Abstract
Background
Atraumatic necrosis of the femoral head is a common cause of hip arthrosis in middle age. In Germany, it affects 5000–7000 patients per year, corresponding to an incidence of 0.01%. Though rarer than primary hip arthrosis, it is still of major clinical and socio-economic significance. Patients with this problem should be diagnosed early and given stage-appropriate treatment.
Method
This review is based on pertinent publications that were retrieved by a selective search in the PubMed, Embase, Medline, and Cochrane Library databases using the terms “osteonecrosis,” “femoral head necrosis,” “diagnosis,” “classification,” “conservative treatment,” “surgical treatment,” “joint preservation,” “osteotomy,” and “arthroplasty,” as well as a recent guideline on atraumatic necrosis of the femoral head in adults.
Results
The etiology and pathogenesis of atraumatic femoral head necrosis in adults are not yet fully clear. The main risk factor is prolonged corticosteroid treatment. Nonspecific complaints and an initially normal plain x-ray of the hip can delay the diagnosis. The diagnosis is established by plain x-ray, computerized tomography, magnetic resonance tomography, and scintigraphy. Conservative treatment alone is not considered adequate. The range of surgical treatments includes joint-preserving and (for more severe necrosis) joint-resecting methods.
Conclusion
Atraumatic femoral head necrosis in adults is a disease that progresses in stages; depending on its stage, it can either be cured or lead to hip arthrosis. A full cure is possible only in early stages. Current research focuses on the effect of new drugs on the intermediate- and long-term outcome.
Non-traumatic femoral head necrosis (FHN) in adults is an acquired ischemic disease of the femoral head characterized by a multifactorial etiology. The initial manifestation is local, usually partial necrosis but the disease has the potential to progress to complete destruction of the femoral head with development of end-stage coxarthrosis. Although confirmed epidemiological data are lacking, FHN seems to be most common in men between the ages of 30 and 50 years (1). Together with hip dysplasia and femoroacetabular impingement, FHN represents one of the leading causes of coxarthrosis in middle age. The necrosis is bilateral in 30 to 70% of cases. If left untreated, FHN leads to severe secondary joint destruction in a high proportion of patients. Extrapolation from the published data suggest an incidence of 0.01% in the German-speaking countries, with 5000 to 7000 persons affected each year (2). Despite the low incidence and prevalence compared with primary coxarthrosis, FHN has a significant economic impact because it largely affects persons in the prime of life (peak age 35 years). For these reasons early diagnosis of FHN and appropriate treatment according to disease stage assume special importance, with prevention of primary and secondary damage in the foreground.
In this article, based on a literature survey and the findings of our own studies, we provide a review of the current strategies for diagnosis and treatment of atraumatic FHN in adults. The literature search was conducted in the PubMed, Embase, Medline, and Cochrane Library databases and included the German-language guideline for atraumatic FHN in adults of the Association of the Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V., AWMF). Relevant publications were selected following the criteria suggested by the German Agency for Quality in Medicine (Ärztliches Zentrum für Qualität in der Medizin, ÄZQ) (3).
Etiology and pathophysiology
The reasons for and mechanisms of FHN are not yet absolutely clear but the consensus in the literature is that a multifactorial process is involved (4).
Long-term corticosteroid treatment is the most frequent and most important risk factor. It is the principal cause of FHN in 10 to 30% of cases (5– 7). Treatment for 2 to 3 months with a daily dose of 2 g prednisolone equivalent or more is regarded as critical (6). Excessive alcohol consumption has also been demonstrated to increase the incidence of non-traumatic FHN. In a prospective study, Matsuo et al. showed a significant dose-dependent increase in risk (8). Intake of up to 320 g ethanol (corresponding to about five bottles of wine) per week raises the risk of non-traumatic FHN by a factor of 2.8. Smoking has also been established as a risk factor, although in contrast to alcohol abuse no dose–effect relationship has been established (8, 9, e1).
FHN develops more frequently in HIV patients, with or without antiretroviral treatment (e2– e4). The causal role of antiretroviral treatment is controversial and is not supported by robust data. Further risk factors are listed in the Box.
Box. Risk factors for atraumatic femoral head necrosis*.
