Abstract
Early detection of risk factors for enhanced primary prevention and novel therapies for treating the chronic consequences of cardiovascular disease are of the utmost importance for reducing morbidity. Recently, fibroblast growth factors (FGFs) have been intensively studied as potential new molecules in the prevention and treatment of cardiovascular disease mainly attributable to metabolic effects and angiogenic actions. Members of the endocrine FGF family have been shown to increase metabolic rate, decrease adiposity, and restore glucose homeostasis, suggesting a multiple metabolic role. Serum levels of FGFs have been associated with established cardiovascular risk factors as well as with the severity and extent of coronary artery disease and could be useful for prediction of cardiovascular death. Furthermore, preclinical investigations and clinical trials have tested FGF administration for therapeutic angiogenesis in ischemic vascular disease, demonstrating a potential role in improving angina and limb function. FGF21 has lately emerged as a potent metabolic regulator with multiple effects that ultimately improve the lipoprotein profile. Early studies show that FGF21 is associated with the presence of atherosclerosis and may play a protective role against plaque formation by improving endothelial function. The present review highlights recent investigations suggesting that FGFs, in particular FGF21, may be useful as markers of cardiovascular risk and may also serve as protective/therapeutic agents in cardiovascular disease.
Keywords: fibroblast growth factor, atherosclerosis, angiogenesis, coronary artery disease, biomarker
cardiovascular disease is a leading cause of morbidity and mortality worldwide, comprising a broad spectrum of disorders of the heart and blood vessels, including coronary artery disease (CAD) and peripheral artery disease. A considerable fall of mortality and morbidity rates attributable to CAD is witnessed in the developed world in the past 50 years, whereas, on the contrary, an increase is observed in developing countries. Life-saving, evidence-based medical therapies introduced in acute myocardial infarction treatment as well as revascularization therapies account for ∼50% of this decrease. The remaining 50% has been attributed to early detection and management of risk factors. Early identification of risk factors for enhanced primary prevention and novel therapies for treating the chronic consequences of cardiovascular disease (e.g., ischemic vascular disease and heart failure) are of the utmost importance for reducing morbidity. Biochemical markers, such as lipoprotein A, homocysteine, and prothrombotic and proinflammatory factors, are being studied for their role in pathophysiology, diagnosis, and prognosis of the disease. Recently, fibroblast growth factors (FGFs) have been intensively studied as potential new molecules in the prevention and treatment of cardiovascular disease.
FGFs constitute a vast family of polypeptides with important biological effects. FGF family in humans includes 22 members (FGF1 to FGF14 and FGF16 to FGF23; FGF15 is the mouse ortholog of FGF19) located in different tissues and having specific functions. Phylogenetic analysis of the human FGF gene family has identified seven subfamilies of FGFs that are also functionally classified according to their intracrine/intracellular, paracrine, and endocrine actions (46). The seven FGF subfamilies are classified as follows: subfamily 1 comprises FGF1 and FGF2; subfamily 4 comprises FGF4, FGF5, and FGF6; subfamily 7 comprises FGF3, FGF7, FGF10, and FGF22; subfamily 8 comprises FGF8, FGF17, and FGF18; subfamily 9 comprises FGF9, FGF16, and FGF20; subfamily 11 comprises FGF11, FGF12, FG13, and FGF14; and subfamily 19 comprises FGF19, FGF21, and FGF23. Subfamily 11 consists of the intracrine factors; subfamily 19 consists of the endocrine factors, and the rest of the subfamilies consist of the paracrine FGFs (24). Intracrine FGFs act as intracellular molecules by regulating electrical excitability (100). The paracrine and endocrine factors are secreted or released from the cell and function by binding to specific FGF receptors (FGFRs). The endocrine FGFs have very low heparin-binding affinity, which enables them to be released in the circulation (46).
Various FGFs along with other cytokines, such as vascular endothelial growth factors (VEGFs), have been examined as therapeutic agents in cardiovascular disease (93–94, 97). Most of them have common effects in biological processes, including angiogenesis, wound healing, neurogenesis, and neuroprotection (19, 48, 86). Recently, the endocrine member FGF21 has been shown to be a potent metabolic regulator and to have multiple beneficial effects in conditions that are considered to be major cardiovascular risk factors, such as hyperlipidemia, obesity, and diabetes (61, 74, 85).
In the present review, we describe the role of FGFs in cardiovascular disease. The potential use of the angiogenic capacity of FGFs for treatment has been the main focus of research, and many studies evaluating this potential have already progressed to advanced-phase clinical trials. We then specifically review the background knowledge and the mechanisms of action of FGF21, the newest member of the FGF family, and the evidence regarding its role and potential protective value in cardiovascular disease and especially atherosclerosis.
Metabolic Effects of FGFs and Association with Cardiovascular Disease
Members of the endocrine family have been shown to exert significant metabolic actions (Table 1). FGF19, a member of the endocrine FGF15/19 family, is characteristically expressed in ileal enterocytes regulating bile acid synthesis (38, 44). In transgenic mouse models and mouse models of dietary- and leptin-induced diabetes, FGF19 increases metabolic rate, decreases adiposity, and restores glucose homeostasis, suggesting a multiple metabolic role (25, 109). FGF19 is also associated with an intense liver-proliferating response and hepatocellular carcinoma in mice overexpressing FGF19 in skeletal muscle although it could also be used as an effective biomarker for patients most likely to respond to anti-FGF19 treatment for hepatocellular carcinoma (79, 95). In patients with type 2 diabetes and metabolic syndrome as well as those with suspected or established CAD, FGF19 is negatively correlated with metabolic indices and known cardiovascular risk factors [e.g., 2-h postprandial glucose, serum fasting insulin, body mass index (BMI), triglycerides, and glycosylated hemoglobin] (4, 34). Patients with CAD were found to have lower levels of FGF19 than those without CAD, adjusting for other factors, whereas FGF19 was also an independent predictor of the extent of CAD (34).
Table 1.
