Abstract
As skill on a sequence of movements is acquired through practice, each movement in the sequence becomes seamlessly associated with another. To study the neural basis of acquired skills, we trained two monkeys (Cebus apella) to perform two sequential reaching tasks. In one task, sequential movements were instructed by visual cues, whereas in the other task, movements were generated from memory after extended practice. Then, we examined neural activity in the dorsal premotor area (PMd) and the effects of its local inactivation during performance of each task. Comparable numbers of neurons in the PMd were active during the two tasks. However, inactivation of the PMd had a marked effect only on the performance of sequential movements that were guided by memory. These results emphasize the importance of the PMd in the internal generation of sequential movements, perhaps through maintaining arbitrary motor–motor associations.
SIGNIFICANCE STATEMENT The dorsal premotor cortex (PMd) has long been thought to be a critical node in the cortical networks responsible for visually guided reaching. Here we show that PMd neurons are active during both visually guided and internally generated sequential movements. In addition, we found that local inactivation of the PMd has a marked effect only on the performance of sequential movements that were internally generated. These observations suggest that, although the PMd may participate in the generation of visually guided sequences, it is more important for the generation of internally guided sequences.
Introduction
As motor skill on sequential movements is acquired through practice, elements in the sequence become seamlessly associated with one another. The process of building associations between the motor elements has features in common with the process of learning arbitrary sensorimotor associations. This similarity raises the possibility that the cortical areas involved in sensorimotor associations also are responsible for the performance of highly practiced sequences of movements.
There is considerable evidence that the dorsal premotor area (PMd) is involved specifically in the guidance of movements based on sensorimotor associations (Passingham, 1988; Mitz et al., 1991; Kurata and Hoffman, 1994). For example, lesions or inactivation of the PMd produce deficits on tasks that rely on arbitrary visuomotor associations (Passingham, 1988; Kurata and Hoffman, 1994), and neurons in the PMd show activity changes that are specifically related to the performance of these tasks (Kurata and Wise, 1988; Mitz et al., 1991). Given the role of PMd in the performance of sensorimotor associations, we wondered whether this cortical area also is involved in the performance of highly practiced sequential movements that are internally generated.
Conversely, the PMd has also been viewed as a critical node of a parietofrontal network for the visual guidance of reaching movements (Johnson et al., 1996; Wise et al., 1997; Hoshi and Tanji 2007; Averbeck et al., 2009; Cisek and Kalaska, 2010). In contrast, the supplementary motor area (SMA) and the adjacent pre-SMA have generally been regarded as the cortical areas responsible for the internal generation of sequential movements (Roland et al., 1980; Tanji and Shima, 1994; Gerloff et al., 1997; Nakamura et al., 1998; Shima and Tanji, 1998; Picard and Strick, 2001; Hikosaka et al., 2002; Dayan and Cohen, 2011). Therefore, to explore the function of the PMd, we trained two monkeys to perform two sequential reaching tasks. In one task, sequential movements were instructed by visual cues, whereas in the other task, movements were generated from memory after extended practice. Then, we examined neural activity in the PMd and the effects of its local inactivation during performance of each task.
Materials and Methods
The care of the monkeys and the experimental protocols adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. All procedures used followed institutional guidelines and were approved by the Institutional Animal Care and Use Committee.
Behavioral task
Two monkeys (Cebus apella, one male weighing 3.2 kg; one female weighing 1.5 kg) were trained to perform two tasks that required sequential reaching movements with their right arms (Matsuzaka et al., 2007; Picard et al., 2013). Because the two tasks have been described in detail previously, they will only be presented here briefly (Fig. 1a,b). In the Random task, the reaching movements were guided by visual targets displayed on a touch screen monitor. Each new target was presented according to a pseudorandom sequence. Contact of the correct target triggered the display of the next target after 100 ms delay. After the monkeys became proficient in the performance of the Random task (∼50 d of practice), they were introduced to the Repeating task. In the Repeating task, new targets were presented according to a three-element repeating sequence. We trained animals on two repeating sequences: 5-3-1-5-3-1 … (Fig. 1a) and 1-2-4-1-2-4 … (Fig. 1b). New targets during the Repeating task were presented 400 ms after contact of the preceding target. This delay promoted the performance of predictive responses during the Repeating task in which the animal moved to the next target in a sequence before the target was presented. Each task was performed continuously in blocks of 200–500 trials. A liquid reward was given after every four to five correct responses.
Figure 1.