Cortisone treatment
Alcohol consumption/abuse
Smoking
Hemoglobinopathy
Sickle-cell anemia
Coagulopathy
Myeloproliferative diseases
Gaucher’s disease
Leukemia
Chemotherapy
Ionizing irradiation
Pregnancy
HIV infection
Genetic predisposition
Collagen type II mutation
Alcohol-metabolizing enzymes
Caisson disease (decompression sickness)
*Modified from (4)
Although the pathophysiological mechanisms of atraumatic FHN have not been conclusively clarified, femoral head ischemia is assumed to be the cause, independent of the etiology. Thrombotic occlusions in the context of intravascular coagulopathy or extravascular compression impair the microcirculation in the subchondral bone and favor the development of necrosis (10).
Diagnosis
Careful diagnostic work-up is particularly important in the early stages of atraumatic FHN. Non-specific symptoms and an initial absence of abnormalities on plain radiographs may lead to a fateful delay in diagnosis and initiation of treatment. Together with the medical history and physical examination, diagnostic imaging plays an important part (1).
Medical history and clinical examination
The patients often report symptoms in the hip joint independent of movement. The complaints are usually unspecific and may depend on disease stage (4). Radiation of pain into the groin and down the thigh as far as the knee joint is possible. Painstaking questioning with regard to potential risk factors is crucial (Box). Intra-articular and extra-articular causes of the symptoms must be considered and excluded (Table 1).
Table 1. Differential diagnosis of hip pain.
| Intra-articular causes | Extra-articular causes |
|---|---|
| Coxarthrosis | Bursitis |
| Hip dysplasia | Snapping hip |
| Femoroacetabular impingement | Insertion tendinopathy |
| Transient ischemic osteoporosis/bone bruise | Para-articular soft-tissue tumor |
| Chondromatosis | Heterotopic ossification |
| Osteochondral lesion | Spinal canal stenosis |
| Infection | Spondylarthrosis/root compression syndrome |
| Fracture | Iliosacral joint symptoms |
| Lesion of round ligament | Piriform muscle syndrome |
| Rheumatoid arthritis | — |
| Pigmented villonodular synovialitis | — |
Transient bone marrow edema, common in men in the fifth and sixth decades of life, may show the appearances of non-traumatic FHN but is a self-limiting process. The debate continues as to whether the changes that occur in the last trimester of pregnancy should be described as non-traumatic FHN or transient bone marrow edema (11).
On clinical examination attention must be paid to the possible presence of abnormal posture or a limp related to pain or limb shortening.
In advanced FHN the findings do not differ from those of primary coxarthrosis and may show a capsular pattern with restriction of internal rotation, flexion, and abduction.
Diagnostic imaging
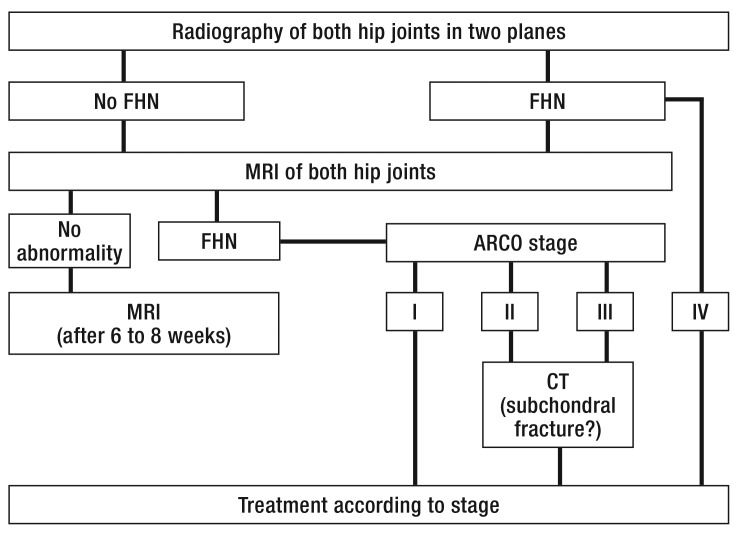
Imaging modalities play an important part in establishing the diagnosis of FHN and determining the stage of disease. Apart from plain radiography and computed tomography, magnetic resonance imaging and scintigraphy enable detection of early changes (12). The diagnostic algorithm in the case of suspected FHN is shown in Figure 1.
Figure 1.