FGF family members and cardiovascular effects
| FGF Subfamily | FGF Involved | Cardiovascular Effect | Studied Cardiovascular End Point in Humans |
|---|---|---|---|
| FGF1 | FGF1 | Angiogenesis | Peripheral artery disease (7, 80, 107) (pharmacological effect) |
| FGF2 | Angiogenesis | Coronary artery disease (94, 103) | |
| Peripheral artery disease (56, 117) (pharmacological effect) | |||
| FGF4 | FGF4 | Angiogenesis | Coronary artery disease (30–31, 37) (pharmacological effect) |
| FGF5 | Angiogenesis | Not available | |
| FGF15/19 | FGF19 | Not established | Coronary artery disease (34) (correlation with serum levels) |
| FGF23 | Cardiomyocyte hypertrophy | Left ventricular hypertrophy in chronic kidney disease (32, 42) | |
| Coronary artery disease (112) | |||
| Cardiovascular death and heart failure (47, 110) (correlation with serum levels) | |||
| FGF21 | Lipid lowering | Lipid-lowering effects (26) (pharmacological effect) | |
| Carotid atherosclerosis (1, 10) | |||
| Coronary artery disease (64, 101) | |||
| Cardiovascular morbidity and mortality (59) | |||
| Pericardial fat deposition (58) | |||
| Atrial fibrillation (33) (correlation with serum levels) |
The cardiovascular-related end point as a result of fibroblast growth factor (FGF) pharmacologic action or observed correlation to FGF serum levels in humans is shown.
FGF23, also a member of the endocrine FGFs, is secreted by osteocytes in the serum, and it is essential in phosphate and vitamin D metabolism (65, 102). FGF23 serum levels have been found to be associated with cardiovascular risk factors such as apolipoprotein A1 and high-density lipoprotein (HDL) in subjects with and without chronic kidney disease (76–77). Elevated serum levels of FGF23 are also associated with the progression and development of left ventricular hypertrophy in patients with chronic kidney disease but are also independently associated with heart disease as well as the severity and extent of CAD in patients undergoing coronary angiography (Table 1) (32, 42, 47, 73, 112). This is supported by evidence that FGF23, except for binding to FGFR and the coreceptor klotho in the kidney and parathyroid gland, acts directly to the heart via a klotho-independent signaling pathway (20). In a recent large study, increased levels of FGF23 were associated with cardiovascular death and incident heart failure in 3,627 patients with stable ischemic heart disease; FGF23 levels also indicated a better response to therapy with angiotensin-converting enzyme inhibitor therapy (110). The link of FGF23 with direct cardiac effects and the potential association of serum levels with cardiovascular risk factors warrant further research.
FGFs and Therapeutic Angiogenesis in Ischemic Vascular Disease
FGF1 was first described as having angiogenic potential in animal studies by Schumacher and associates (97–98). Available data on this molecule are not clear despite encouraging results from phase 1 and 2 clinical trials for therapeutic angiogenesis in ischemic vascular disease (6, 80); a phase 3 trial showed no positive effects from the treatment with this factor (7). FGF2, namely basic FGF, along with VEGFs, is known to act in multiple stages of angiogenesis (Table 1) (92, 107). In animal studies, FGF2 leads to improved myocardial perfusion, can provide cardioprotection during ischemia-perfusion injury (5, 35, 39, 41, 43, 55), and has a strong link with cardiac hypertrophy (40, 49, 96). In humans, FGF2 protein delivery resulted in significant improvement in angina (Table 1) (94, 103). Recombinant FGF2 has also been tested in peripheral artery disease with improvements of limb function (Table 1) (56, 117).
FGF4 (a member of the FGF4 subfamily that includes FGF4, FGF5, and FGF6) promotes the growth of human embryonic stem cells and their pluripotency (75). In a rabbit hind-limb ischemia model, FGF4 administered by adenoviral gene transfer induced vascular permeability, therapeutic angiogenesis, and arteriogenesis (91). Subsequently, a series of clinical trials, Angiogenic GENe Therapy (AGENT), AGENT2, AGENT3, and AGENT4, were carried out to test the safety and efficacy of FGF4 as gene therapy. During these studies FGF4 was found to have no difference between the treated and the placebo group in myocardial perfusion in patients with stable angina and reversible ischemia and to have no significant effect on exercise tolerance at 12 wk (Table 1) (30–31, 37).
FGF5, also a member of the FGF4 subfamily, is known to be highly expressed in the central nervous system and in the skin, where it regulates hair growth (36, 105). FGF5 is reported to increase myocardial blood flow and function, decrease myocyte apoptosis, and increase myocyte number after gene transfer of the growth factor in swine (28, 70, 104). There are no available results from extensive studies on FGF5 and its effects in human cardiovascular disease, which would provide more definitive insight into the role of this molecule.
FGF21: Expression and Mode of Action
FGF21 is emerging as the newest candidate from the FGF family with a potentially critical role in the cardiovascular system. Many of the above-mentioned members of the FGF family are different from FGF21, as they are paracrine factors acting as secreted or released extracellular proteins instead of being released in the blood stream as endocrine FGF21 (46). The initially purified from bovine brain FGF1 and FGF2 were identified to be mitogenic for a wide variety of mesoderm-derived cells, whereas FGF21 is mostly known for its metabolic effects rather than the mitogenic ones, with so far only one study in the literature documenting a potential angiogenic effect of this molecule (29, 115).
FGF21 was initially identified in mouse embryos, and it is a member of the subfamily 15/19 of the FGFs, together with FGF19 and FGF23 in humans (24, 81). Gene expression in C57/BL6 mice has shown that FGF21 is expressed predominantly in pancreas, testis, liver, and brown adipose tissue and in lower amounts in other tissues, including the aorta (24). FGF21 acts through the known FGFRs as a complex with β-klotho protein (50, 82), and, following the formation of the complex on the cell surface, a signaling cascade is activated (45, 50, 82). The β-klotho gene was identified in mice to encode for a type I membrane protein β-klotho, member of the klotho family, and it is predominantly expressed in liver, pancreas, and adipose tissue and in lower amounts in mouse aorta (24, 45). The expression of mRNA-encoding FGFRs in cultured human endothelial cells (ECs) and smooth muscle cells showed that both cell types express FGFR1, but ECs also express low levels of FGFR4, whereas smooth muscle cells also express low levels of FGFR2 (8). Importantly, mouse cardiomyocytes express FGFR1 and FGFR3, as well as β-klotho (66).
Recent in vivo findings demonstrate that FGF21 has several metabolic effects, and its serum levels have also been correlated with many cardiovascular risk factors, summarized in Fig. 1.
Fig. 1.
