Abstract
Background
Improved quantitative assessment of cerebral hemodynamics in newborns might enable us to optimize cerebral perfusion. Our objective was to develop an approach to assess cerebral hemodynamics across multiple time scales during the first 72 hours of life in newborns during hypothermia therapy.
Methods
Spontaneous oscillations in mean arterial pressure (MAP) and regional cerebral tissue oxygen saturation (SctO2) were analyzed using a moving window correlation (MWC) method with time scales ranging from 0.15 to 8 hours in this pilot methodology study. Abnormal neurodevelopmental outcome was defined by Bayley III scores and/or cerebral palsy by 24 months of age using receiver operating curve (ROC).
Results
Multiple-time-scale correlations between MAP and SctO2 oscillations were tested in 10 asphyxiated newborns undergoing hypothermia therapy. Large non induced fluctuations in the blood pressure were observed during cooling in all five infants with abnormal outcomes. Notably, these infants had two distinct patterns of correlation: a positive in-phase correlation at the short time scales (15 min), and/or a negative anti-phase correlations observed at long time scales (4 hrs.). Both the in-phase (AUC 0.6, [95% CI 0.2–0.95]) and anti-phase correlations (AUC 0.75, [95% CI 0.4–0.95]) appeared to be related to an abnormal outcome.
Conclusions
Our observations suggest that the time scale is an important factor that needs to be standardized in the assessment of neonatal cerebral hemodynamics.
Keywords: neonate, hypoxic-ischemic encephalopathy (HIE), hypothermia, cerebral hemodynamics, near infrared spectroscopy (NIRS)
Introduction
The healthy brain is protected by cerebral autoregulation, which maintains cerebral blood flow relatively constant across a wide range of changes in perfusion pressure.1 In asphyxiated newborns, invasive positron emission tomography studies have reported impairment of cerebral hemodynamics with impaired cerebral vasomotor control associated with death and abnormal outcomes. 2 While hypothermic therapy provides neuroprotection via reduction in cerebral metabolism as well as cerebral blood flow, 3–5 a significant knowledge gap exists regarding how to quantify hemodynamics in real time during this therapy. Since approximately 40% of asphyxiated newborns still have neurodevelopmental abnormalities at 24 months of age despite hypothermia therapy,6–8 a better real-time understanding of pathophysiological mechanisms of brain injury is essential to identify those in need of the additional neuroprotective strategies.
Cerebral autoregulation is dynamic and possesses multiple-time-scale properties. 9,10 Assessment of cerebral autoregulation in neonates must be noninvasive and allow for the application of external perturbations, which are often used and have proven helpful in the study of cerebral autoregulation in adults. 21 The heterogeneity and complexity of associated confounding factors in these sick newborns with HIE represents a challenge to the conventional fixed scales which have been previously used in other patient populations. 11,12 The primary aim of this pilot study was to test a multiple-time-scale approach in order to assess the most optimal time in which to measure cerebral hemodynamics of newborns with neonatal encephalopathy during hypothermia in the first 72 hours of life. Spontaneous oscillations in mean arterial pressure (MAP) from an indwelling umbilical arterial catheter and regional cerebral tissue oxygen saturation (SctO2) by near-infrared spectroscopy (NIRS) were measured. A time domain moving window correlation (MWC) method was employed to quantify the relationship between changes in MAP and SctO2 across multiple time scales.
