Abstract
We present a case of proton pump inhibitor-responsive eosinophilic esophagitis (PPI-REE) in a patient with severe dysphagia and markedly elevated baseline esophageal eosinophilia that was previously deemed unresponsive to PPI. Upon reintroduction to PPI therapy with monthly endoscopy and dilation over the course of 4 months, the patient improved clinically and resolved her mucosal eosinophilia. Our case suggests that a longer duration of PPI therapy may be required for histologic improvement, especially in patients with very high mucosal eosinophil count.
Introduction
Eosinophilic esophagitis (EoE) has become an increasingly recognized and prevalent condition over the past 2 decades. EoE is defined as a chronic, immune/antigen-mediated esophageal disease characterized clinically by symptoms related to esophageal dysfunction and histologically by eosinophil predominant inflammation (≥15 eosinophils per high powered field [HPF]).1,2 Esophageal eosinophilia is a non-specific inflammatory finding seen in several diseases, including gastroesophageal reflux disease (GERD). An initial report of proton pump inhibitorresponsive eosinophilic esophagitis (PPI-REE) was described in a series of 3 patients with suspected EoE who had clinical, endoscopic, and pathologic resolution of esophageal eosinophilia with PPI therapy.3
According to the 2013 American College of Gastroenterology clinical guidelines, patients with suspected EoE should be given a 2-month course of PPIs followed by endoscopy with biopsies.4 In accordance with this recommendation, PPI-REE is diagnosed when patients with mucosal eosinophilia demonstrate symptomatic and histologic response to PPIs.4 This is a pivotal point in the treatment of mucosal eosinophilia, as the PPI-REE rate is considerable, ranging from 35–74%.5 Response of esophageal eosinophilia to PPI therapy is not yet fully understood; however, recent reports have shown eosinophils exposed to omeprazole stimulate cytokines IL-13 and IL-4, which can block eotaxin-3, known to be involved in mobilizing eosinophils and thought to play a role in the development of EoE.6
Case Report
A 49-year-old white woman presented with dysphagia for solid food since her early in her second decade. She had one food impaction requiring a hospital visit, and had symptoms suggestive of food impaction almost every week. She rarely reported heartburn. Her past medical history included celiac sprue treated with a strict glutenfree diet for the last 20 years. Ten years prior, endoscopy with bougie dilation was associated with severe chest pain for several days. An endoscopic biopsy 5 years prior showed >15 eosinophils in 2 HPF consistent with EoE.
Subsequently, she was treated with fluticasone (2 puffs BID) then budesonide (1 gm BID slurry); both were associated with side effects (headaches, sore throat, and thrush) resulting in termination after 2–3 weeks. She had taken PPIs in the past, including dexlansoprazole 30 mg for several months in the last year, but these medications did not help her symptoms. Repeat biopsy was not performed. Six months prior to her presentation she was placed on a 6-food elimination diet, to which she was adhering with the aid of a dietician. She avoids nuts and seafood due to an oral allergy syndrome, wheat, and now eggs, dairy products, and soy. Currently, this is her only active treatment for EoE.
On initial endoscopy, the 10-mm adult scope could not be passed due to esophageal narrowing 20 mm from the teeth; a 5 mm pediatric scope was used. The entire esophagus was pale and narrowed throughout, with no exudate, rare furrows, and incomplete rings in the mid-esophagus. At the Z line (37 cm) was a peptic-appearing ring with a 2-cm hiatal hernia. Four biopsies were obtained from the distal and 2 from the proximal esophagus. Savary dilation was performed from 9 mm to 12 mm with moderate resistance. Following the dilation, she had mild-moderate pain for 5 days, requiring narcotic analgesia.
Initial biopsies showed 400 eosinophils per HPF from both the proximal and distal esophagus, associated with lamina propria fibrosis, despite 6 months of the 6-food elimination diet (Figure 1). She was started on dexlansoprazole 60 mg AM. Three further endoscopies and dilation followed at monthly intervals (Figure 2). The final 2 dilations were performed with Maloney bougies.
Figure 1.
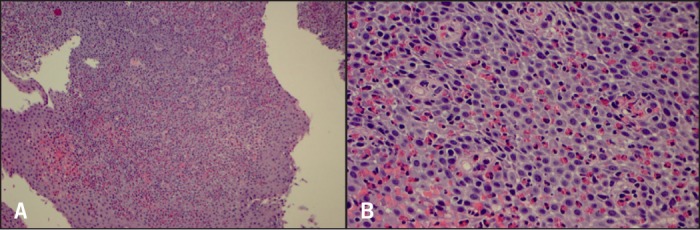
Baseline biopsies. (A) Tangential esophageal muscosa with marked eosinophilia. H&E stain x100 magnification. (B) Intraepithelial eosinophils up to 400/hpf with intercellular edema. H&E stain x400 magnification.
Figure 2.
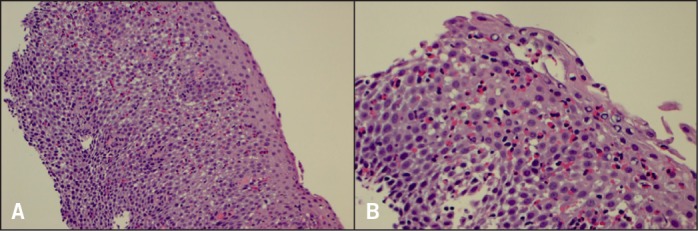
Biopsies 1 month after dexlansoprazole. (A) Esophageal mucosa with eosinophilia and intercellular edema. H&E stain x100 magnification. (B) Marked intraepithelial eosinophils, up to 200/hpf with intercellular edema and microabscesses. H&E stain x400 magnification.