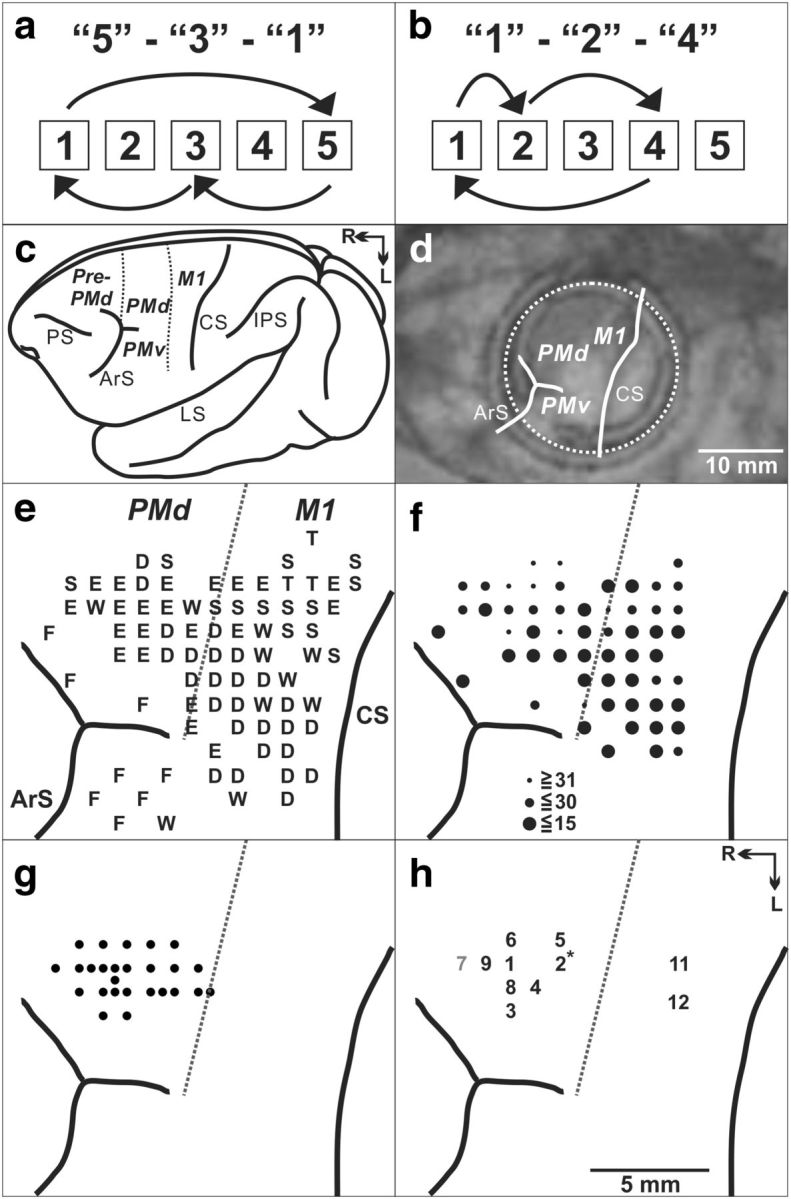
Task and cortical maps for monkey S. a, Repeating task, targets, and movements for sequence 5-3-1. b, Repeating task, targets, and movements for sequence 1-2-4. c, Lateral view of cebus brain. Dashed lines indicate the M1–PMd border and the pre-PMd–PMd border. PS, Principal sulcus; ArS, arcuate sulcus; CS, central sulcus; IPS, intraparietal sulcus; LS, lateral sulcus; R, rostral; L, lateral. d, MR image after the chamber implantation for monkey S. The white dotted circle indicates the chamber outline. e, Intracortical stimulation map from monkey S. Letters indicate the movements evoked at each site: S, shoulder; E, elbow; W, wrist; D, digit; F, face; T, trunk. f, Intracortical stimulation thresholds are indicated by the filled circle size. g, Penetration sites for single-unit recordings. h, Muscimol injection sites in the PMd (sites 1–10) and M1 (sites 11 and 12). * indicates that site 2 was injected twice. Black numbers, Injection sites with a significant effect; gray number, injection site in which no significant effect was observed.
Surgery
We implanted a recording chamber over the left hemisphere and devices for head fixation using conventional procedures. After the surgery, the placement of the chamber was verified using structural MR images (Fig. 1c,d).