Methods
The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and informed consent was obtained from parents before enrollment. Inborn infants 36 weeks of gestation and birth weight 1800 grams who were admitted to the neonatal intensive care unit (NICU) at the Parkland Hospital of Dallas from June 2010 to January 2012 with perinatal asphyxia or metabolic acidosis, and a clinical exam showing moderate to severe encephalopathy in the first six hours were cooled according to the published protocols as defined by the NICHD Neonatal Research Network study of whole body hypothermia.13 Infants undergoing cooling who had an indwelling arterial catheter in order to allow continuous recording were included. Exclusion criteria included the presence of congenital anomalies, unstable oxygenation status due to pulmonary hypertension, imminent death, or transfer to another facility. Whole body hypothermia was started within 6 hours after birth and achieved by placing the newborn on a cooling blanket (Blanketrol II, Cincinnati Sub-Zero) and maintaining the esophageal temperature at 33.5 °C by the blanket servomechanism for 72 hours. Afterward, rewarming was obtained by 0.5 degrees per hour with incremental changes per published protocols. 13 Magnetic resonance imaging (MRI) studies (3T, Philips Healthcare Systems) were performed between five and eight days of life for evidence of neurological abnormalities and injuries. Out-patient neurodevelopmental follow-up assessments were performed at 24 months of age as specified by our protocol.
14 Neurodevelopmental delay was identified by a Bayley-III equal or less 85 in any of the domains listed (motor, cognitive or language) or documentation of cerebral palsy by the developmental pediatrician at the follow-up assessment.13,15
Continuous monitoring of cerebral hemodynamics
Continuous MAP was measured from an indwelling umbilical arterial catheter. Regional SctO2 was measured using a spatially resolved NIRS cerebral oximeter (INVOS 4100–5100; Somanetics, Troy, MI). The probe (neonatal, Soma Sensor) was placed on the left frontoparietal side of the infant’s head. Both MAP and SctO2 data were recorded simultaneously with a Vital Sync™ system (Somanetics Corporation, Troy, Michigan). Additionally, a pulse oximeter (Massimo Corporation, Irvine, CA) was used to measure arterial oxygen saturation (SaO2) and was set to maximal sensitivity with 2-second averaging of measurements. Blood pressure variance range was calculated by averaging the maximum and minimum values. Recordings of SaO2 were synchronized with those of MAP and SctO2. Fractional tissue O2 extraction, defined as FTOE = (SaO2 − SctO2)/SaO2, was calculated to reflect oxygen utilization of regional brain tissue. 16 EEG using standard montage was obtained starting day 1 and continued until the end of the hypothermia therapy. Fourteen channels of scalp EEG data were referentially recorded using Stellate Harmonia acquisition systems sampling at 200 Hz, using the international 10–20 and modified combinatorial nomenclature system of electrode placement.
Multiple-time-scale correlation analysis
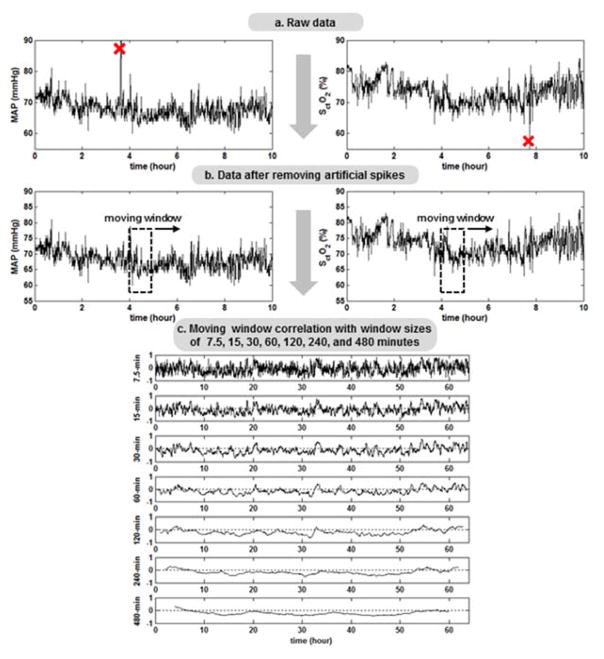
A schematic diagram of multiple-time-scale MWC data processing and analysis procedures is shown in Figure 1. First, both MAP and SctO2 time series were inspected by an author (FT) who was blinded to clinical outcomes, through a spike-detection algorithm to identify outliers that were defined as a transient swing of 15% or larger from the baseline. The spike-like outliers usually resulted from body movements or other technical artifacts, and, therefore, were replaced with the values linearly interpolated from the nearest data points. The Pearson’s correlation coefficients (R) between MAP and SctO2 were calculated based on the MWC method with the sizes of the moving window (i.e., the time scales) over 1/8, 1/4, 1/2, 1, 2, 4, 6 and 8 hours. To evaluate the overall extent of correlation between MAP and SctO2 at each time scale, we defined a priori a cerebral hemodynamic index (CH index) as the percentage of data points with significant R < −0.4 or R > 0.4 over the entire data.17–20
Figure 1.