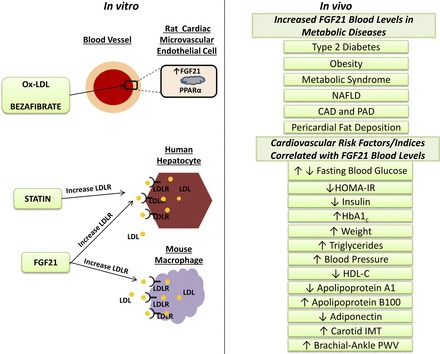
Left: data from in vitro experiments showing that fibroblast growth factor 21 (FGF21) and peroxisome proliferator-activated receptor-α (PPAR-α) are expressed in rat endothelial cells; incubation of rat endothelial cells with oxidized low-density lipoprotein (Ox-LDL) or bezafibrate induced an upregulation in the expression of FGF21. Human hepatocytes and mouse macrophages increase the LDL uptake as a result of increased LDL receptor (LDLR) expression after stimulation with FGF21; the known effect of statins on hepatocytes is included. Right: results from in vivo findings including a list of metabolic conditions where FGF21 serum levels are documented to be increased and a list of cardiovascular risk factors/indicators positively or negatively correlated with FGF21 serum levels. NAFLD, nonalcoholic fatty liver disease; CAD, coronary artery disease; PAD, peripheral artery disease; HOMA-IR, homeostasis model assessment-estimated insulin resistance; HbA1c, glycosylated hemoglobin; HDL-C, high-density lipoprotein cholesterol; IMT, intima media thickness; PWV, pulse-wave velocity.
Metabolic Effects of FGF21
Many animal and, recently, clinical studies have highlighted the actions and beneficial role of FGF21 in metabolic diseases (Fig. 2). It was initially found to increase glucose uptake in mouse 3T3-L1 and primary human adipocytes (51). Reduced plasma glucose and triglyceride levels were observed when FGF21 was administered in genetic mouse models of obesity and type 2 diabetes, leptin-deficient ob/ob mice, and leptin receptor-deficient db/db mice (51). Furthermore, FGF21 administration in diet-induced obese mouse models lowered their mean body weight and fat mass, increased their energy expenditure, ameliorated their lipid and glucose metabolism, improved hepatic and peripheral insulin sensitivity, and reduced hepatic steatosis (12, 113). When FGF21 was administered daily for 6 wk in diabetic obese rhesus monkeys, a dramatic decrease in fasting blood glucose and insulin was observed along with significant improvements in the lipid and lipoprotein profile, including a decrease in triglycerides and low-density lipoprotein (LDL) and an increase in HDL (52). FGF21 has also been shown to be critical in promoting adaptation in ketotic states and fasting according to animal studies (15). When mice consume a ketogenic diet and when they are on a fasting regime, serum FGF21 levels increase, whereas they rapidly decrease after animals are refed from the fasted condition (3). When mice lacking FGF21 are put on a ketogenic diet, multiple metabolic impairments including hyperlipidemia, impaired glucose tolerance, and steatosis are observed (2–3). FGF21 has a potent role in various tissues such as in white adipose tissue by regulating browning in adaptive thermogenesis and in liver, which constitutes a target tissue for FGF21 through which FGF21 effects on lipid and glucose metabolism are mediated (22–23).
Fig. 2.
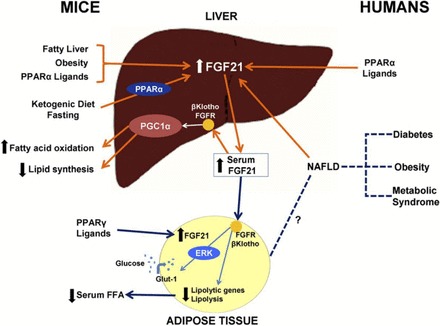
Summary of FGF21 physiology in mice and humans. In mice, consumption of a ketogenic diet leads to a PPAR-α-dependent increase of FGF21 in the liver and an increase in serum FGF21 concentrations. FGF21 expression in the liver is also induced by fatty liver disease, obesity, and PPAR-α ligands in mice. PPAR-α ligands, such as fenobibrate, also increase FGF21 messenger RNA expression in human hepatocytes. FGF21 interacts with the FGF receptor (FGFR) in the presence of β-klotho in the mouse liver and adipose tissue. This interaction leads to a PPAR-γ coactivator protein-1α (PGC-1α)-dependent upregulation of fatty acid oxidation and downregulation of lipid synthesis in the liver. In mouse adipose tissue, the presence of PPAR-γ ligands leads to the production of FGF21, and the short-term effect of FGF21 results in a decreased expression of lipolytic genes and leads to lower concentrations of circulating free fatty acids (FFA). FGF21-induced phosphorylation of extracellular signal-regulated kinase-1 (ERK) leads to the activation of glucose transporter-1 (Glut-1) and glucose uptake in mouse 3T3-L1 adipocytes and primary human adipocytes. In humans, serum concentrations of FGF21 are higher in diabetes, obesity, metabolic syndrome, and NAFLD. This effect may be mediated by increased FGF21 liver expression. [From Domouzoglou and Maratos-Flier (15).]
FGF21: A biomarker for metabolic diseases
Studies in humans with nonalcoholic fatty liver disease, obesity, and diabetes document increased serum levels of FGF21 (Fig. 1). Baseline FGF21 serum levels are significantly higher in obese people and patients with type 2 diabetes compared with controls, and treatment with fenofibrate further increases these levels (78, 85). Greater FGF21 concentrations were observed in a group of patients with type 2 diabetes, and these were significantly correlated to adiponectin, weight, glucose, HDL cholesterol, and triglycerides (74). In two other studies of patients with type 2 diabetes, fasting serum FGF21 was increased and correlated with multiple metabolic parameters such as blood glucose and lipids, blood pressure, and HbA1c (Fig. 1). Thus a role of FGF21 in the pathogenesis of insulin resistance and type 2 diabetes was suggested (9, 61).
The metabolic syndrome has also been associated with increased serum FGF21 levels, whereas an increase in FGF21 serum levels has been suggested as a new biomarker for nonalcoholic fatty liver disease or steatohepatitis (17, 54, 60, 62, 116, 118). A study on obese children confirmed that increased serum FGF21 is correlated to BMI and free fatty acids (90). When serum FGF21 levels were tested after an oral load of fructose, it was interestingly shown that FGF21 values acutely spike, presenting a similar curve as serum glucose and insulin after a glucose load. This finding shows that FGF21 presents a typical hormonal response possibly mediated by carbohydrate-responsive element-binding protein that is activated by fructose (18).