Microstimulation and recording
We used glass-coated Elgiloy microelectrodes (0.6–1.5 MΩ at 1 kHz) to deliver intracortical microstimuli and for single-unit recording. A constant-current stimulator was used to deliver cathodal pulses (12–32 pulses, 0.2 ms duration, 333 Hz, 1–50 μA intensity) at a depth of 1500 μm below the cortical surface (Dum and Strick, 2005). Stimulus intensity was measured with a current monitor (Ion Physics). The motor response evoked by stimulation was determined by visual observation and muscle palpation. The response threshold was defined as the lowest stimulus intensity necessary to evoke a response on ∼80% of the trials.
Muscimol injections
We injected 1–3 μl of the muscimol solution (5 μg/μl in saline, 0.2 μl every 30–60 s; Sigma) at 1.5 mm below the cortical surface using a 30 gauge cannula connected to a 10 μl Hamilton syringe. The cortical sites injected in one monkey (monkey S) are displayed in Figure 1h. At least two days separated experimental sessions with muscimol injections.
To ensure that the alterations in behavior at the beginning or end of a daily training session did not influence our results, we limited our analysis to the period of each daily session when the animals' performance was stable. To do this, we excluded the first 10 trials at the start of each session and then collected a block of “control” trials (500 Random and 500 Repeating movements). Next, we injected muscimol and waited 20 min. Then, we collected a block of “test” trials (1000 Random and 1000 Repeating movements). One of the two monkeys (monkey J) displayed a large performance deficit after some muscimol injections. We terminated data collection early when the animal stopped reliable performance of the task (after ∼1000 trials). To confirm that task performance was stable across control and test trials, we analyzed various features of performance during daily training sessions (e.g., error rate, percentage of predictive responses, etc.). We found no significant change in performance measures throughout the daily training session.
Data analysis
Neuron recording.
We defined task-related neurons as single units that displayed phasic increases or decreases in discharge temporally coupled to the performance of the task (Matsuzaka et al., 2007; Picard et al., 2013). To compare data from the two tasks, we limited our analysis during the Random task to the same moves that were performed during the Repeating task (e.g., to moves 5 to 3, 3 to 1, …). For each Random or Repeating move, we measured the mean firing rate of the neuron in a 200 ms interval centered on target contact. We used this value to calculate a modulation index (MI) for each move: MI = [Repeating − Random]/[Repeating + Random]. Six comparisons were possible for each neuron. We calculated the mean MI based on all the MIs of all the task-related neurons (0.034 ± 0.155, mean ± SD). A neuron was considered to be Repeating enhanced if the MI of one or more of its moves was 1 SD above the mean MI and none of its moves were 1 SD below the mean MI. A neuron was considered to be Random enhanced if one or more of its MI was 1 SD below the mean MI and none of the MIs were 1 SD above the mean MI. A neuron was considered to be Mixed if some responses were Repeating enhanced and some were Random enhanced. We excluded data from this analysis only as a result of low trial number or low activity for both tasks. Additional statistical analyses using other tests (e.g., t tests and ANCOVA tests, p < 0.05) yielded comparable results.
Behavioral effects of muscimol injections.
We collected a variety of kinematic measures during the performance of the Random and Repeating tasks, including response times (RTs), movement times (MTs), and contact points on the touch screen. We defined MT as the interval between the release of contact from one target to touch of the next target. We defined RT during the Random task as the time between the presentation of a new target and contact of that target. We defined RT during the Repeating task as the time between contact of two targets in a sequence. We subtracted 400 ms to account for the delay in the cue presentation. This could result in a negative RT if the monkey moved quickly to the next target in the sequence before the presentation of a cue. RTs <150 were considered to be predictive. We divided behavioral analysis into two task periods: (1) a pre-injection, control period; and (2) a post-injection, test period. We used χ2 tests with Holm–Bonferroni's correction to examine the significance of changes in success rate and predictive responses. We used t tests with Holm–Bonferroni's correction to examine MT and RT changes. We excluded trials after errors from analysis because in case of errors the trial was repeated.