a. Data processing and analysis procedures. Mean arterial pressure (MAP) and cerebral tissue oxygen saturation (SctO2) were recorded every 30 seconds. The raw data were inspected through a spike-detection algorithm to identify any measurement artifacts. 1b. Spike-like data points were replaced with linearly interpolated values from their nearest neighbors. 1c. Pearson’s correlation coefficient (R) between MAP and SctO2 was computed within a moving window. The window size increased over time scales of 1/8, 1/4, 1/2, 1, 2, 4, and 8 hours.
Data were analyzed during steady-state changes in arterial partial pressure CO2 (PaCO2, 40–50 mm Hg) and hemoglobin level (12–15 mg/dl) and normal blood glucose concentrations. Therefore, the actual length of data processed was less than 72 hours in some instances. Clinically unstable patients, with less than 48 hours of continuous recording were further excluded from the multiple-time-scale data analysis. Patients with a constant SctO2 ≥95% for six hours or more were excluded by convention as oscillations cannot be recorded in such infants. The latter high SctO2 have already been studied and correlated with abnormal outcomes, so there is no need to measure a hemodynamic index in these scenarios.21,22
Statistical analysis
Since multiple-time-scale assessment of cerebral autoregulation in newborns undergoing hypothermia therapy have not been described in prior literature, an empirical sample size of sequentially cooled newborns was used in this pilot study. One author (TF) was blinded to clinical outcomes and determined the 10 infants whose tracing met the predefined criteria for MWC analysis. Data were summarized as means ± SD or as median and ranges where appropriate. Differences in patient characteristics between neonates with adverse and favorable outcomes were compared using Student’s t-test, Chi-squared test or Fisher’s exact test where appropriate and predictive values as well as likelihood ratios were calculated. A receiver operator characteristic (ROC) curve was generated for various time scale measures in order to assess the sensitivity and specificity of these measures in detecting abnormal neurodevelopmental outcomes. The optimal time scale cut-off value was selected based on the area under the ROC curve compared with a 45° line of equality (p<0.05).
RESULTS
Cohort Characteristics in cooled newborns
During the pilot study period, 20 newborns received whole body hypothermia therapy for 72 hours, of which 10 met the entry criteria and were recruited for the study. Subjects were excluded for clinical instability with hypoxia and pulmonary hypertension (n=3), NIRS signal ≥ 95 (n=4), use of pressors (n=2), lack of continuous arterial recording (n=1). Infants had an average gestational age of 39±2 weeks and all were in our institution. All had umbilical arterial evidence of severe fetal acidosis with multiple organ involvement and moderate (n=8) or severe encephalopathy (n=2). None of the infants studied had seizures during the duration of hypothermia (Table 1).
Table 1.
Patient Characteristics
| Maternal age (years) | 27± 6 |
|
| |
| Mode of Delivery | |
|
| |
| C/section Emergent | 12 (60%) |
|
| |
| C/section Elective | 5 (25%) |
|
| |
| Vaginal Delivery | 3 (15%) |
|
| |
| Weight (gm) | 3025 ± 520 |
|
| |
| Gestational age | 39 ± 2 |
|
| |
| Race | Hispanic 75% |
|
| |
| Black 14% | |
|
| |
| Sex | 14 (70%) M |
| 6 (30%) F | |
|
| |
| Apgar Score 1 min | 2 (2–3) |
|
| |
| Apgar Score 5 min | 6 (5–7) |
|
| |
| Umbilical Arterial pH at birth | 6.98 ± 0.09 |
| O2 (mmHg) | 16 (11–20) |
| CO2 (mmHg) | 77 (66–99) |
| Base deficit (mmHg) | 19 ± 6 |
|
| |
| Mechanical Ventilation | 12 (60%) |
|
| |
| Days in hospital | 24 (10–50) |
Data presented as mean ± SD or median and IQR.