Diagnostic procedure in the event of suspected atraumatic femoral head necrosis (FHN). MRI, magnetic resonance imaging; FHN, femoral head necrosis; CT, computed tomography; ARCO, Association Research Circulation Osseous
Radiography
Plain radiography in two planes (anteroposterior pelvic and hip joint according to Lauenstein) is the initial step in diagnostic imaging and serves to compare the two sides, exclude differential diagnoses, and determine the disease stage in advanced cases. The signs of non-traumatic FHN on plain radiography are sclerosis and cysts with a sclerotic margin. Later in the disease process subchondral fractures and areas of joint collapse are found. Early FHN (ARCO stage I; ARCO = Association Research Circulation Osseous) cannot be visualized by plain radiography.
Computed tomography
Computed tomography (CT) has higher sensitivity and specificity than plain radiography, but is also incapable of visualizing early FHN (ARCO I) (13). The introduction of high-resolution CT with slice thickness of 1 to 2 mm enables very good demonstration of the trabecular structures. Thus the prognostically important subchondral fractures in the early phase of mechanical instability are visualized better on CT than on magnetic resonance imaging (MRI) (13). Patients in ARCO stage II with suspected subchondral fractures on MRI should therefore be referred for CT.
Magnetic resonance imaging
Magnetic resonance imaging has sensitivity and specificity of >95% and therefore represents the gold standard for non-invasive diagnostic investigation (14, 15, e5, e6). It visualizes bone marrow changes and enables confirmation of the diagnosis even in early stages of FHN when no abnormalities are detected by plain radiography (e7). In advanced disease MRI enables good evaluation of the size and location of the osteonecrotic zone, the extent of involvement of acetabular cartilage, and the depth of joint collapse. These serve as prognostic factors and help to determine the appropriate treatment (16).
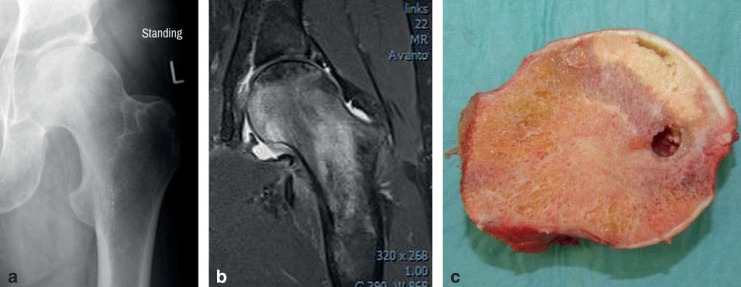
Owing to the high proportion of cases in which the disease is bilateral, assessment of the contralateral hip joint with the aid of MRI is recommended even in the absence of clinical or radiographic signs of FHN (17). MRI distinguishes transient bone marrow edema from FHN on the basis of homogeneous edema extending into the intertrochanteric region without subchondral changes (Figure 2).
Figure 2.
Femoral head necrosis in a 41-year-old patient, ARCO stage III: a) on radiography; b) magnetic resonance imaging (MRI); c) after resection of the femoral head. Plain radiography with the patient standing shows no subchondral fractures or areas of joint surface collapse. The fat-suppressed MRI sequence reveals pronounced bone marrow edema and the zone of necrosis. The resected and divided femoral head (1 month after imaging) displays an area of pronounced necrosis, cystic changes, and a subchondral fracture zone.
Skeletal scintigraphy
Like MRI, skeletal scintigraphy visualizes early changes of bone and bone marrow. Despite high sensitivity, however, the specificity of scintigraphy is low, so the location and extent of FHN cannot be adequately quantified (14, e5).
Single-photon emission computed tomography
Single-photon emission computed tomography (SPECT) is a scintigraphic modality with improved spatial resolution and tomographic image presentation. It has higher sensitivity than planar scintigraphy, but lower sensitivity and specificity than MRI (18).
Classification of femoral head necrosis
There are numerous classifications of the pathophysiological progression of FHN (19). The ARCO classification is the one most often used in Europe, particularly in the German-speaking countries (20) (Table 2).
