Abstract
BACKGROUND: Macrophage activation by cytokines provides only a partial explanation of antimycobacterial immunity in man. Because cytolytic T lymphocytes have been shown to contribute to immunity in animal models of intracellular infection, the generation of mycobacterial antigen specific cytotoxic T cells was examined in the peripheral blood of patients with tuberculosis. METHODS: Subjects comprised 36 patients with active tuberculosis (18 newly diagnosed) and 32 healthy volunteers, of whom 25 had had BCG vaccination and seven were Mantoux negative. The ability of purified protein derivative (PPD) stimulated peripheral blood lymphocytes to lyse autologous, mycobacterial antigen bearing macrophages was examined by using a chromium 51 release assay. RESULTS: PPD stimulated lymphocytes from normal, Mantoux positive, BCG vaccinated subjects produced high levels of PPD specific cytolysis, whereas lymphocytes from unvaccinated, uninfected subjects caused little or no cytolysis. The generation of cytolytic T lymphocytes by patients with tuberculosis was related to their clinical state. Those with cavitating pulmonary disease or lymph node tuberculosis generated PPD specific lymphocytes with cytotoxic ability similar to that of those from Mantoux positive control subjects, whereas lymphocytes from patients with non-cavitating pulmonary infiltrates showed poor antigen specific cytolysis. After seven days of stimulation with PPD in vitro, lymphoblasts contained both CD4+ and CD8+ cells. Mycobacterial antigen specific cytolysis was restricted to the CD4+ cell population and was blocked by monoclonal antibodies directed against major histocompatibility class II (MHC) antigens. CONCLUSION: CD4+ cytolytic T cells can lyse autologous macrophages presenting mycobacterial antigen and were found in patients with cavitating pulmonary tuberculosis or tuberculous lymphadenitis and in normal, Mantoux positive control subjects. The ability to generate these T cell responses seems to be a marker for response to mycobacteria and may contribute to tissue damage in tuberculosis. These responses do not provide protective immunity against Mycobacterium tuberculosis but may help in disease localisation.