FGF21 is involved in glucose and lipid metabolism, which indicates that elevated serum concentrations of this molecule in conditions such as obesity, diabetes, hyperlipidemia, and fatty liver disease could be used as a biomarker, although one could also speculate that FGF21 plays a protective role in the course of pathophysiological processes involved in atherosclerosis in humans. The prognostic value of serum FGF21 was assessed in patients with type 2 diabetes, and it was shown that FGF21 levels are predictive of combined cardiovascular morbidity and mortality (Fig. 3) (59). Increased baseline serum levels of this molecule were found to be associated with a higher risk for cardiovascular events in patients with type 2 diabetes in the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, and interestingly this association tended to be stronger in the patient group that presented higher total cholesterol levels (84). The authors speculate that the increased basal levels of FGF21 in this group of patients may be an indication of the potential role of FGF21 as a biomarker for the early detection of cardiometabolic risk and furthermore that it may reflect a compensatory response or the need of supraphysiological doses of FGF21 as a result of FGF21 resistance, a hypothesis proven in obese mouse models (21). In the clinical setting, when an FGF21 analog was administered to patients with obesity and type 2 diabetes, there was improvement in dyslipidemia with modest effects on glucose homeostasis (26).
Fig. 3.
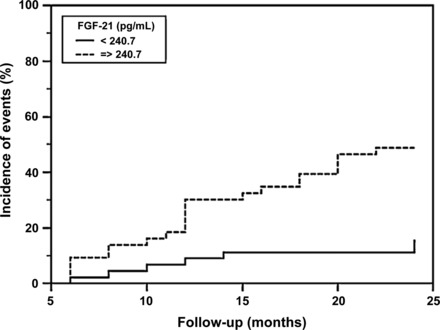
Kaplan-Maier curve of combined cardiovascular morbidity and mortality at 24-mo follow-up in patients with type 2 diabetes mellitus according to the level of FGF21 (median value 240.7 pg/ml). Dashed line, patients with FGF21 > 240.7 pg/ml; solid line, patients with FGF21 ≤ 240.7 pg/ml. Log rank test: P = 0.0013. [From Lenart-Lipinska et al. (59).].
A strong link between FGF21 and mitochondrial diseases was recently uncovered when serum levels of this protein were shown to be significantly elevated and when FGF21 was found to be the most sensitive predictor compared with other known predictors in detecting the presence of the disease (13). This aspect of FGF21 properties may be considered similar to the known increase of FGF21 in states of fasting, as mitochondrial diseases create a pseudo-starvation condition at the muscle level.
Association of FGF21 with Cardiovascular Risk and Atherosclerosis
FGF21 association with type 2 diabetes, dyslipidemia, and metabolic syndrome, conditions that are potential precursors to cardiovascular disease, suggest a possible link of this hormone to the atherosclerosis process. Evidence toward such a link was provided by showing that serum levels of FGF21 are increased in patients with CAD (64, 101). Patients with CAD and comorbidities such as diabetes and hypertension had even higher FGF21 levels than the patients with no comorbidities. FGF21 serum levels were also found to be positively correlated with the homeostasis model assessment-estimated insulin-resistance index (HOMA-IR), insulin, fasting blood glucose, triglycerides, and apolipoprotein B100, whereas there was a negative correlation with HDL cholesterol and apolipoprotein A1 (64). In patients with type 2 diabetes, serum FGF21 levels were associated with the presence of atherosclerosis in carotid arteries, further confirming the importance of this molecule in diabetes-related atherosclerosis (1).
In contrast to the above findings, when subjects strictly matched for BMI were evaluated for any association between serum FGF21, glucose/lipid metabolism, CAD, and pericardial fat deposition, there was a strong correlation of FGF21 serum levels with the presence of metabolic syndrome but not with CAD, diabetes, and obesity, as assessed by multi-detector computed tomography (58). In this study, FGF21 levels were found to be strongly correlated with serum lipids and especially triglycerides, insulin, and HOMA-IR, whereas there was a negative correlation with HDL cholesterol. The discrepancy between this and the previous studies on FGF21 serum levels and their association with CAD could be attributed to the fact that the patients in the first study were not strictly matched for BMI and might have had a significantly worse lipid profile, suggesting that the correlation was more attributable to the preexisting cardio-metabolic factors than the CAD per se.
Interestingly, increased FGF21 serum levels have also been associated with pericardial fat accumulation (Table 1), indicating that FGF21 may be related to fat deposition and dyslipidemia independent of obesity (58). Pericardial fat has been previously shown to be related to cardio-metabolic risk factors and contribute to CAD; this could further extend the role of FGF21 as a biomarker for cardiovascular risk (72). Obesity and pericardial fat are also associated with the presence and the severity of atrial fibrillation (106), and an association between elevated FGF21 serum levels and atrial fibrillation has been shown independently of risk factors, such as BMI, presence of hypertension, and levels of high-sensitivity C-reactive protein (33).
Markers of subclinical atherosclerosis have been evaluated in relation to serum FGF21 levels, which were found to be positively correlated to carotid intima-media thickness in female patients (10). When 744 community-dwelling adults participating in the Baltimore Longitudinal Study of Aging were assessed for FGF21 serum levels, an independent association with hypertension was discovered (99). Moreover, measurements of brachial-ankle pulse-wave velocity, indicative of arterial stiffness, in obese nondiabetic women were found to be correlated with serum FGF21 levels. When the same women were followed up with after a 3-mo exercise program, FGF21 levels decreased along with improvement of brachial-ankle pulse-wave velocity measurements (114).
Protective Role of FGF21 in Cardiovascular Disease
The protective role of FGF21 for the cardiovascular system is supported by in vitro studies, which provide evidence that one of the pharmacological effects of FGF21 is to increase cholesterol uptake in human hepatocytes and mouse macrophages, by increasing LDL receptor (LDL-R) through reduction of the myosin regulatory light chain-interacting protein/inducible degrader of the LDL-R, an effect additive to that of statins (14, 16, 53, 71) (Fig. 1).