MWC analysis was done on all eligible 10 patients. No differences were seen in the general characteristics of the selected 10 infants for the MWC analysis, when compared to the overall cohort of cooled infants. All enrolled infants survived to NICU discharge. MRI was available on all patients and was performed at a median age of 7 days. The MRI was abnormal in 5 (50%) neonates, and all had evidence of diffuse white matter injury. In addition, one also had imaging abnormalities involving the basal ganglia. Abnormal Bayley III scores occurred in 5 (50%) of the cooled infants. Of the five infants with an abnormal outcome, all had cognitive scores < 70, while two had scores 70–85 in the motor domains and one additional infant had a severe cerebral palsy.
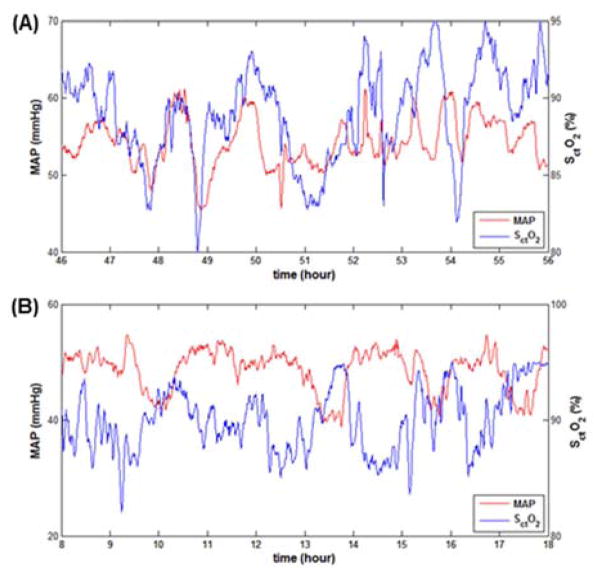
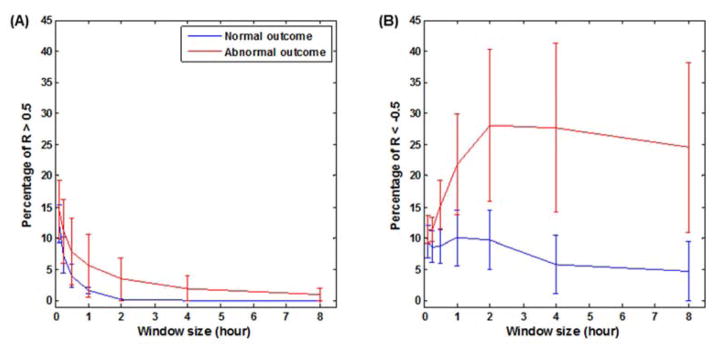
Key findings were presence of the large intermittent spontaneous fluctuations in the blood pressure with a variance range of 30± 4 mmHg, which were observed during hypothermia. Notably, these swings in blood pressure were observed during steady state without influences from medications, activity, seizures or pressors infusions. Figure 2 illustrates in two newborns the presence of different patterns of correlations between MAP and SctO2 which were both observed in the patients with abnormal outcomes. Positive correlations were observed at short time scale of 7 minutes (R > 0.4 during 32% of the individual patient total recording time). Conversely, predominantly negative correlations were observed in another patient at the long time-scale 4 hours (R < − 0.4 during 39% of the individual patient total recording time). The infants with normal outcomes all had CH index <10% at any of the time scales studied, when compared to infants with abnormal outcomes (Figure 3) below. Infants with abnormal outcomes were best detected either by a positive CH index using the conventionally utilized 7.5 minutes short time scale, i.e. window at 1/8 hrs. (AUC 0.6, [95% CI 0.2–0.95]), or alternatively by a negative CH index at the long 4 hour time scale (AUC 0.75, [95% CI 0.4–0.95]).