After 2 months on PPI, there was a dramatic decrease in eosinophils, but still in the abnormal range (55 distal; 15 proximal; Figure 3). By 4 months, esophageal histology had normalized (5 distal; 2 proximal; Figure 4). The esophageal lumen was progressively dilated to 17 mm diameter; all signs of inflammation had resolved and the normal vascular pattern returned. The patient is currently eating more foods without difficulty or transient food impactions. She will remain on dexlansoprazole 60 mg AM, and the eliminated foods will be slowly re-introduced into her diet.
Figure 3.
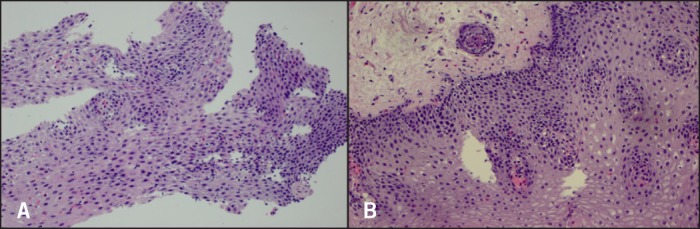
Biopsies 2 months after dexlansoprazole. H&E stain x200 magnification. (A) Proximal biopsies with up to 55 eosinophils/hpf. (B) Distal biopsies have about 15 eosinophils/hpf.
Figure 4.
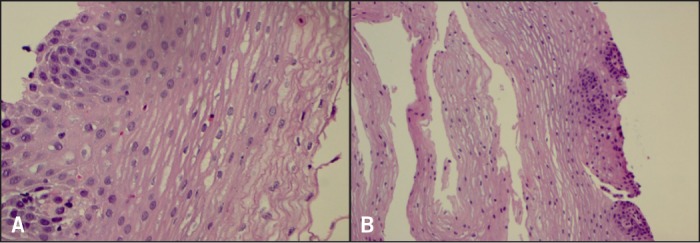
Biopsies 4 months after dexlansoprazole. (A) Distal esophageal mucosa with rare intraepithelial eosinophils (5/hpf). H&E stain x400 magnification. (B) Proximal esophageal mucosa nearly normal. H&E stain x200 magnification.
Discussion
Our case reinforces that repeating a PPI trial is important, as it is a pivotal point in the management of EoE patients. PPI compliance can be an issue for many patients and dosing strategies should be optimized, including dosing on an empty stomach 30 minutes before meals. An alternative is to use dexlansoprazole, in which dosing is independent of meals, but this PPI is expensive and often not covered by insurance. Furthermore, all patients need repeat esophageal biopsies while on PPIs before declaring the trial a failure, as symptoms may not be reliable.
Our case also suggests that the degree of esophageal eosinophilia may be a factor in the rate of improvement after PPI therapy. In our experience and in the literature, most patients have mucosal eosinophilia in the range of 25–200 eosinophils per HPF.9 Our patient had 400 eosinophils per HPF at baseline in both proximal and distal esophageal biopsies. After 2 months of high-dose PPI therapy, the count decreased to 15–55 eosinophils per HPF, but required almost 4 months before the esophageal eosinophilia normalized. Based on current guidelines, this patient would be considered a “non-responder” and dietary and/or topical steroid treatment would follow.1,4 However, we suggest that 2 months is only a minimal duration for a PPI trial, and it may need to be extended if the patient has a very high initial mucosal eosinophil count.10
Disclosures
Author contributions: All authors contributed equally to manuscript creation. JE Richter is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Liacouras CA, Futura GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. [DOI] [PubMed] [Google Scholar]
- 2.Futura GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–63. [DOI] [PubMed] [Google Scholar]
- 3.Ngo P, Futura GT, Antonioli DA, et al. Eosinophils in the esophagus—peptic or allergic eosinophilic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101(7):1666–70. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES, Gonsalves N, Hirano I, et al. ACG clinical guideline: Evidence-based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am J Gastroenterol. 2013;108(5):679–92. [DOI] [PubMed] [Google Scholar]
- 5.Dellon ES, Speck O, Woodward K, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: A prospective cohort study. Am J Gastroenterol. 2013;108(12):1854–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng E, Zhang X, Huo X, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic esophagitis and GERD. Gut. 2013;62(6):302250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrison AF, Jarboe LA, Weinberg BM, et al. Pattern of proton inhibitor use in clinical practice. Am J Med. 2001;111(6):469–473. [DOI] [PubMed] [Google Scholar]
- 8.Gunaratnam NT, Jessup TP, Inadomi J, Lascouksis DP. Sub-optimal PPI dosing is prevalent in patients with poorly controlled GERD. Aliment Pharm Ther. 2006;23(10):1473–7. [DOI] [PubMed] [Google Scholar]
- 9.Dellon ES. Diagnosis and management of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2012;10(10):1066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipka S, Muhammad A, Champeaux A, Richter JE. Case report of proton pump inhibitor responsive esophageal eosinophilia: Why 2 months of proton pump inhibitors is required. Dis Esophagus. Published online ahead of print May 20, 2014. [DOI] [PubMed] [Google Scholar]