FGF21 is upregulated by peroxisome proliferator-activated receptor-α (PPAR-α) in the animal and human liver and by PPAR-γ in adipose tissue (27, 69, 111). PPAR-α and PPAR-γ are both known to play an important role in lipid and glucose regulation and in cardiovascular disease (57). A recent in vitro study has provided evidence that both FGF21 and PPAR-α mRNA are expressed in rat cardiovascular ECs (Fig. 1) (68). In this study, a mechanistic insight into the dual role for FGF21 was provided; taking into account the lipid-lowering effects of both FGF21 and PPAR-α, the two molecules could cooperate to create an autocrine feedback loop in conditions of increased presence of lipids. Moreover, the finding in the same study that, by inducing apoptosis of cardiovascular ECs with oxidized LDL (ox-LDL), a dose-dependent induction of FGF21 was observed (Fig. 1) and the apoptotic effect of ox-LDL was influenced by the FGF21 response leads to the speculation that FGF21 could be a biological marker of EC stress and injury and, more importantly, that it may play a physiological role in improving endothelial function at an early stage of atherosclerosis, as the authors conclude. Use of bezafibrate, a PPAR-α ligand, has been shown to induce FGF21 expression, further supporting a protective role of FGF21 (Fig. 1) (68).
FGF21, both endogenous and exogenously administered, successfully interacts with cardiomyocytes to protect them after myocardial ischemia/reperfusion injury (Fig. 4) (66). FGF21 was shown to be the main mediator (via β-klotho-dependent signaling pathways) of a cardioprotective endocrine response in the liver and adipose tissue activated by experimental myocardial ischemia (66). These findings indicate β-klotho-dependent pathways of action of FGF21 on cardiac tissue and support a potential therapeutic role of this molecule. The protective action of FGF21 on ischemic/injured cardiac cells is further supported by more studies in vitro and in vivo (11, 67, 87). By regulating genes in antioxidant pathways, such as uncoupling protein 3 and peroxiredoxin 5, FGF21 prevents oxidative stress mediated by reactive oxygen species in cardiomyocytes (88). Protection of the heart from hypertrophy is another targeted action of FGF21 that has been discovered in mice, as FGF21 knockout mice seem to be unprotected from hypertrophic insults, whereas protection is restored as soon as FGF21 is replaced (89).
Fig. 4.
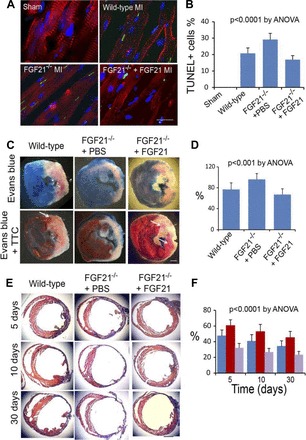
Cardioprotective action of FGF21 in myocardial ischemia/reperfusion injury. A: immunofluorescence micrographs showing cells undergoing DNA fragmentation in the ischemic myocardium (MI) by the TUNEL assay. Red, cardiac troponin I; green, TUNEL-positive cell nuclei; blue, cell nuclei. Scale bar = 10 mm. B: graphic representation of the fraction of TUNEL-positive cell nuclei in the ischemic myocardium calculated in reference to the total cell nuclei. Means and SDs are presented (n = 8). The P value was estimated by ANOVA among all groups. C: left ventricular slices from wild-type mice and FGF21 2/2 mice with administration of PBS or recombinant FGF21 at 24 h after myocardial ischemia/reperfusion injury, showing the influence of FGF21 on the fraction of acute myocardial infarcts (by the triphenyltetrazolium chloride, TTC, assay) in reference to the area at risk (by the Evans blue assay). Note that the left ventricular wall thickness is thinner in FGF21 2/2 mice with PBS administration than that in wild-type mice and FGF21 2/2 mice with FGF21 administration. Arrows, TTC-positive (red) myocardium within the area at risk. Scale bar = 1 mm. D: graphic representation of the influence of FGF21 on the fraction of acute myocardial infarcts in reference to the area at risk. Means and SDs are presented (n = 8). The P value was estimated by ANOVA among all groups. E: Azan-stained left ventricular sections from wild-type mice and FGF21 2/2 mice with administration of PBS or recombinant FGF21 at 5, 10, and 30 days after myocardial ischemia/reperfusion injury. Red, intact myocardium; blue, myocardial infarcts and fibrous tissue. Scale bar = 1 mm. F: graphic representation of the fraction of myocardial infarcts in wild-type mice (blue) and FGF21 2/2 mice with administration of PBS (red) or recombinant FGF21 (purple) at 5, 10, and 30 days after myocardial injury. Means and SDs are presented. The P value was estimated by ANOVA and is <0.0001 for both time- and treatment-based comparisons. [From Liu et al. (66).]
There is also evidence that, in adipose tissue, β-klotho-independent signaling pathways of FGF21 exist, highlighting the necessity to assess signaling alternatives in other tissues as well, such as the coronary artery wall (108). In a double knockout mouse model of atherosclerosis, with both FGF21 and apolipoprotein E deficiency, an accelerated plaque-formation process was observed compared with the single apolipoprotein E deficiency control group (63). The protective effects of FGF21 presence were found to be connected to metabolic effects, such as cholesterol lowering by suppressing sterol-responsive element protein 2 expression in the liver as well as induction of the antiatherosclerotic molecule adiponectin in adipose tissue (63, 83).
Conclusions
FGF family members have a potent intracellular, paracrine, and endocrine activity with multiple effects on metabolism and potential actions on the cardiovascular system. Members of the endocrine family have been shown to be associated with metabolic markers and the presence/extent of CAD predicting cardiovascular morbidity. The therapeutic value of FGFs related to angiogenesis has been investigated in clinical trials, but current evidence remains inconclusive; thus further research is required.
FGF21 is emerging as a new promising member of the FGF family with a potentially important role in cardiovascular disease and especially atherosclerosis. FGF21 levels have been shown to be strongly related to traditional cardiovascular risk factors and conditions such as hyperlipidemia, hypertension, diabetes, and obesity in humans. However, multiple beneficial metabolic effects of FGF21 have been previously demonstrated in experimental and animal models, suggesting that FGF21 is not a simple marker of cardiovascular risk but may have a protective effect on the cardiovascular system, contributing to a reduction in risk. Emerging evidence demonstrating the protective role of FGF21 against endothelial damage, atherosclerotic plaque formation, and ischemic injury of cardiomyocytes related to oxidative stress has provided a greater insight toward that direction. The role of FGF21 in the development of atherosclerosis and whether FGF21 could serve as a novel therapeutic agent during the early stages of atherosclerotic disease need to be studied further.