Results
Activity of PMd neurons
We identified the arm area of the PMd using intracortical stimulation (Fig. 1e,f). Electrode penetrations for neuron recording were placed in the caudal portion of the PMd (Fig. 1g) in which intracortical stimulation evoked shoulder, elbow, wrist, or finger movements at stimulus thresholds ≤50 μA. We recorded the activity of 327 task-related neurons in the PMd during performance of the two tasks (monkey S, n = 106; monkey J, n = 221). Forty-three percent of neurons (140 of 327 neurons) displayed responses that were enhanced in one task compared with the other (i.e., differential neurons). More than half of the differential neurons (52%) displayed enhanced activity during the Repeating task (73 of 140 differential; Fig. 2a). A similar number of neurons displayed enhanced activity during the Random task (67 of 140 differential neurons, 48%; Fig. 2b). The remainder of the task-related neurons displayed nondifferential responses (163 of 327 neurons, ∼50%) except for a small number of neurons that displayed mixed responses (24 of 327 neurons, 7%). Neurons with differential, nondifferential, or mixed responses were intermingled in the PMd. Taken together, these results indicate that changes in PMd activity are associated with performance of internally instructed and visually guided sequences.
Figure 2.
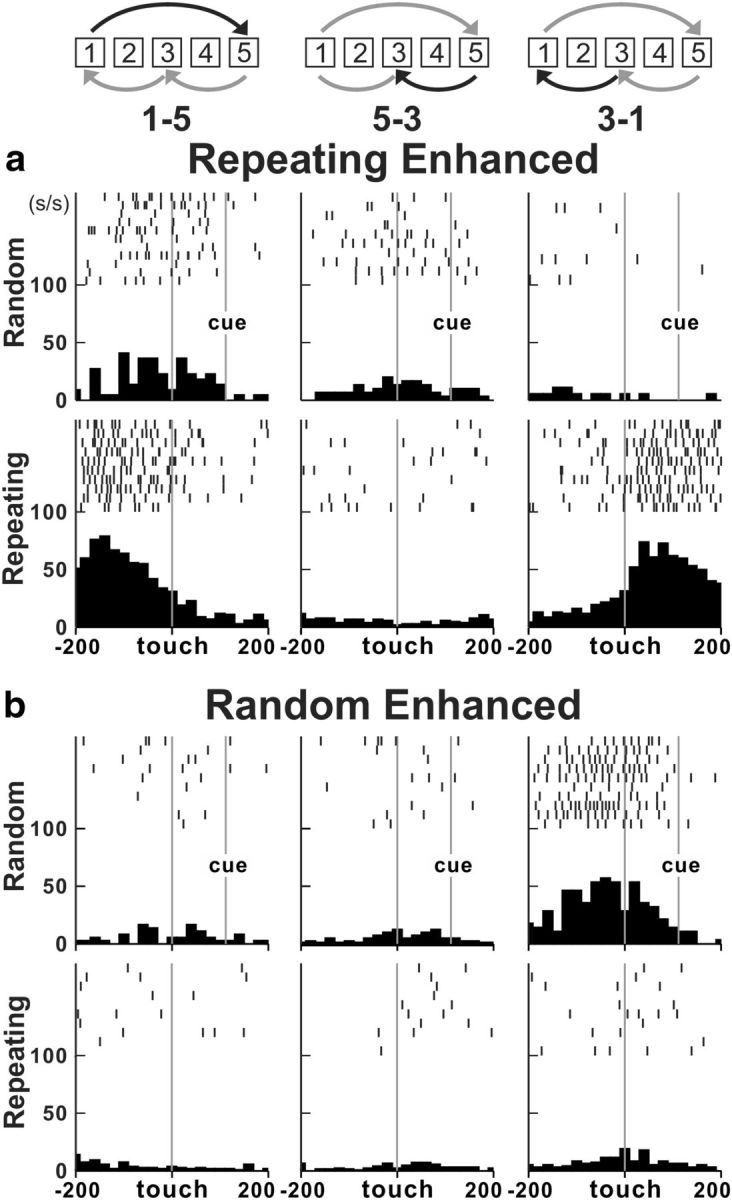
Neural activity in the PMd during the two tasks. a, Activity of a Repeating enhanced neuron. The activity was enhanced for movements from target 1 to target 5. The increase was present after contact with target 1 (right; MI = 0.9) and before the contact with target 5 (left). This neuron displayed only modest or no changes in activity when the same movements were made during the Random task (t test, p < 0.001 for all moves). b, Activity of a Random enhanced neuron. The activity was enhanced for movements from target 3 to target 1 (right; MI = −0.6). This neuron displayed only modest or no changes in activity during the Repeating task (bottom; t test, p < 0.001 for move 1-5, p = 0.01 for move 5-3, p < 0.001 for move 3-1). The movement performed is indicated by the symbols and numbers at the top of each column. Each tick mark represents a spike. The rasters and histograms are aligned on target contact. The rasters show 10 trials. Histograms include all correct trials. Histogram bin width, 20 ms.