Figure 2.
Examples of the different patterns of hemodynamics noted during cooling in infants with abnormal outcomes. Mean arterial pressure (MAP) and cerebral tissue oxygen saturation (SctO2) are depicted on the y axis, time on the x axis in: (2a.) newborn with positive correlation, and (2b.) newborn with negative correlation both of whom had abnormal outcomes.
Figure 3.
Cerebral hemodynamics index (CH index) derived from the multiple-time-scale correlation analysis. The CH index values in the selected 10 newborns are plotted against the five time scales of 7min, 15min, 30 min, 1hr, 2hr, 4hr and 8 hr. At each time scale, the CH index was calculated for significant correlation coefficient R>0.5 (left) and R< −0.5 (right).
In further analyses, we inspected the dependence of MAP-SctO2 correlation on the range of MAP by measuring the binned averages of R against MAP at each of selected time scales. Most infants optimal MAP was found to be around 50 to 55 mm HG in all time scales studied, while the amount of time from the total recording, which included this critical MAP, greatly varied amongst patients. Secondary analyses were also performed following initiation rewarming, by comparing data to the 12 preceding hours of hypothermia respectively. Significant hemodynamic differences were seen during rewarming with respect to heart rate (122 ±15 versus. 103 ±12, p 0.002), and MAP (50 ±2 versus. 56 ± 8, p 002). No infants had any documented hypotension or fluid bolus need. No differences were seen in NIRS SctO2 (80 ±8 versus. 80 ± 7, p 0.7) or fractional tissue oxygen extraction (18±11 vs. 19±7, p 0.8) during rewarming vs hypothermia. However, fractional tissue oxygen extraction was lower in the infants with abnormal outcomes as compared to the normal outcomes (13± 4% vs. 21±5%).
Discussion
In this pilot study, large non induced fluctuations in the blood pressure and multiple-time- scale correlations between MAP and SctO2 oscillations were observed in the asphyxiated newborns undergoing hypothermia therapy. This is the first report to highlight that infants with abnormal outcomes despite cooling had two different patterns of impaired cerebral hemodynamics during hypothermia, depending on the time scale studied. In addition to the well reported positive correlation between changes in MAP and SctO2 at short time scales of 10 minutes, we observed negative correlations at the larger time scales of 4 hours which were also associated with abnormal neurodevelopmental outcomes. These findings would suggest that cerebral hemodynamics is a time-scale-dependent phenomenon, potentially associated with abnormal outcomes at 18–24 months. Since prior studies have only utilized a short time scale window of correlation, the new findings at larger time scales emphasize the need of standardized methodology assessing different time scales in specific patient populations.
Asphyxia starts with a fetal insult due to ischemia that impairs cerebral blood flow regulation as a consequence of a substantial interruption of maternal and/or fetal placental blood flow and gas exchange.23 The timing, severity, pattern and duration of the fetal insult as well as the degree of recovery via fetal adaptive mechanisms determine the spectrum of disease, outcomes and possibly the responses to the hypothermia therapy. Cerebral autoregulation represents a key physiological mechanism essential to maintaining a relatively constant brain perfusion in the face of changing arterial blood pressure. Invasive methodology in animal and previous clinical studies reported impaired autoregulation in the face of hypoxia24, hypercarbia25, and acidosis26, all of which are integral components of HIE. For example, a linear relationship between cerebral blood flow and arterial blood pressure indicating an impaired autoregulation was observed in asphyxiated newborns with the Xenon clearance method.27–28 Such a loss of cerebral autoregulation was reported also using a PET scan 2 or arterial spin labeling MRI.29 Recent non- invasive NIRS studies showed that high SctO2 was associated with abnormal outcomes in HIE prior to 21–22 and following hypothermia therapy. 30,31 These studies demonstrated high SctO2 with low FTOE indicative of a luxury brain perfusion associated with a complete cerebral vasoparalysis to be related abnormal outcomes. 30,31 Our studies are different in that we include the blood pressure to evaluate hemodynamics, rather than isolated NIRS values. Moreover we have excluded the infants with high SctO2, from our MWC analysis as these infants have already been studied and by convention, it is not possible to record oscillatory hemodynamics on a saturated NIRS signal. We focused on early recognition of spontaneous oscillations in blood pressure; as such infants have not been studied, and if recognized early, might benefit from added stabilizing neuro-vascular interventions.