GRANTS
This work was supported by the Alexander S. Onassis Public Benefit Foundation (to E. Domouzoglou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.M.D., K.K.N., and M.I.P. conception and design of research; E.M.D. prepared figures; E.M.D. drafted manuscript; E.M.D., K.K.N., A.P.V., M.I.P., L.K.M., A.T., and E.M.-F. approved final version of manuscript; K.K.N., A.P.V., M.I.P., L.K.M., A.T., and E.M.-F. edited and revised manuscript.
REFERENCES
- 1.An SY, Lee MS, Yi SA, Ha ES, Han SJ, Kim HJ, Kim DJ, Lee KW. Serum fibroblast growth factor 21 was elevated in subjects with type 2 diabetes mellitus and was associated with the presence of carotid artery plaques. Diabetes Res Clin Pract 96: 196–203, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150: 4931–4940, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barutcuoglu B, Basol G, Cakir Y, Cetinkalp S, Parildar Z, Kabaroglu C, Ozmen D, Mutaf I, Bayindir O. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann Clin Lab Sci 41: 390–396, 2011. [PubMed] [Google Scholar]
- 5.Battler A, Scheinowitz M, Bor A, Hasdai D, Vered Z, Di Segni E, Varda-Bloom N, Nass D, Engelberg S, Eldar M, Belkin M, Savion N. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol 22: 2001–2006, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Baumgartner I, Chronos N, Comerota A, Henry T, Pasquet JP, Finiels F, Caron A, Dedieu JF, Pilsudski R, Delaere P. Local gene transfer and expression following intramuscular administration of FGF-1 plasmid DNA in patients with critical limb ischemia. Mol Ther 17: 914–921, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belch J, Hiatt WR, Baumgartner I, Driver IV, Nikol S, Norgren L, Van Belle E. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet 377: 1929–1937, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Brogi E, Winkles JA, Underwood R, Clinton SK, Alberts GF, Libby P. Distinct patterns of expression of fibroblast growth factors and their receptors in human atheroma and nonatherosclerotic arteries. Association of acidic FGF with plaque microvessels and macrophages. J Clin Invest 92: 2408–2418, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 116: 65–68, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol 33: 2454–2459, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Cong WT, Ling J, Tian HS, Ling R, Wang Y, Huang BB, Zhao T, Duan YM, Jin LT, Li XK. Proteomic study on the protective mechanism of fibroblast growth factor 21 to ischemia-reperfusion injury. Can J Physiol Pharmacol 91: 973–984, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Davis RL, Liang C, Edema-Hildebrand F, Riley C, Needham M, Sue CM. Fibroblast growth factor 21 is a sensitive biomarker of mitochondrial disease. Neurology 81: 1819–1826, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Do HT, Tselykh TV, Makela J, Ho TH, Olkkonen VM, Bornhauser BC, Korhonen L, Zelcer N, Lindholm D. Fibroblast growth factor-21 (FGF21) regulates low-density lipoprotein receptor (LDLR) levels in cells via the E3-ubiquitin ligase Mylip/Idol and the Canopy2 (Cnpy2)/Mylip-interacting saposin-like protein (Msap). J Biol Chem 287: 12602–12611, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domouzoglou EM, Maratos-Flier E. Fibroblast growth factor 21 is a metabolic regulator that plays a role in the adaptation to ketosis. Am J Clin Nutr 93: 901S–905S, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong B, Wu M, Cao A, Li H, Liu J. Suppression of Idol expression is an additional mechanism underlying statin-induced up-regulation of hepatic LDL receptor expression. Int J Mol Med 27: 103–110, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139: 456–463, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab 4: 51–57, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelmann GL, Dionne CA, Jaye MC. Acidic fibroblast growth factor and heart development. Role in myocyte proliferation and capillary angiogenesis. Circ Res 72: 7–19, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 121: 4393–4408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59: 2781–2789, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS, Kharitonenkov A, Spiegelman BM, Maratos-Flier E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 152: 2996–3004, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24: 2050–2064, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu L, John LM, Adams SH, Yu XX, Tomlinson E, Renz M, Williams PM, Soriano R, Corpuz R, Moffat B, Vandlen R, Simmons L, Foster J, Stephan JP, Tsai SP, Stewart TA. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 145: 2594–2603, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 18: 333–340, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I, Dahlin M, Amark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8: 169–174, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Giordano FJ, Ping P, McKirnan MD, Nozaki S, DeMaria AN, Dillmann WH, Mathieu-Costello O, Hammond HK. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med 2: 534–539, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Gospodarowicz D, Bialecki H, Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem 253: 3736–3743, 1978. [PubMed] [Google Scholar]
- 30.Grines C, Rubanyi GM, Kleiman NS, Marrott P, Watkins MW. Angiogenic gene therapy with adenovirus 5 fibroblast growth factor-4 (Ad5FGF-4): a new option for the treatment of coronary artery disease. Am J Cardiol 92: 24N–31N, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Grines CL, Watkins MW, Mahmarian JJ, Iskandrian AE, Rade JJ, Marrott P, Pratt C, Kleiman N. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol 42: 1339–1347, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119: 2545–2552, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X, Chen C, Cheng G, Xie C, Yang M, Shou X, Sun C. Serum fibroblast growth factor 21 levels are increased in atrial fibrillation patients. Cytokine 73: 176–180, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Hao Y, Zhou J, Zhou M, Ma X, Lu Z, Gao M, Pan X, Tang J, Bao Y, Jia W. Serum levels of fibroblast growth factor 19 are inversely associated with coronary artery disease in Chinese individuals. PLoS One 8: e72345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada K, Grossman W, Friedman M, Edelman ER, Prasad PV, Keighley CS, Manning WJ, Sellke FW, Simons M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J Clin Invest 94: 623–630, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haub O, Drucker B, Goldfarb M. Expression of the murine fibroblast growth factor 5 gene in the adult central nervous system. Proc Natl Acad Sci USA 87: 8022–8026, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R, Andrasfay T, Engler RL. Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials. J Am Coll Cardiol 50: 1038–1046, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 17: 1581–1591, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.House SL, Branch K, Newman G, Doetschman T, Schultz Jel J. Cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2 is mediated by the MAPK cascade. Am J Physiol Heart Circ Physiol 289: H2167–H2175, 2005. [DOI] [PubMed] [Google Scholar]