Effect of PMd inactivation
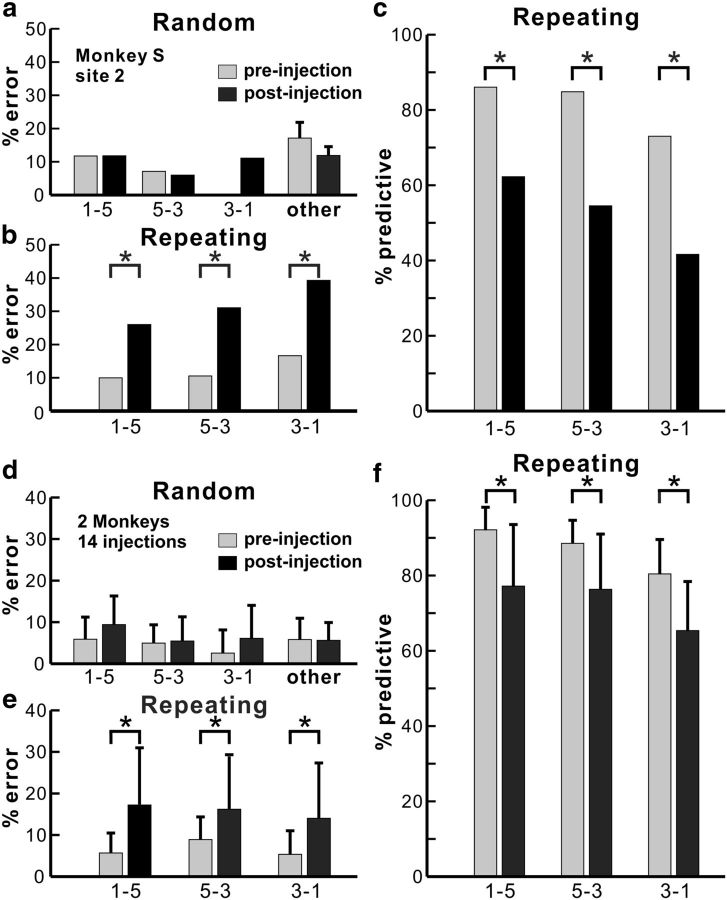
To test the causal contribution of the PMd to motor performance of the two tasks, we used small injections of muscimol, a GABA agonist (5 μg/μl, 1 or 3 μl) to inactivate localized regions of the PMd. We placed a total of 22 injections of muscimol (monkey S, n = 10; monkey J, n = 12) at 21 different sites in which intracortical stimulation evoked shoulder, elbow, or wrist movements (Fig. 1h). One site was tested twice (Fig. 1h, site 2). We observed a change in the animal's performance after 14 of the 22 injections.
The muscimol injections had a significant effect only on the performance of movements during the Repeating task (Figs. 3, 4). Localized inactivation of the PMd resulted in a significant increase in the number of incorrect responses and a significant decrease in the number of predictive responses during the Repeating task (Fig. 4; 14 injection sessions, χ2 test, p < 0.03). The number of incorrect responses during the Repeating task increased by as much as three times (Figs. 3a, 4b). Overall, errors doubled during the Repeating task after inactivation of the PMd (Fig. 4e). In contrast, localized inactivation of the PMd did not produce a statistically significant effect on the performance of movements during the Random task (Figs. 3, 4a,d). Specifically, we did not see a significant increase in the number of incorrect responses for any of the individual movements made during the Random task (Fig. 4a,d; 14 injections, χ2 test, p > 0.05).
Figure 3.