Full text
PDF


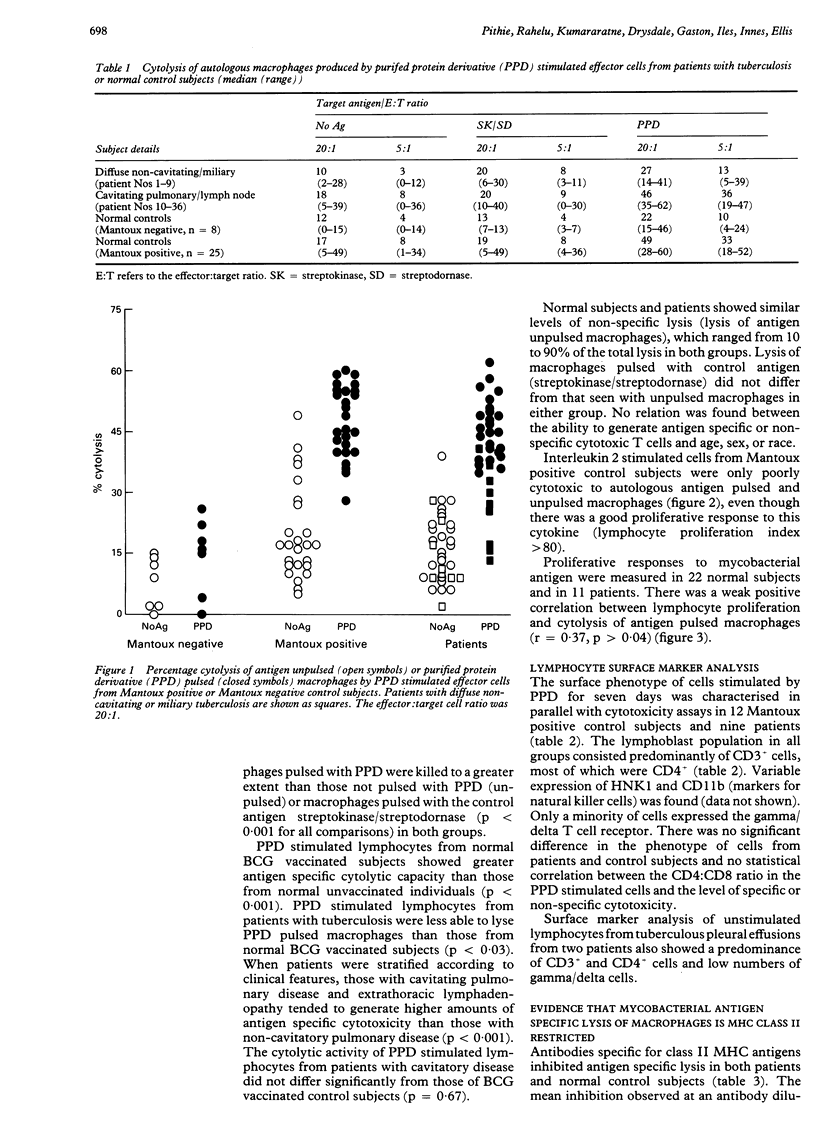
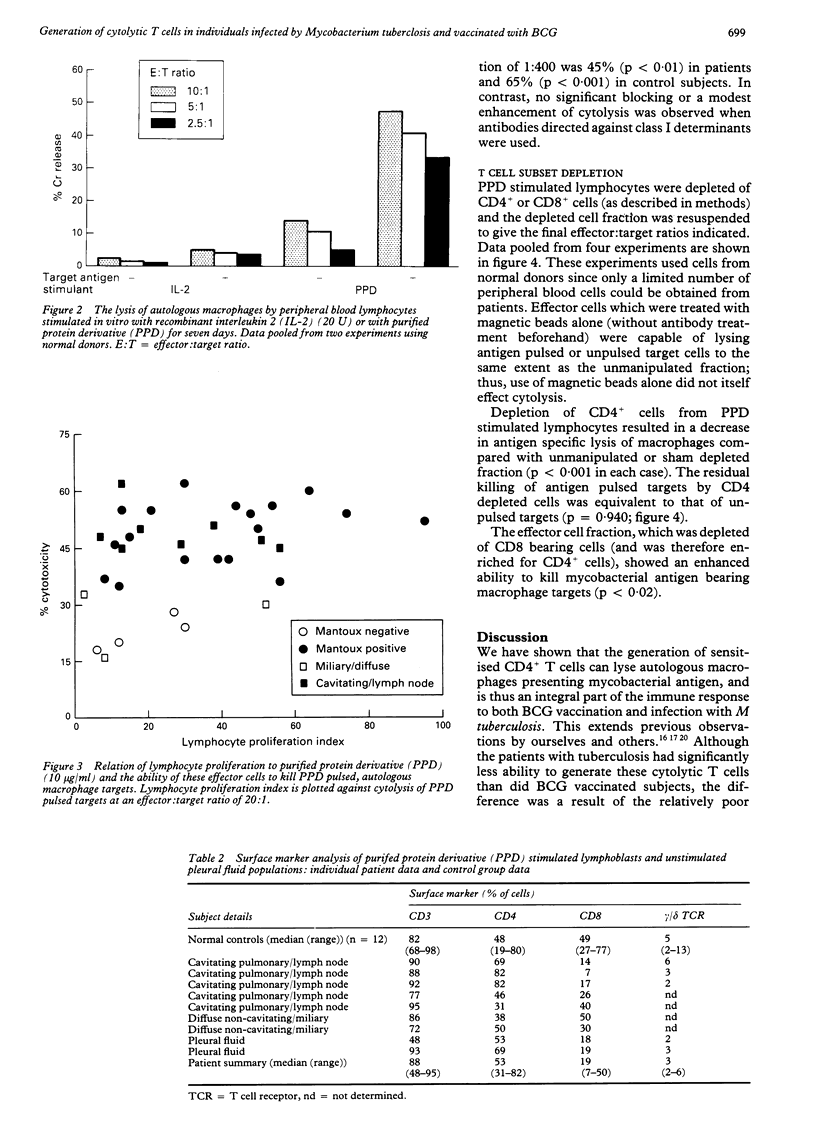
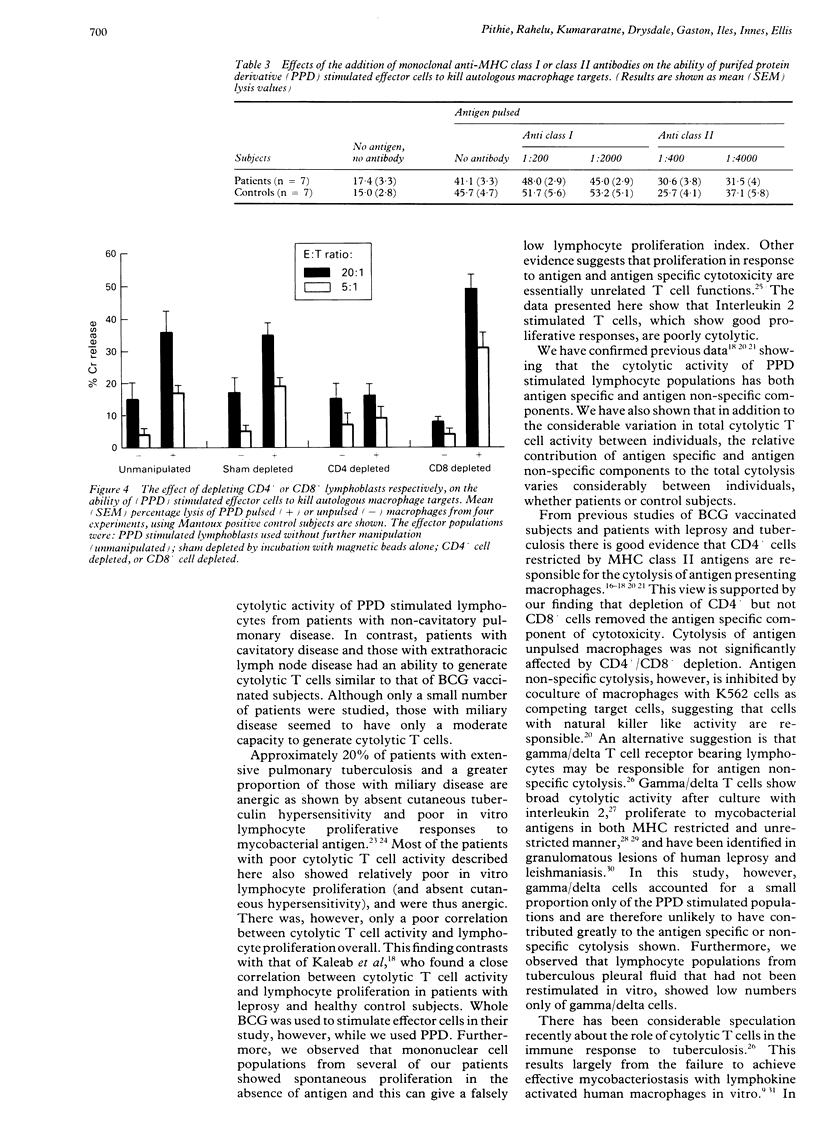

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- De Libero G., Flesch I., Kaufmann S. H. Mycobacteria-reactive Lyt-2+ T cell lines. Eur J Immunol. 1988 Jan;18(1):59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini S., Zarcone D., Viale M., Cerruti G., Millo R., Moretta A., Grossi C. E. Morphologic and functional characterization of human peripheral blood T cells expressing the T cell receptor gamma/delta. Eur J Immunol. 1989 Jul;19(7):1183–1188. doi: 10.1002/eji.1830190705. [DOI] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988 Jun;56(6):1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hansen P. W., Kristensen T. Cell mediated PPD specific cytotoxicity against human monocyte targets: III. Cellular typing with CTLs restricted by class II HLA antigens. Tissue Antigens. 1986 Apr;27(4):227–238. doi: 10.1111/j.1399-0039.1986.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Schondelmaier S., Schoel B., Kaufmann S. H. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. 1990 Mar 1;171(3):667–679. doi: 10.1084/jem.171.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleab B., Ottenoff T., Converse P., Halapi E., Tadesse G., Rottenberg M., Kiessling R. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990 Dec;20(12):2651–2659. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Kiessling R., Teklemariam S., Hancock G., Sheftel G., Job C. K., Converse P., Ottenhoff T. H., Becx-Bleumink M., Dietz M. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J Exp Med. 1989 Mar 1;169(3):893–907. doi: 10.1084/jem.169.