- 40.House SL, House BE, Glascock B, Kimball T, Nusayr E, Schultz JE, Doetschman T. Fibroblast growth factor 2 mediates isoproterenol-induced cardiac hypertrophy through activation of the extracellular regulated kinase. Mol Cell Pharmacol 2: 143–154, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.House SL, Melhorn SJ, Newman G, Doetschman T, Schultz Jel J. The protein kinase C pathway mediates cardioprotection induced by cardiac-specific overexpression of fibroblast growth factor-2. Am J Physiol Heart Circ Physiol 293: H354–H365, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Hsu HJ, Wu MS. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337: 116–122, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Hughes GC, Biswas SS, Yin B, Coleman RE, DeGrado TR, Landolfo CK, Lowe JE, Annex BH, Landolfo KP. Therapeutic angiogenesis in chronically ischemic porcine myocardium: comparative effects of bFGF and VEGF. Ann Thorac Surg 77: 812–818, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2: 217–225, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI. Molecular cloning and expression analyses of mouse betaKlotho, which encodes a novel Klotho family protein. Mech Dev 98: 115–119, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149: 121–130, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). J Am Coll Cardiol 60: 200–207, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci USA 102: 18189–18194, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaye D, Pimental D, Prasad S, Maki T, Berger HJ, McNeil PL, Smith TW, Kelly RA. Role of transiently altered sarcolemmal membrane permeability and basic fibroblast growth factor release in the hypertrophic response of adult rat ventricular myocytes to increased mechanical activity in vitro. J Clin Invest 97: 281–291, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 215: 1–7, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148: 774–781, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Knopp RH. Drug treatment of lipid disorders. N Engl J Med 341: 498–511, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Kralisch S, Tonjes A, Krause K, Richter J, Lossner U, Kovacs P, Ebert T, Bluher M, Stumvoll M, Fasshauer M. Fibroblast growth factor-21 serum concentrations are associated with metabolic and hepatic markers in humans. J Endocrinol 216: 135–143, 2013. [DOI] [PubMed] [Google Scholar]
- 55.Laham RJ, Rezaee M, Post M, Novicki D, Sellke FW, Pearlman JD, Simons M, Hung D. Intrapericardial delivery of fibroblast growth factor-2 induces neovascularization in a porcine model of chronic myocardial ischemia. J Pharmacol Exp Ther 292: 795–802, 2000. [PubMed] [Google Scholar]
- 56.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 359: 2053–2058, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Lee TI, Kao YH, Chen YC, Huang JH, Hsiao FC, Chen YJ. Peroxisome proliferator-activated receptors modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes Res Clin Pract 100: 330–339, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Lee Y, Lim S, Hong ES, Kim JH, Moon MK, Chun EJ, Choi SI, Kim YB, Park YJ, Park KS, Jang HC, Choi SH. Serum FGF21 concentration is associated with hypertriglyceridemia, hyperinsulinemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol (Oxf) 80: 57–64, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Lenart-Lipinska M, Matyjaszek-Matuszek B, Gernand W, Nowakowski A, Solski J. Serum fibroblast growth factor 21 is predictive of combined cardiovascular morbidity and mortality in patients with type 2 diabetes at a relatively short-term follow-up. Diabetes Res Clin Pract 101: 194–200, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 53: 934–940, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Yang G, Ning H, Yang M, Liu H, Chen W. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract 82: 209–213, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Li X, Fan X, Ren F, Zhang Y, Shen C, Ren G, Sun J, Zhang N, Wang W, Ning G, Yang J. Serum FGF21 levels are increased in newly diagnosed type 2 diabetes with nonalcoholic fatty liver disease and associated with hsCRP levels independently. Diabetes Res Clin Pract 93: 10–16, 2011. [DOI] [PubMed] [Google Scholar]
- 63.Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, Jin L, Lian Q, Huang Y, Ding H, Triggle C, Wang K, Li X, Xu A. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation 131: 1861–1871, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One 5: e15534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291: E38–E49, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep 3: 2767, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu SQ, Tefft BJ, Roberts DT, Zhang LQ, Ren Y, Li YC, Huang Y, Zhang D, Phillips HR, Wu YH. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol 303: H1446–H1458, 2012. [DOI] [PubMed] [Google Scholar]
- 68.Lu Y, Liu JH, Zhang LK, Du J, Zeng XJ, Hao G, Huang J, Zhao DH, Wang GZ, Zhang YC. Fibroblast growth factor 21 as a possible endogenous factor inhibits apoptosis in cardiac endothelial cells. Chin Med J (Engl) 123: 3417–3421, 2010. [PubMed] [Google Scholar]
- 69.Lundasen T, Hunt MC, Nilsson LM, Sanyal S, Angelin B, Alexson SE, Rudling M. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 360: 437–440, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Lynch P, Lee TC, Fallavollita JA, Canty JM Jr, Suzuki G. Intracoronary administration of AdvFGF-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation 116: I71–I76, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Ma PT, Gil G, Sudhof TC, Bilheimer DW, Goldstein JL, Brown MS. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci USA 83: 8370–8374, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, O'Donnell CJ, Fox CS, Hoffmann U. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 30: 850–856, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masai H, Joki N, Sugi K, Moroi M. A preliminary study of the potential role of FGF-23 in coronary calcification in patients with suspected coronary artery disease. Atherosclerosis 226: 228–233, 2013. [DOI] [PubMed] [Google Scholar]
- 74.Matuszek B, Lenart-Lipinska M, Duma D, Solski J, Nowakowski A. Evaluation of concentrations of FGF-21 - a new adipocytokine in type 2 diabetes. Endokrynol Pol 61: 50–54, 2010. [PubMed] [Google Scholar]
- 75.Mayshar Y, Rom E, Chumakov I, Kronman A, Yayon A, Benvenisty N. Fibroblast growth factor 4 and its novel splice isoform have opposing effects on the maintenance of human embryonic stem cell self-renewal. Stem Cells 26: 767–774, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, Larsson TE. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant 24: 3125–3131, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 205: 385–390, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 71: 369–375, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, Pham TA, Dillard-Telm L, Tsai SP, Stephan JP, Stinson J, Stewart T, French DM. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol 160: 2295–2307, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikol S, Baumgartner I, Van Belle E, Diehm C, Visona A, Capogrossi MC, Ferreira-Maldent N, Gallino A, Wyatt MG, Wijesinghe LD, Fusari M, Stephan D, Emmerich J, Pompilio G, Vermassen F, Pham E, Grek V, Coleman M, Meyer F. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther 16: 972–978, 2008. [DOI] [PubMed] [Google Scholar]