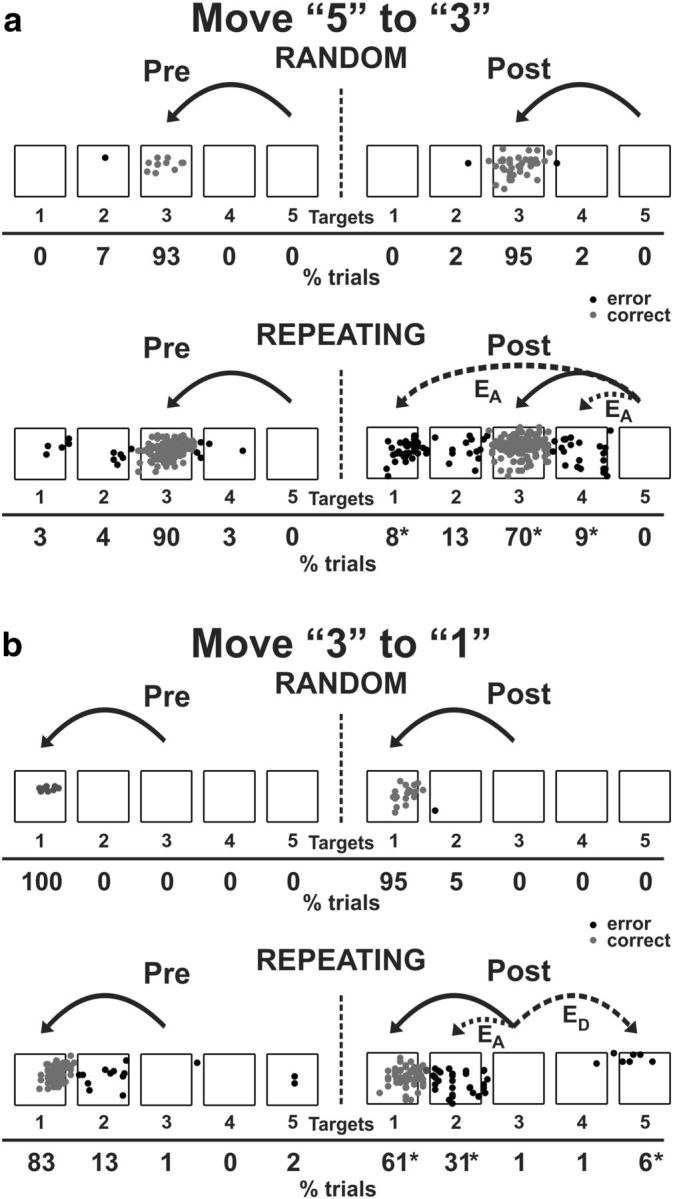
Reaching end points before and after muscimol injection. Left, Preinjection; right, postinjection. EA, Accuracy errors; ED, Direction errors; gray dots, correct response; black dots, error response. The muscimol injection was placed at site 2 in monkey S (Fig. 1 h). a, End points for move 5 to 3. b, End points for move 3 to 1. Percentage of trials ending in each target are given below the targets. Touches between targets were counted as touches to the closest target. *p < 0.05.
Figure 4.
Effects of PMd inactivation. a–c, The performance data for an injection at site 2 in monkey S (Fig. 1h). a, Error rate in the Random task. PMd inactivation did not have any effect on number of errors (χ2 test, p = 0.992 for move 1-5, p = 1.000 for move 5-3, p = 1.000 for move 3-1, df = 1; others, t test, p = 0.415). b, Error rate in the Repeating task. After the muscimol injection, the number of errors increased dramatically with all movements in the Repeating task (χ2 test, p < 0.001 for all moves, df = 1). c, Predictive responses. The percentage of predictive responses decreased significantly after the muscimol injection (χ2 test, p = 0.006 for move 1-5, p < 0.001 for move 5-3, p = 0.014 for move 3-1, df = 1). d–f, Population data for 14 effective injections at 13 cortical sites. d, Average error rate in the Random task. PMd inactivation did not have an effect on the number of error responses in the Random task (paired t test with 14 injection experiments, p = 0.260 for move 1-5, p = 1.000 for move 5-3, p = 0.273 for move 3-1, p = 0.753 for others, df = 13). e, Average error rate in the Repeating task. The error rate in the Repeating task increased significantly after the PMd inactivation (paired t test, p = 0.015 for move 1-5, p = 0.03 for move 5-3, p = 0.014 for move 3-1, df = 13). f, Average predictive responses. The percentage of predictive responses decreased after localized inactivation of the PMd (paired t test, p = 0.004 for move 1-5, p = 0.004 for move 5-3, p < 0.001 for move 3-1, df = 13). Other, Movements that were not a part of the Repeating sequence (5-3, 3-1, or 1-5). *p < 0.05.
The errors during the Repeating task can be categorized as two types: (1) errors of accuracy; and (2) errors in direction. An accuracy error was considered to be a reach performed in the correct direction but to an endpoint outside of the correct target (Fig. 3a,b, EA). For example, movement 5-3 requires a leftward movement from target 5 to target 3. Before the muscimol injection at site 2 in monkey S (Fig. 1h), the animal made accuracy errors by moving to targets 1, 2, or 4 on 10% of the trials for the 5-3 movement. After the muscimol injection, the number of accuracy errors increased to 30% of the trials (Figs. 3a, 4b).
