 237–246, 2002. [PubMed] [Google Scholar]
- 32.Ferreira J, Santos AR, Calixto JB. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci 65: 1059–1066, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira PH, Beckenkamp P, Maher CG, Hopper JL, Ferreira ML. Nature or nurture in low back pain? Results of a systematic review of studies based on twin samples. Eur J Pain 17: 957–971, 2013. [DOI] [PubMed] [Google Scholar]
- 33a.Fibromyalgia: poorly understood; treatments are disappointing. Prescrire Intl 18: 169–173, 2009. [PubMed] [Google Scholar]
- 34.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain 140: 254–264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain 140: 292–304, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J 27: 793–802, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gautam M, Benson CJ, Ranier JD, Light AR, Sluka KA. ASICs do not play a role in maintaining hyperalgesia induced by repeated intramuscular acid injections. Pain Res Treat 2012: 817347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautam M, Benson CJ, Sluka KA. Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience 170: 893–900, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geborek P, Saxne T, Pettersson H, Wollheim FA. Synovial fluid acidosis correlates with radiological joint destruction in rheumatoid arthritis knee joints. J Rheumatol 16: 468–472, 1989. [PubMed] [Google Scholar]
- 40.Goldie I, Nachemson A. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop Scand 40: 634–641, 1969. [DOI] [PubMed] [Google Scholar]
- 41.Goldie I, Nachemson A. Synovial pH in rheumatoid knee joints. II. The effect of local corticosteroid treatment. Acta Orthop Scand 41: 354–362, 1970. [DOI] [PubMed] [Google Scholar]
- 42.Gong W, Kolker SJ, Usachev Y, Walder RY, Boyle DL, Firestein GS, Sluka KA. Acid-sensing ion channel 3 decreases phosphorylation of extracellular signal-regulated kinases and induces synoviocyte cell death by increasing intracellular calcium. Arthritis Res Ther 16: R121, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory NS, Brito RG, Fusaro MC, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol. In press. [DOI] [PMC free article] [PubMed]
- 44.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex-dependent manner. Pain 154: 2668–2676, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain 14: 1255–1269, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grunder S, Geissler HS, Bassler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport 11: 1607–1611, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Harding AM, Kusama N, Hattori T, Gautam M, Benson CJ. ASIC2 subunits facilitate expression at the cell surface and confer regulation by PSD-95. PLos One 9: e93797, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem 279: 11006–11015, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 23: 5437–5445, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain 149: 393–405, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Ikeuchi M, Kolker SJ, Burnes LA, Walder RY, Sluka KA. Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137: 662–669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain 10: 336–342, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 208: 191–194, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Izumi M, Ikeuchi M, Ji Q, Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci 19: 77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jancic CC, Cabrini M, Gabelloni ML, Rodriguez Rodrigues C, Salamone G, Trevani AS, Geffner J. Low extracellular pH stimulates the production of IL-1beta by human monocytes. Cytokine 57: 258–268, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, Ding C. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis 74: 703–710, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Kandahari AM, Yang X, Dighe AS, Pan D, Cui Q. Recognition of immune response for the early diagnosis and treatment of osteoarthritis. J Immunol Res 2015: 192415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 161: 950–960, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katsura K, Kristian T, Smith ML, Siesjo BK. Acidosis induced by hypercapnia exaggerates ischemic brain damage. J Cereb Blood Flow Metab 14: 243–250, 1994. [DOI] [PubMed] [Google Scholar]
- 61.Kolker SJ, Walder RY, Usachev Y, Hillman J, Boyle DL, Firestein GS, Sluka KA. Acid-sensing ion channel 3 expressed in type B synoviocytes and chondrocytes modulates hyaluronan expression and release. Ann Rheum Dis 69: 903–909, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong X, Tang X, Du W, Tong J, Yan Y, Zheng F, Fang M, Gong F, Tan Z. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol 281: 44–50, 2013. [DOI] [PubMed] [Google Scholar]