Table 2. ARCO classification*.
| Stage | Clinical findings, pathology, imaging |
|---|---|
| 0 |
|
| I |
|
| II |
|
| III |
|
| IV |
|
*From (20)
Treatment
If left untreated, FHN leads to subchondral fractures of the femoral head within 2–3 years, and is then no longer amenable to joint-preserving treatment (21, e8). The disease stage and the extent and location of necrosis are crucial predictive factors with regard to joint collapse and treatment success (21, 22). Pain at the time of diagnosis, age <40 years, and the continued existence of risk factors are secondary criteria of disease progress. Restitutio ad integrum, i.e., complete healing, is a realistic goal only in the early stages of FHN, but even then the spectrum of available treatments may not achieve success. In advanced cases the aim is to postpone mechanical failure of the hip and eventual implantation of a prosthetic joint. The following sections describe the conservative and surgical options for treatment of FHN embodied in the current German-language guideline on atraumatic FHN in adults (Tables 3, 4).
Table 3. Treatment options for femoral head necrosis.
| Conservative treatment | Level of evidence* |
|---|---|
| Restriction of weight bearing | 2+ |
| Medication | |
| Anticoagulants – | 2+ |
| Prostaglandin analogs – | 2+ |
| Bisphosphonates – | 2+ |
| Extracorporeal shock-wave treatment | 1–2+ |
| Electromagnetic stimulation | 2+ |
| Hyperbaric oxygen treatment | 2+ |
| Surgical treatment | |
| Decompression | 2++ |
| Osteotomy | 2++ |
| Bone transplantation | 2+ |
| Joint replacement | 2++ |
*Based on ssessment of study quality according to the recommendations of the Scottish Intercollegiate Guidelines Network (SIGN) (40).
Table 4. The SIGN classification of evidence (40).
| Levels of evidence | Description |
|---|---|
| 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with very low risk of systematic error (bias) |
| 1+ | Well-conducted meta-analyses, systematic reviews of RCTs, or RCTs with low risk of systematic error (bias) |
| 1− | Meta-analyses, systematic reviews of RCTs, or RCTs with high risk of systematic error (bias) |
| 2++ | High-quality systematic reviews of case control or cohort studies or high-quality case control or cohort studies with very low risk of systematic distortion (confounding, bias, chance) and high probability that the relationship is causal |
| 2+ | Well-conducted case control or cohort studies with low risk of systematic distortion (confounding, bias, chance) and moderate probability that the relationship is causal |
| 2− | Case control or cohort studies with high risk of systematic distortion (confounding, bias, chance) and significant risk that the relationship is not causal |
| 3 | Non-analytical studies, e.g., case reports or case series |
| 4 | Expert opinion |
RCT, randomized controlled trial
Conservative treatment
The conservative treatment of atraumatic FHN is the subject of heated debate in the literature (23). The options include the following:
Physical measures
Medication
Hyperbaric oxygen treatment
Electrical stimulation
Extracorporeal shock-wave treatment
Restriction of weight bearing
The aim of mechanical load reduction is to support the body’s own regeneration processes and to halt progression of the disease. In a meta-analysis, Mont et al. found that around 75% of patients treated by restriction of weight bearing showed worsening of symptoms and radiological progression at 34 months (21). Load reduction does not seem suitable as sole treatment for FHN.
Medication
Vasodilators
Prostaglandin analogs are thought to influence revascularization. In the early stages of FHN (ARCO I–II) they can alleviate pain and lead to a reduction in bone marrow edema (24). Off-label treatment with these vasodilators is recommended if operative treatment is contraindicated or the patient refuses surgery.
Bisphosphonates
Alendronate may relieve the pain from early-stage FHN. A number of highly rated studies show this effect and point to postponement of radiological progression (25, 26, e9). In a randomized controlled trial (RCT), Lai et al. conclude that administration of 70 mg alendronate/week seems to slow the progression of FHN (26). All of these studies had a short duration of follow-up, however, so no final conclusions can be drawn with regard to progression.
In the studies published to date, anticoagulants and statins have achieved no relevant amelioration of pain or reliable prevention of disease progression (27, 28). In view of the sometimes substantial side effects, these substances should not be used to treat FHN.
The other substances used for the treatment of FHN have mostly been reported in studies with small case numbers and are therefore not described here.
Surgical treatment
Joint-preserving procedures
Joint-preserving operations are performed principally in ARCO stage I and II FHN, but can also be carried out in stage III disease, depending on the severity and site of necrosis (29). Liebermann et al. conducted a meta-analysis to compare various joint-preserving operative procedures and investigate the influence of the magnitude of necrosis on disease progression. They found that none of the methods of joint-preserving surgical intervention was superior to the others (30).