A direction error was considered to be a reach performed in the direction opposite to the correct target (Fig. 3b, ED). For example, movement 3-1 requires a leftward movement from target 3 to target 1. Before the muscimol injection at site 2 in monkey S, the animal made direction errors by moving in the rightward direction to target 4 or 5 on 2% of the trials for the 3-1 movement. After the muscimol injection, the number of direction errors increased to 7% of the trials (Fig. 3b).
As noted above, the muscimol injections also caused a change in the number of predictive movements during the Repeating task. We defined predictive movements as responses <150 ms after the presentation of a visual cue (see Materials and Methods). Before the muscimol injection at site 2 in monkey S, the animal made predictive movements on ∼82% of the trials. After the muscimol injection, the number of predictive movements decreased to ∼53% of the trials (Fig. 4c). Overall, we observed a 14% decrease in the number of predictive movements during the Repeating task after inactivation of the PMd (Fig. 4f).
In general, movements during the Random task had slightly longer MTs than movements during the Repeating task (255.39 ± 30.16 vs 219.37 ± 31.85 ms for move 3-1; 188.79 ± 24.01 vs 147.17 ± 19.67 ms for move 5-3; 222.66 ± 99.99 vs 150.05 ± 66.41 ms for move 1-5). Muscimol injections into the PMd did not cause a significant change in MTs for most of the movements performed during the Random and Repeating tasks. A small but significant increase in MT only occurred after muscimol injections for the move during the Repeating task. These results suggest that simple alterations in movement speed after muscimol injections were not the cause of the errors on the Repeating task.
To control for the possibility that inactivation of any motor area might preferentially disrupt the Repeating task because it is the faster-paced task, we performed two control experiments. In two separate experimental sessions, we injected muscimol into the shoulder representation of M1 (at points 11 and 12 on the map shown in Fig. 1h). We examined the performance of the animal on the Random and Repeating tasks just as we did for muscimol injections into the PMd. In both cases, we found that M1 inactivation impaired performance on both the Random and Repeating tasks (χ2 test, p < 0.05).
Discussion
The most important observation of the present study is that localized inactivation of the PMd results in a selective deficit in the performance of sequential movements during the Repeating task. This observation emphasizes the importance of the PMd for the generation of sequential movements that are internally guided. Overall, we found comparable numbers of neurons that displayed enhanced activity during the Random and Repeating tasks. Even so, movement performance during the Random task was unaffected by PMd inactivation. These results suggest that, although the PMd may be involved in the performance of visually guided sequences, it is more important for the performance of internally generated sequences. Other cortical and subcortical areas, such as the ventral premotor area and superior colliculus, may be more critical for the visual guidance of reaching movements (Mushiake et al., 1991; Kurata and Hoffman, 1994; Werner et al., 1997).
The function of the PMd has been the subject of some controversy. Many authors have focused on the importance of the PMd for the “visual guidance of motor behavior” (Johnson et al., 1996; Wise et al., 1997; Hoshi and Tanji, 2007; Averbeck et al., 2009). Averbeck et al. (2009, p. 1917) concluded that the PMd is a part of the “dorsal premotor cluster,” which represents “the frontal node of the network underlying visually guided reaching (Kalaska and Crammond, 1995; Johnson et al., 1996), mental rehearsal (Cisek and Kalaska, 2004), and aspects of decision making in the reach system (Cisek and Kalaska, 2004; Pesaran et al., 2008).” These conclusions have generally come from studies that used single neuron recording or functional imaging during visually guided reaching. In contrast, other authors using lesion and neuron recording have emphasized the role of the PMd in building arbitrary sensorimotor associations (Passingham, 1988; Mitz et al., 1991; Kurata and Hoffman, 1994).
Our inactivation results emphasize the role of the PMd in guiding sequential movements based on internal instructions. At the start of training on our task, the three elements in the sequence were independent of one another and were guided by visual instructions. After considerable practice, the individual elements in the sequence became associated with each other. In other words, practice enabled the animal to build arbitrary motor–motor associations. The practiced sequences could then be internally generated in a seamless and predictive manner. One interpretation of our results is that localized inactivation of the PMd disrupted the arbitrary motor–motor associations in very much the same way as lesions of the premotor cortex disrupt the ability of an animal to perform arbitrary sensorimotor associations (Passingham, 1988). Hardwick et al. (2013) advanced a similar proposal based on a meta-analysis of neuroimaging data collected during the Serial Reaction Time task. In essence, they concluded that the left PMd of humans “is a critical node in the motor learning network” for sequential movements (Hardwick et al., 2013, p. 283). Our results provide a clear demonstration of the importance of the PMd for the performance of practiced sequential movements.