- 81.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 1492: 203–206, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104: 7432–7437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106: 2767–2770, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Ong KL, Januszewski AS, O'Connell R, Jenkins AJ, Xu A, Sullivan DR, Barter PJ, Hung WT, Scott RS, Taskinen MR, Keech AC, Rye KA. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia 58: 464–473, 2015. [DOI] [PubMed] [Google Scholar]
- 85.Ong KL, Rye KA, O'Connell R, Jenkins AJ, Brown C, Xu A, Sullivan DR, Barter PJ, Keech AC. Long-term fenofibrate therapy increases fibroblast growth factor 21 and retinol-binding protein 4 in subjects with type 2 diabetes. J Clin Endocrinol Metab 97: 4701–4708, 2012. [DOI] [PubMed] [Google Scholar]
- 86.Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA 95: 5672–5677, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel V, Adya R, Chen J, Ramanjaneya M, Bari MF, Bhudia SK, Hillhouse EW, Tan BK, Randeva HS. Novel insights into the cardio-protective effects of FGF21 in lean and obese rat hearts. PLoS One 9: e87102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M, Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 106: 19–31, 2015. [DOI] [PubMed] [Google Scholar]
- 89.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun 4: 2019, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab 97: 2143–2150, 2012. [DOI] [PubMed] [Google Scholar]
- 91.Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, Taipale P, Kauppinen RA, Rubanyi GM, Yla-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J 17: 100–102, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Rosenblatt-Velin N, Lepore MG, Cartoni C, Beermann F, Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J Clin Invest 115: 1724–1733, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosengart TK, Lee LY, Patel SR, Sanborn TA, Parikh M, Bergman GW, Hachamovitch R, Szulc M, Kligfield PD, Okin PM, Hahn RT, Devereux RB, Post MR, Hackett NR, Foster T, Grasso TM, Lesser ML, Isom OW, Crystal RG. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 100: 468–474, 1999. [DOI] [PubMed] [Google Scholar]
- 94.Ruel M, Laham RJ, Parker JA, Post MJ, Ware JA, Simons M, Sellke FW. Long-term effects of surgical angiogenic therapy with fibroblast growth factor 2 protein. J Thorac Cardiovasc Surg 124: 28–34, 2002. [DOI] [PubMed] [Google Scholar]
- 95.Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, French DM, Finn RS, Lowe SW, Powers S. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by Oncogenomic screening. Cancer Cell 19: 347–358, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scheinowitz M, Kotlyar A, Zimand S, Ohad D, Leibovitz I, Bloom N, Goldberg I, Nass D, Engelberg S, Savion N, Eldar M. Basic fibroblast growth factor induces myocardial hypertrophy following acute infarction in rats. Exp Physiol 83: 585–593, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation 97: 645–650, 1998. [DOI] [PubMed] [Google Scholar]
- 98.Schumacher B, von Specht BU, Haberstroh J, Pecher P. The stimulation of neo-angiogenesis in the ischemic heart by the human growth factor FGF. J Cardiovasc Surg (Torino) 39: 445–453, 1998. [PubMed] [Google Scholar]
- 99.Semba RD, Crasto C, Strait J, Sun K, Schaumberg DA, Ferrucci L. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J Hum Hypertens 27: 397–399, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shakkottai VG, Xiao M, Xu L, Wong M, Nerbonne JM, Ornitz DM, Yamada KA. FGF14 regulates the intrinsic excitability of cerebellar Purkinje neurons. Neurobiol Dis 33: 81–88, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol 12: 124, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation 105: 788–793, 2002. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki G, Lee TC, Fallavollita JA, Canty JM Jr. Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767–775, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki S, Kato T, Takimoto H, Masui S, Oshima H, Ozawa K, Imamura T. Localization of rat FGF-5 protein in skin macrophage-like cells and FGF-5S protein in hair follicle: possible involvement of two Fgf-5 gene products in hair growth cycle regulation. J Invest Dermatol 111: 963–972, 1998. [DOI] [PubMed] [Google Scholar]
- 106.Thanassoulis G, Massaro JM, O'Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol 3: 345–350, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tomanek RJ, Sandra A, Zheng W, Brock T, Bjercke RJ, Holifield JS. Vascular endothelial growth factor and basic fibroblast growth factor differentially modulate early postnatal coronary angiogenesis. Circ Res 88: 1135–1141, 2001. [DOI] [PubMed] [Google Scholar]
- 108.Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, Nakao K, Imura A. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA 107: 1666–1671, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, Stephan JP, Tsai SP, Powell-Braxton L, French D, Stewart TA. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 143: 1741–1747, 2002. [DOI] [PubMed] [Google Scholar]
- 110.Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O'Donnell TF, Vora AN, Omland T, Solomon SD, Pfeffer MA, Braunwald E, Sabatine MS. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. J Am Coll Cardiol 63: 2421–2428, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 28: 188–200, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao Y, Peng C, Huang W, Zhang J, Xia M, Zhang Y, Ling W. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS One 8: e72545, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang SJ, Hong HC, Choi HY, Yoo HJ, Cho GJ, Hwang TG, Baik SH, Choi DS, Kim SM, Choi KM. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-A levels and arterial stiffness in obese women. Clin Endocrinol (Oxf) 75: 464–469, 2011. [DOI] [PubMed] [Google Scholar]
- 115.Yaqoob U, Jagavelu K, Shergill U, de Assuncao T, Cao S, Shah VH. FGF21 promotes endothelial cell angiogenesis through a dynamin-2 and Rab5 dependent pathway. PLoS One 9: e98130, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest 40: 887–892, 2010. [DOI] [PubMed] [Google Scholar]
- 117.Yonemitsu Y, Matsumoto T, Itoh H, Okazaki J, Uchiyama M, Yoshida K, Onimaru M, Onohara T, Inoguchi H, Kyuragi R, Shimokawa M, Ban H, Tanaka M, Inoue M, Shu T, Hasegawa M, Nakanishi Y, Maehara Y. DVC1-0101 to treat peripheral arterial disease: a Phase I/IIa open-label dose-escalation clinical trial. Mol Ther 21: 707–714, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57: 1246–1253, 2008. [DOI] [PubMed] [Google Scholar]