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kumararatne D. S., Pithie A. S., Drysdale P., Gaston J. S., Kiessling R., Iles P. B., Ellis C. J., Innes J., Wise R. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990 Jun;80(3):314–323. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N. R., Richardson P. R. A critical appraisal of the direct antibody-rosette test for the detection of cell surface antigens. J Immunol Methods. 1981;47(3):265–274. doi: 10.1016/0022-1759(81)90282-9. [DOI] [PubMed] [Google Scholar]
- Lowrie D. B. Is macrophage death on the field of battle essential to victory, or a tactical weakness in immunity against tuberculosis? Clin Exp Immunol. 1990 Jun;80(3):301–303. doi: 10.1111/j.1365-2249.1990.tb03285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987 Aug;69(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Müller I., Cobbold S. P., Waldmann H., Kaufmann S. H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987 Sep;55(9):2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D. R., Douglass J. E. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980 Jan;77(1):32–37. doi: 10.1378/chest.77.1.32. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Ainsworth M., Champion B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology. 1986 Nov;59(3):333–338. [PMC free article] [PubMed] [Google Scholar]
- Sekaly R. P., MacDonald H. R., Zaech P., Glasebrook A. L., Cerottini J. C. Cytolytic T lymphocyte function is independent of growth phase and position in the mitotic cycle. J Exp Med. 1981 Aug 1;154(2):575–580. doi: 10.1084/jem.154.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga M., Dannenberg A. M., Jr, Higuchi S. Macrophage functional heterogeneity in vivo. Macrolocal and microlocal macrophage activation, identified by double-staining tissue sections of BCG granulomas for pairs of enzymes. Am J Pathol. 1980 May;99(2):305–323. [PMC free article] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]