Core decompression
Core decompression is the surgical treatment technique most frequently used in the early stages of FHN (31). The aim is decompression of the femoral head with reduction of the intraosseous pressure, thus alleviating pain, improving blood flow, and leading to regeneration of the necrotic zones. Core decompression is a promising treatment option particularly in the early stages ARCO I and II, provided the necrosis in stage II does not extend around more than 30% of the femoral head circumference and—by definition—no subchondral fractures are present (32). Superiority of core decompression to conservative treatment alone is described by Mont et al. and supported by two RCTs in their meta-analysis (33). Restitutio ad integrum cannot reliably be achieved with joint-preserving surgery in ARCO stage II, but decompression leads to alleviation of pain and can slow down the destructive process (33).
In ARCO stage III core decompression may result in short-term pain relief, but is associated with much worse results in terms of progression and prognosis than in stage II.
Corrective osteotomy
Various two- and three-dimensional corrective osteotomies are available. Common to all techniques is rotation of the necrotic segment of the femoral head out of the main loading zone and away from the action of axial forces. Corrective osteotomies are not routine interventions: they are technically demanding and have a relatively high complication rate. One must also bear in mind that insertion of an implant is often harder to accomplish in patients with previous corrective osteotomy (34).
Vascularized and non-vascularized bone transplants
After removal of the necrotic material, bone, usually cortical (e.g., from the fibula), is implanted into the femoral head. Numerous articles reporting vascularized and non-vascularized bone transplants have been published, with widely varying results (35, 36, e10). The use of growth factors (BMP) in combination with non-vascularized bone transplants has shown encouraging results in a small number of studies, but is not standard (e11, e12).
Joint resection
Joint resection and replacement can be considered in patients with advanced FHN (ARCO stages III and IV) and end-stage changes in the hip joint whose prognosis is unfavorable (risk factors, underlying disease, large defect, good mobility needed). The results of hip arthroplasty in advanced FHN are good, with short- and long-term outcomes comparable with those for the treatment of primary coxarthrosis (37, 38, e13). A systematic review of national registers of total arthroplasty revealed 6-year survival rates of 95 to 97% for both primary coxarthrosis and FHN (39). However, the survival and revision rates appear to depend on the patient’s age and the etiology of the FHN. Younger patients should always be treated with procedures that preserve as much bone as possible because of the high likelihood that several revisions will be necessary. On the basis of the literature to date, the outcomes with the various implant designs do not differ. The long-term results after cemented and cementless arthroplasty are comparable with the outcomes for primary coxarthrosis (39). The currently available data do not permit any conclusions with regard to the use of short-stem implants in FHN.
Conclusion
Atraumatic FHN in adults is a locally destructive disease of multifactorial origin. If left untreated, it may lead within a few years to severe joint destruction and development of coxarthrosis. Although the causes and mechanisms of atraumatic FHN have not yet been finally clarified, certain risk factors are known to be associated with increased incidence of osteonecrosis of the femoral head. Atraumatic FHN often affects relatively young people with active private and working lives. This underlines the importance of early confirmation of the diagnosis and initiation of the corresponding treatment, with the aim of preserving as much joint function as possible and minimizing later damage. In the early stages the patient’s symptom’s are frequently unspecific and radiography often reveals no abnormality, so careful evaluation of the risk factors and timely MRI are particularly important. The MRI examination should include the contralateral (asymptomatic) hip joint. The ARCO classification of atraumatic FHN has been widely adopted in the German-speaking countries, permitting assessment of the disease course, comparison of the efficacy of the different treatment methods, and providing assistance in determining the best treatment. Complete recovery can be attained in ARCO stage I, but in stage II joint-preserving procedures often do no more than slow the pace of progression. Stages III and IV of atraumatic FHN are an expression of mechanical failure of the hip joint. The efficacy of conservative treatment alone is a topic of heated debate in the literature. Joint-preserving procedures may be successful in the early stages (ARCO I and II) and are superior to conservative treatment alone. Joint replacement should be considered in late-stage atraumatic FHN and is in many cases the only feasible treatment option. The results of arthroplasty for atraumatic FHN have improved greatly in recent years and are now equivalent to those for primary coxarthrosis.
Key Messages.
The reasons for and mechanisms of femoral head necrosis are not yet absolutely clear but are regarded in the literature as a multifactorial process.
MRI shows bone marrow changes and confirms the diagnosis of femoral head necrosis at an early stage, before any abnormality is detected by radiography.