- 63.Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res 214: 150–154, 1981. [DOI] [PubMed] [Google Scholar]
- 64.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience 5: 2325–2327, 1980. [DOI] [PubMed] [Google Scholar]
- 65.Kweon HJ, Suh BC. Acid-sensing ion channels (ASICs): therapeutic targets for neurological diseases and their regulation. BMB Rep 46: 295–304, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li WG, Yu Y, Zhang ZD, Cao H, Xu TL. ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 6: 88, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem 272: 29778–29783, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 64: 885–897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majithia V, Geraci SA. Rheumatoid arthritis: diagnosis and management. Am J Med 120: 936–939, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem 278: 48907–48913, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Macrophages in rheumatoid arthritis. Histol Histopathol 22: 581–586, 2007. [DOI] [PubMed] [Google Scholar]
- 74.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best practice & research. Clin Rheum 21: 403–425, 2007. [DOI] [PubMed] [Google Scholar]
- 75.McCleskey EW. A molecular sensor for the baroreceptor reflex? Neuron 64: 776–777, 2009. [DOI] [PubMed] [Google Scholar]
- 76.Meeus M, Nijs J, Meirleir KD. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: a systematic review. Eur J Pain 11: 377–386, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain 126: 54–63, 2006. [DOI] [PubMed] [Google Scholar]
- 78.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagakura Y, Oe T, Aoki T, Matsuoka N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain 146: 26–33, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Nagakura Y, Takahashi M, Noto T, Sekizawa T, Oe T, Yoshimi E, Tamaki K, Shimizu Y. Different pathophysiology underlying animal models of fibromyalgia and neuropathic pain: comparison of reserpine-induced myalgia and chronic constriction injury rats. Behav Brain Res 226: 242–249, 2012. [DOI] [PubMed] [Google Scholar]
- 81.Olson TH, Riedl MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport 9: 1109–1113, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Omerbasic D, Schuhmacher LN, Bernal Sierra YA, Smith ES, Lewin GR. ASICs and mammalian mechanoreceptor function. Neuropharmacology 94: 80–86, 2014. [DOI] [PubMed] [Google Scholar]
- 83.Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S. The burden of musculoskeletal conditions. PLos One 9: e90633, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paljarvi L, Rehncrona S, Soderfeldt B, Olsson Y, Kalimo H. Brain lactic acidosis and ischemic cell damage: quantitative ultrastructural changes in capillaries of rat cerebral cortex. Acta Neuropathol (Berl) 60: 232–240, 1983. [DOI] [PubMed] [Google Scholar]
- 85.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature 407: 1007–1011, 2000. [DOI] [PubMed] [Google Scholar]
- 86.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem 271: 7879–7882, 1996. [DOI] [PubMed] [Google Scholar]
- 88.Radhakrishnan R, Bement MK, Skyba D, Sluka KA, Kehl LJ. Models of muscle pain: carrageenan model and acidic saline model. Curr Protoc Pharmacol Chapter 5: Unit 5.35, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Radhakrishnan R, Moore SA, Sluka KA. Unilateral carrageenan injection into muscle or joint induces chronic bilateral hyperalgesia in rats. Pain 104: 567–577, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett 457: 141–145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev 60: 57–64, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand Suppl 556: 83–91, 1986. [PubMed] [Google Scholar]
- 93.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol 99: 1977–1984, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Sharma NK, Ryals JM, Liu H, Liu W, Wright DE. Acidic saline-induced primary and secondary mechanical hyperalgesia in mice. J Pain 10: 1231–1241, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci 31: 9723–9734, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simao AP, Avelar NC, Tossige-Gomes R, Neves CD, Mendonca VA, Miranda AS, Teixeira MM, Teixeira AL, Andrade AP, Coimbra CC, Lacerda AC. Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch Phys Med Rehabil 93: 1692–1700, 2012. [DOI] [PubMed] [Google Scholar]