The observed blood pressure variability in our newborns with HIE, without medications or surgical interventions, and in the absence of seizures, is in agreement with studies on preterm infants where blood pressure variability was also found to exceed the autoregulatory capacity. 32
A recent study of asphyxiated newborns by Howlett et al.33 reported median optimal MAP to be around 50 mmHg during hypothermia, and neonates with MRI injury spent a greater proportion of time with MAP below that cutoff than neonates with no or mild injury. Our findings of an optimal range MAP of 50–55 mmHg associated with the CH index concur with above study. Variations in the time spent within an optimal MAP range during cooling were reported in both studies and further emphasize the importance of using continuous, real-time autoregulation monitoring to individualize hemodynamic goals.
Other researchers have studied preterm newborns non-invasively by quantifying frequency domain coherence between oscillations in MAP and NIRS oxy/deoxy-hemoglobin difference (HbD). 11,12,34 Such studies have used a time scale of moving window correlation fixed at 10 min and reported a pressure-passive status of impaired cerebral autoregulation, with a positive correlation between these two variables. 11,12 The observed positive correlation in newborns with HIE at the small time scales are consistent with above previous studies done in preterm newborns using NIRS HbD. In the present study, we elected to use NIRS SctO2 rather than HbD as an index of cerebral blood flow hemodynamics, because such food and drug administration (FDA) approved sensors are less sensitive to movement artifact,35,36 and can be easily integrated in the clinical care. NIRS SctO2 have also been correlated to HbD37, as well as to MRI arterial spin label cerebral blood flow in the setting of HIE38. Our findings of negative correlations between changes in MAP and SctO2 within the time scales of 4 hours are new and have not been done prior with neither NIRS SctO2 or HbD signals. We speculate that the underlying mechanisms for the observed negative correlations are related to large blood pressure variability. Changes in SctO2 in reality represent changes in blood oxygenation from the arterioles, capillary vascular beds, and post-capillary venues, 16,39 It is possible that after a decreased blood pressure, redistribution of oxygenated blood among the arterioles, capillary vascular beds, and venules, can result in an increase in SctO2, at the long time scale studied. The changes still need to be studied in larger clinical settings to understand their role in the observed abnormal outcomes. Overall the mean value of FTOE was reduced in the above newborns indicating greater severity of injury as reported in other studies. 28,29
Limitations of this study include a small sample size, which limits our ability to separately analyze moderate and severe encephalopathy, and other confounders of outcomes. We emphasize that these findings are very preliminary, and their predictive values will need to be studied in larger groups of patients.
We do not know whether these findings would apply in settings using other type of NIRS sensors.40 Alternative measures of CBF, such as Doppler flow velocity, were not measured, as they are not recommended for long term monitoring. The effects of rewarming, vasoactive infusions or seizures on autoregulation were not examined, as these patients were excluded in order to validate the MTS methodology in a steady state, without external modifiers. Strengths of this study include a follow-up of clinical outcomes at 18–24 months, continuous monitoring of MAP and SctO2 during the first 72 hours of life in newborns during hypothermia therapy, and testing the effect of multiple time scales on cerebral hemodynamics. Future studies should also be performed in patients prior to initiation of cooling to distinguish the effects of hypothermia from the severity of the asphyxia insult. 41
In summary, we report the presence of multiple-time-scale correlations between oscillations in MAP and SctO2 in the first 72 hours of life during hypothermic therapy which suggest impairment of cerebral hemodynamics in newborns with HIE. Preliminary findings of this study highlight the significance of development of rigorous approaches to assess early impairment of cerebral autoregulation in real time in order to guide future neuroprotective interventions in newborns with HIE.