The high incidence of bilateral atraumatic femoral head necrosis justifies MRI examination of the contralateral hip joint, even in the absence of clinical and radiographic signs.
If left untreated, femoral head necrosis leads to subchondral fractures of the femoral head within 2–3 years, and is then no longer amenable to joint-preserving treatment.
Joint-preserving procedures may be successful in the early stages of femoral head necrosis (ARCO I and II) and are superior to conservative treatment alone.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Lieberman JR, Berry DJ, Mont MA, et al. Osteonecrosis of the hip: Management in the twenty-firstcentury. J Bone Joint Surg Am. 2002;84:834–853. [Google Scholar]
- 2.Hofmann S, Kramer J, Plenk H. Osteonekrose des Hüftgelenkes im Erwachsenenalter. Orthopaede. 2005;34:171–184. doi: 10.1007/s00132-005-0762-4. [DOI] [PubMed] [Google Scholar]
- 3.Ärztliches Zentrum für Qualität in der Medizin (ÄZQ) Curriculum Evidenz basierte Medizin (EbM) des Ärztlichen Zentrums für Qualität in der Medizin2007. www.aezq.de/mdb/edocs/pdf/info/curriculum-ebm-2005.pdf. (last accessed on 29. November 2015)
- 4.Aldridge JM III, Urbaniak JR. Avascular necrosis of the femoral head: etiology, pathophysiology, classification, and current treatment guidelines. Am J Orthop. 2004;33:327–332. [PubMed] [Google Scholar]
- 5.Wang GJ, Cui Q, Balian G. The Nicolas Andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res. 2000;370:295–310. doi: 10.1097/00003086-200001000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Griffith JF, Antonio GE, Kumta SM, et al. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology. 2005;235:168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 7.Koo KH, Kim R, Kim YS, et al. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21:299–303. doi: 10.1007/s100670200078. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo K, Hirohata T, Sugioka Y, Ikeda M, Fukuda A. Influence of alcohol intake, cigarette smoking, and occupational status on idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res. 1988;234:115–123. [PubMed] [Google Scholar]
- 9.Gullihorn L, Karpman R, Lippiello L. Differential effects of nicotine and smoke condensate on bone cell metabolic activity. J Orthop Trauma. 2005;19:17–22. doi: 10.1097/00005131-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Glueck CJ, Freiberg RA, Fontaine RN, Tracy T, Wang P. Hypofibrinolysis, thrombophilia, osteonecrosis. Clin Orthop Relat Res. 2001;386:19–33. doi: 10.1097/00003086-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22:455–464. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 12.Reppenhagen S, Rackwitz L, Kenn W, et al. Diagnostik der atraumatischen Femurkopfnekrose des Erwachsenen. Osteologie. 2010;19:10–17. [Google Scholar]
- 13.Stevens K, Tao C, Lee SU, et al. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. Am J Roentgenol. 2003;180:363–368. doi: 10.2214/ajr.180.2.1800363. [DOI] [PubMed] [Google Scholar]
- 14.Beltran J, Burk JM, Herman LJ, et al. Avascular necrosis of the femoral head: early MRI detection and radiological correlation. Magn Reson Imaging. 1987;5:431–442. doi: 10.1016/0730-725x(87)90377-8. [DOI] [PubMed] [Google Scholar]
- 15.Grimm J, Hopf C, Higer HP. Die Femurkopfnekrose. Diagnostik und morphologische Analyse mittels Röntgen, Szintigraphie, Computertomographie und Magnetresonanztomographie. Z Orthop. 1989;127:680–690. doi: 10.1055/s-2008-1040312. [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Sugano N, Nishii T, Hananouchi T, Yoshikawa H. Extent of osteonecrosis on MRI predicts humeral head collapse. Clin Orthop Relat Res. 2008;466:1074–1080. doi: 10.1007/s11999-008-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fordyce MJ, Solomon L. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg Br. 1993;75:365–367. doi: 10.1302/0301-620X.75B3.8496201. [DOI] [PubMed] [Google Scholar]