- 97.Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain 98: 69–78, 2002. [DOI] [PubMed] [Google Scholar]
- 98.Sluka KA, Gregory NS. The dichotomized role for acid sensing ion channels in musculoskeletal pain and inflammation. Neuropharmacology 94: 58–63, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sluka KA, International Association for the Study of Pain. Mechanisms and Management of Pain for the Physical Therapist. Seattle, WA: IASP Press, 2009. [Google Scholar]
- 100.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 24: 37–46, 2001. [DOI] [PubMed] [Google Scholar]
- 101.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106: 229–239, 2003. [DOI] [PubMed] [Google Scholar]
- 102.Sluka KA, Rasmussen LA, Edgar MM, O'Donnell JM, Walder RY, Kolker SJ, Boyle DL, Firestein GS. Acid-sensing ion channel 3 deficiency increases inflammation but decreases pain behavior in murine arthritis. Arthritis Rheum 65: 1194–1202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel 12: 693–704, 2009. [PMC free article] [PubMed] [Google Scholar]
- 104.Steen AE, Reeh PW, Geisslinger G, Steen KH. Plasma levels after peroral and topical ibuprofen and effects upon low pH-induced cutaneous and muscle pain. Eur J Pain 4: 195–209, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Steen KH, Steen AE, Reeh PW. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci 15: 3982–3989, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steen KH, Wegner H, Meller ST. Analgesic profile of peroral and topical ketoprofen upon low pH-induced muscle pain. Pain 93: 23–33, 2001. [DOI] [PubMed] [Google Scholar]
- 107.Steinhagen C, Hirche HJ, Nestle HW, Bovenkamp U, Hosselmann I. The interstitial pH of the working gastrocnemius muscle of the dog. Pflügers Arch 367: 151–156, 1976. [DOI] [PubMed] [Google Scholar]
- 108.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci USA 98: 711–716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taguchi T, Katanosaka K, Yasui M, Hayashi K, Yamashita M, Wakatsuki K, Kiyama H, Yamanaka A, Mizumura K. Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain 156: 415–427, 2015. [DOI] [PubMed] [Google Scholar]
- 110.Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol 33: 400–405, 2006. [DOI] [PubMed] [Google Scholar]
- 111.Tong J, Wu WN, Kong X, Wu PF, Tian L, Du W, Fang M, Zheng F, Chen JG, Tan Z, Gong F. Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J Immunol 186: 3686–3692, 2011. [DOI] [PubMed] [Google Scholar]
- 112.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci 21: 8026–8033, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163–2196, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain 152: 2348–2356, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J Pain 11: 210–218, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem 272: 20975–20978, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997. [DOI] [PubMed] [Google Scholar]
- 118.Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol 8: 418–424, 1998. [DOI] [PubMed] [Google Scholar]
- 119.Wang X, Li WG, Yu Y, Xiao X, Cheng J, Zeng WZ, Peng Z, Xi Zhu M, Xu TL. Serotonin facilitates peripheral pain sensitivity in a manner that depends on the nonproton ligand sensing domain of ASIC3 channel. J Neurosci 33: 4265–4279, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain 109: 497–499, 2004. [DOI] [PubMed] [Google Scholar]
- 121.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem 279: 43716–43724, 2004. [DOI] [PubMed] [Google Scholar]
- 122.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol 89: 2459–2465, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Xu J, Gu H, Brennan TJ. Increased sensitivity of group III and group IV afferents from incised muscle in vitro. Pain 151: 744–755, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res 99: 501–509, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Yen YT, Tu PH, Chen CJ, Lin YW, Hsieh ST, Chen CC. Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol Pain 5: 1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yokoyama T, Lisi TL, Moore SA, Sluka KA. Muscle fatigue increases the probability of developing hyperalgesia in mice. J Pain 8: 692–699, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68: 61–72, 2010. [DOI] [PubMed] [Google Scholar]
- 128.Zha XM. Acid-sensing ion channels: trafficking and synaptic function. Mol Brain 6: 1, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci 29: 8438–8446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