The PMd proper is densely interconnected with the primary motor cortex (M1) and is an important source of input to M1 (Dum and Strick, 2005). We demonstrated recently that extended practice on a Repeating sequence results in dramatic alterations in the functional activation and neural responses of M1 (Matsuzaka et al., 2007; Picard et al., 2013). The current results are consistent with the PMd functioning as a major source of input to M1 to guide the performance of internally generated sequences. This conclusion is surprising given the longstanding view that the preparation for and generation of sequential movements depends on the SMA and the pre-SMA (Roland et al., 1980; Tanji and Shima, 1994; Gerloff et al., 1997; Nakamura et al., 1998; Shima and Tanji, 1998; Picard and Strick, 2001; Hikosaka et al., 2002; Dayan and Cohen, 2011). Thus, our results raise an important question: how do the SMA and PMd differ in their contributions to sequential movement control? Tanji (2001) has suggested one potential answer, namely, spatial and nonspatial sequences might be learned and controlled by different cortical systems. For example, Tanji and colleagues found that the SMA was critical to the performance of movement sequences that relied on largely temporal order (Shima and Tanji, 1998; Tanji, 2001). Indeed, Nakamura et al. (1999) reported that inactivation of the SMA and pre-SMA had little effect on the performance of well learned spatial sequences. These observations along with ours suggest that the SMA may be critical to the performance of temporal sequences, whereas the PMd may be essential to the performance of spatial sequences. This possibility and others, such as the involvement of these cortical areas in different stages of skill acquisition (Sakai et al., 1998; Hikosaka et al., 2002; Dayan and Cohen, 2011), should be explored in future experiments.
Footnotes
This material is based on work supported in part by National Institutes of Health Grants R01 NS24328 (P.L.S.), P01 NS044393 (P.L.S.), and P30 NS076405 (P.L.S.) and Japan Society for the Promotion of Science (M.O.). We are grateful to Mike Page for the development of computer programs.
The authors declare no competing financial interests.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License Creative Commons Attribution 4.0 International, which permits unrestricted use, distribution and reproduction in any medium provided that the original work is properly attributed.
References
- Averbeck BB, Battaglia-Mayer A, Guglielmo C, Caminiti R. Statistical analysis of parieto-frontal cognitive-motor networks. J Neurophysiol. 2009;102:1911–1920. doi: 10.1152/jn.00519.2009. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25:1375–1386. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. Stimulation over the human supplementary motor area interferes with the organization of future elements in complex motor sequences. Brain. 1997;120:1587–1602. doi: 10.1093/brain/120.9.1587. [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283–297. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/S0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Distinctions between dorsal and ventral premotor areas: anatomical connectivity and functional properties. Curr Opin Neurobiol. 2007;17:234–242. doi: 10.1016/j.conb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Kalaska JF, Crammond DJ. Deciding not to GO: neuronal correlates of response selection in a GO/NOGO task in primate premotor and parietal cortex. Cereb Cortex. 1995;5:410–428. doi: 10.1093/cercor/5.5.410. [DOI] [PubMed] [Google Scholar]
- Kurata K, Hoffman DS. Differential effects of muscimol microinjection into dorsal and ventral aspects of the premotor cortex of monkeys. J Neurophysiol. 1994;71:1151–1164. doi: 10.1152/jn.1994.71.3.1151. [DOI] [PubMed] [Google Scholar]
- Kurata K, Wise SP. Premotor cortex of rhesus monkeys: set-related activity during two conditional motor tasks. Exp Brain Res. 1988;69:327–343. doi: 10.1007/BF00247578. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godschalk M, Wise SP. Learning-dependent neuronal activity in the premotor cortex: activity during the acquisition of conditional motor associations. J Neurosci. 1991;11:1855–1872. doi: 10.1523/JNEUROSCI.11-06-01855.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sakai K, Hikosaka O. Effects of local inactivation of monkey medial frontal cortex in learning of sequential procedures. J Neurophysiol. 1999;82:1063–1068. doi: 10.1152/jn.1999.82.2.1063. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Premotor cortex and preparation for movement. Exp Brain Res. 1988;70:590–596. doi: 10.1007/BF00247607. [DOI] [PubMed] [Google Scholar]
- Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453:406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/S0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci. 2013;16:1340–1347. doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Larsen B, Lassen NA, Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/PL00005690. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]