- 18.Miller IL, Savory CG, Polly DW, Jr, et al. Femoral head osteonecrosis. Detection by magnetic resonance imaging versus single-photon emission computed tomography. Clin Orthop. 1989;247:152–162. [PubMed] [Google Scholar]
- 19.Mont MA, Marulanda GA, Jones LC, et al. Systemic analysis of classification systems for osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88:16–26. doi: 10.2106/JBJS.F.00457. [DOI] [PubMed] [Google Scholar]
- 20.Gardeniers JWM. Report of the committee of staging and nomenclature. ARCO News Letter. 1993;5:79–82. [Google Scholar]
- 21.Mont MA, Zywiel MG, Marker DR, McGrath MS, Delanois RE. The natural history of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2010;92:2165–2170. doi: 10.2106/JBJS.I.00575. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann S, Mazieres B. Osteonekrose: Natürlicher Verlauf und konservative Therapie. Orthopäde. 2000;29:403–410. doi: 10.1007/s001320050461. [DOI] [PubMed] [Google Scholar]
- 23.Lüring C, Beckmann J, Pennekamp PH, Linhardt O, Grifka J, Tingart M. Die konservative Therapie der aseptischen Femurkopfnekrose. Gibt es evidenzbasierte Konzepte? Orthopäde. 2007;36:441–442. doi: 10.1007/s00132-007-1083-6. [DOI] [PubMed] [Google Scholar]
- 24.Jäger M, Werner A, Lentrodt S, Mödder U, Krauspe R. Schmerztherapie bei nichtjuvenilen, aseptischen Osteonekrosen. Schmerz. 2004;18:481–491. doi: 10.1007/s00482-004-0356-9. [DOI] [PubMed] [Google Scholar]
- 25.Nishii T, Sugano N, Miki H, Hashimoto J, Yoshikawa H. Does alendronate prevent collapse in osteonecrosis of the femoral head? Clin Orthop Rel Res. 2006;443:273–279. doi: 10.1097/01.blo.0000194078.32776.31. [DOI] [PubMed] [Google Scholar]
- 26.Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am. 2005;87:2155–2159. doi: 10.2106/JBJS.D.02959. [DOI] [PubMed] [Google Scholar]
- 27.Ajmal M, Matas AJ, Kuskowski M, Cheng EY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population? Orthop Clin North Am. 2009;40:235–239. doi: 10.1016/j.ocl.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasawa K, Tada Y, Koarada S, et al. Prevention of steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus by anti-coagulant. Lupus. 2006;15:354–357. doi: 10.1191/0961203306lu2311oa. [DOI] [PubMed] [Google Scholar]
- 29.Im GI, Kim DY, Shin JH, Cho WH, Lee CJ. Degeneration of the acetabular cartilage in osteonecrosis of the femoral head: histopathologic examination of 15 hips. Acta Orthop Scand. 2000;71:28–30. doi: 10.1080/00016470052943847. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman JR, Engstrom SM, Meneghini RM, SooHoo NF. Which factors influence preservation of the osteonecrotic femoral head? Clin Orthop Relat Res. 2012;470:525–534. doi: 10.1007/s11999-011-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozic KJ, Zurakowski D, Thornhill TS. Survivorship analysis of hips treated with core decompression for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 1999;81:200–209. doi: 10.2106/00004623-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Fairbank AC, Bhatia D, Jinnah RH, Hungerford DS. Long-term results of core decompression for ischaemic necrosis of the femoral head. J Bone Joint Surg Br. 1995;77:42–49. [PubMed] [Google Scholar]
- 33.Mont MA, Carbone JJ, Fairbank AC. Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res. 1996;324:169–178. doi: 10.1097/00003086-199603000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Shannon BD, Trousdale RT. Femoral osteotomies for avascular necrosis of the femoral head. Clin Orthop. 2004;418:34–40. doi: 10.1097/00003086-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Rijnen WH, Gardeniers JW, Buma P, et al. Treatment of femoral head osteonecrosis using bone impactation grafting. Clin Orthop. 2003;417:74–83. doi: 10.1097/01.blo.0000096823.67494.64. [DOI] [PubMed] [Google Scholar]