Acknowledgments
Support: Dr Chalak is supported by a K23 5K23HD069521-03 and Gerber Foundation award
Footnotes
Author Contributions: All authors contributed to 1) the conception and design, acquisition of data, or analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the submitted version.
Specifically, 1) Lina F Chalak, MD, conceptualized the study and wrote the first draft, participated in data interpretation, critical review and revision of the manuscript, and finalized the manuscript as submitted after feedback from the other authors. 2) Tian Fenghua, Ph.D., performed data acquisition and participated in interpretation, and approved the final submitted version.3) Takashi Tarumi, PHD, participated with methodology, data interpretation and manuscript revision.4) Rong Zhang PHD, participated in study concept, data interpretation as well as manuscript revision.
Disclosure/conflict of interest. None of the authors has any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiological reviews. 1959 Apr;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- 2.Pryds O, Greisen G, Lou H, Friis-Hansen B. Vasoparalysis associated with brain damage in asphyxiated term infants. J Pediatr. 1990 Jul;117(1 Pt 1):119–125. doi: 10.1016/s0022-3476(05)72459-8. [DOI] [PubMed] [Google Scholar]
- 3.Laptook AR. Use of therapeutic hypothermia for term infants with hypoxic-ischemic encephalopathy. Pediatr Clin North Am. 2009 Jun;56(3):601–616. doi: 10.1016/j.pcl.2009.03.007. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 4.Laptook AR, Corbett RJ, Burns DK, Sterett R. A limited interval of delayed modest hypothermia for ischemic brain resuscitation is not beneficial in neonatal swine. Pediatr Res. 1999 Oct;46(4):383–389. doi: 10.1203/00006450-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Laptook AR, Shalak L, Corbett RJ. Differences in brain temperature and cerebral blood flow during selective head versus whole-body cooling. Pediatrics. 2001 Nov;108(5):1103–1110. doi: 10.1542/peds.108.5.1103. [DOI] [PubMed] [Google Scholar]
- 6.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005 Mar-Apr;365(9465):1147–1152. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 7.Higgins RD, Raju T, Edwards AD, et al. Hypothermia and Other Treatment Options for Neonatal Encephalopathy: An Executive Summary of the Eunice Kennedy Shriver NICHD Workshop. J Pediatr. 2011 Aug 27; doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nature neuroscience. 2003 Jan;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998 Jan;274(1 Pt 2):H233–241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 10.Zhang R, Zuckerman JH, Levine BD. Spontaneous fluctuations in cerebral blood flow: insights from extended-duration recordings in humans. Am J Physiol Heart Circ Physiol. 2000 Jun;278(6):H1848–1855. doi: 10.1152/ajpheart.2000.278.6.H1848. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji M, Saul JP, du Plessis A, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000 Oct;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 12.Soul JS, Hammer PE, Tsuji M, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007 Apr;61(4):467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 13.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005 Oct 13;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 14.Chalak LF, Dupont TL, Sanchez PJ, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol. 2014 Apr 17; doi: 10.1038/jp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran S. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy (vol 366, pg 2085, 2012) New Engl J Med. 2012 Sep 13;367(11):1073–1073. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greisen G. Is near-infrared spectroscopy living up to its promises? Semin Fetal Neonatal Med. 2006 Dec;11(6):498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 18.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010 Sep;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brady KM, Mytar JO, Lee JK, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. 