- 36.Mont MA, Etienne G, Ragland PS. Outcome of nonvascularized bone grafting for osteonecrosis of the femoral head. Clin Orthop. 2003;417:84–92. doi: 10.1097/01.blo.0000096826.67494.38. [DOI] [PubMed] [Google Scholar]
- 37.Mont MA, Seyler TM, Plate JF, Delanois RE, Parvizi J. Uncemented total hip arthroplasty in young adults with osteonecrosis of the femoral head: a comparative study. J Bone Joint Surg Am. 2006;88:104–109. doi: 10.2106/JBJS.F.00451. [DOI] [PubMed] [Google Scholar]
- 38.Ortiguera CJ, Pulliam IT, Cabanela ME. Total hip arthroplasty for osteonecrosis matched-pair analysis of 188 hips with long-term follow-up. J Arthroplasty. 1999;14:21–28. doi: 10.1016/s0883-5403(99)90197-3. [DOI] [PubMed] [Google Scholar]
- 39.Johannson HR, Zywiel MG, Marker DR, Jones LC, McGrath MS, Mont MA. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review. Int Orthop. 2011;35:465–473. doi: 10.1007/s00264-010-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–336. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Hirota Y, Hirohata T, Fukuda K, et al. Association of alcohol intake, cigarette smoking, and occupational status with the risk of idiopathic osteonecrosis of the femoral head. Am J Epidemiol. 1993;137:530–538. doi: 10.1093/oxfordjournals.aje.a116706. [DOI] [PubMed] [Google Scholar]
- e2.Allison GT, Bostrom MP, Glesby MJ. Osteonecrosis in HIV disease: epidemiology, etiologies, and clinical management. AIDS. 2003;17:1–9. doi: 10.1097/01.aids.0000042940.55529.93. [DOI] [PubMed] [Google Scholar]
- e3.Hasse B, Ledergerber B, Egger M, et al. Swiss HIV Cohort Study. Antiretroviral treatment and osteonecrosis in patients of the Swiss HIV Cohort Study: a nested case-control study. AIDS Res Hum Retroviruses. 2004;20:909–915. doi: 10.1089/aid.2004.20.909. [DOI] [PubMed] [Google Scholar]
- e4.Ries MD, Barcohana B, Davidson A, Jergesen HE, Paiement GD. Association between human immunodeficiency virus and osteonecrosis of the femoral head. J Arthroplasty. 2002;17:135–139. doi: 10.1054/arth.2002.28727. [DOI] [PubMed] [Google Scholar]
- e5.Markisz JA, Knowles RJ, Altchek DW, Schneider R, Whalen JP, Cahill PT. Segmental patterns of avascular necrosis of the femoral heads: early detection with MR imaging. Radiology. 1987;162:717–720. doi: 10.1148/radiology.162.3.3809485. [DOI] [PubMed] [Google Scholar]
- e6.Mitchell DG. Using MR imaging to probe the pathophysiology of osteonecrosis. Radiology. 1989;71:25–26. doi: 10.1148/radiology.171.1.2928533. [DOI] [PubMed] [Google Scholar]
- e7.Bassett LW, Gold RH, Reicher M, Bennett LR, Tooke SM. Magnetic resonance imaging in the early diagnosis of ischemic necrosis of the femoral head. Preliminary results. Clin Orthop Relat Res. 1987;214:237–248. [PubMed] [Google Scholar]
- e8.Castro FP, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop. 2000;29:187–194. [PubMed] [Google Scholar]
- e9.Agarwala S, Jain D, Joshi VR, Sule A. Efficacy of alendronate, a bisphosphonate, in the treatment of AVN of the hip. A prospective open-label study. Rheumatology. 2005;44:352–359. doi: 10.1093/rheumatology/keh481. [DOI] [PubMed] [Google Scholar]
- e10.Berend KR, Gunneson EE, Urbaniak JR. Free vascularized fibular grafting for the treatment of postcollapse osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:987–993. doi: 10.2106/00004623-200306000-00001. [DOI] [PubMed] [Google Scholar]
- e11.Hungerford DS, Mont MA. Die potentielle Anwendung von Zytokinen und Wachstumsfaktoren bei der Behandlung der Osteonekrose. Orthopäde. 2000;29:442–456. doi: 10.1007/s001320050465. [DOI] [PubMed] [Google Scholar]
- e12.Liebermann JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop. 2004;429:139–145. doi: 10.1097/01.blo.0000150312.53937.6f. [DOI] [PubMed] [Google Scholar]
- e13.Kim OH, Oh SH, Kim JS, Koo KH. Contemporary total hip arthroplasty with and without cement in patients with osteonecrosis of the femoral head. J Bone Joint Surg Am. 2003;85:675–681. doi: 10.2106/00004623-200304000-00014. [DOI] [PubMed] [Google Scholar]