2010 Sep;41(9):1957–1962. doi: 10.1161/STROKEAHA.109.575167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi B, Brady K, Lee J, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010 Feb 1;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toet MC, Lemmers PM, van Schelven LJ, van Bel F. Cerebral oxygenation and electrical activity after birth asphyxia: their relation to outcome. Pediatrics. 2006 Feb;117(2):333–339. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- 22.Ancora G, Maranella E, Locatelli C, Pierantoni L, Faldella G. Changes in cerebral hemodynamics and amplitude integrated EEG in an asphyxiated newborn during and after cool cap treatment. Brain Dev. 2009 Jun;31(6):442–444. doi: 10.1016/j.braindev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Shalak L, Perlman JM. Hypoxic-ischemic brain injury in the term infant-current concepts. Early Hum Dev. 2004 Nov;80(2):125–141. doi: 10.1016/j.earlhumdev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Tweed A, Cote J, Lou H, Gregory G, Wade J. Impairment of cerebral blood flow autoregulation in the newborn lamb by hypoxia. Pediatr Res. 1986 Jun;20(6):516–519. doi: 10.1203/00006450-198606000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990 Summer;2(2):161–192. [PubMed] [Google Scholar]
- 26.Ong BY, Greengrass R, Bose D, Gregory G, Palahniuk RJ. Acidemia impairs autoregulation of cerebral blood flow in newborn lambs. Can Anaesth Soc J. 1986 Jan;33(1):5–9. doi: 10.1007/BF03010901. [DOI] [PubMed] [Google Scholar]
- 27.Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979 Jan;94(1):118–121. doi: 10.1016/s0022-3476(79)80373-x. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt JS. Near-infrared spectroscopy in asphyxial brain injury. Clin Perinatol. 1993 Jun;20(2):369–378. [PubMed] [Google Scholar]
- 29.Wintermark P, Lechpammer M, Warfield SK, et al. Perfusion Imaging of Focal Cortical Dysplasia Using Arterial Spin Labeling: Correlation With Histopathological Vascular Density. J Child Neurol. 2013 Nov;28(11):1474–1482. doi: 10.1177/0883073813488666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meek JH, Elwell CE, McCormick DC, et al. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed. 1999 Sep;81(2):F110–115. doi: 10.1136/fn.81.2.f110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemmers PM, Zwanenburg RJ, Benders MJ, et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr Res. 2013 Aug;74(2):180–185. doi: 10.1038/pr.2013.84. [DOI] [PubMed] [Google Scholar]
- 32.Howlett JA, Northington FJ, Gilmore MM, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2013 Aug 13; doi: 10.1038/pr.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong FY, Silas R, Hew S, Samarasinghe T, Walker AM. Cerebral oxygenation is highly sensitive to blood pressure variability in sick preterm infants. PLoS One. 2012;7(8):e43165. doi: 10.1371/journal.pone.0043165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011 Nov;31(11):722–729. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 35.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007 Oct;38(10):2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008 Sep;39(9):2531–2537. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caicedo A, De Smet D, Naulaers G, et al. Cerebral tissue oxygenation and regional oxygen saturation can be used to study cerebral autoregulation in prematurely born infants. Pediatr Res. 2011 Jun;69(6):548–553. doi: 10.1203/PDR.0b013e3182176d85. [DOI] [PubMed] [Google Scholar]
- 38.Wintermark P, Hansen A, Warfield SK, Dukhovny D, Soul JS. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage. 2014 Jan 15;85(Pt 1):287–293. doi: 10.1016/j.neuroimage.2013.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liem KD, Greisen G. Monitoring of cerebral haemodynamics in newborn infants. Early Hum Dev. 2010 Mar;86(3):155–158. doi: 10.1016/j.earlhumdev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Dix LM, van Bel F, Baerts W, Lemmers PM. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr Res. 2013 Aug 13; doi: 10.1038/pr.2013.133. [DOI] [PubMed] [Google Scholar]
- 41.Haaland K, Karlsson B, Skovlund E, Lagercrantz H, Thoresen M. Postnatal development of the cerebral blood flow velocity response to changes in CO2 and mean arterial blood pressure in the piglet. Acta Paediatr. 1995 Dec;84(12):1414–1420. doi: 10.1111/j.1651-2227.1995.tb13579.x. [DOI] [PubMed] [Google Scholar]