Abstract
Whole brain computed tomography perfusion (CTP) has the potential to select eligible patients for reperfusion therapy. We aimed to find the optimal thresholds on baseline CTP for ischemic core and penumbra in acute ischemic stroke. We reviewed patients with acute ischemic stroke in the anterior circulation, who underwent baseline whole brain CTP, followed by intravenous thrombolysis and perfusion imaging at 24 hours. Patients were divided into those with major reperfusion (to define the ischemic core) and minimal reperfusion (to define the extent of penumbra). Receiver operating characteristic (ROC) analysis and volumetric consistency analysis were performed separately to determine the optimal threshold by Youden’s Index and mean magnitude of volume difference, respectively. From a series of 103 patients, 22 patients with minimal-reperfusion and 47 with major reperfusion were included. Analysis revealed delay time ≥ 3 s most accurately defined penumbra (AUC = 0.813; 95% CI, 0.812-0.814, mean magnitude of volume difference = 29.1 ml). The optimal threshold for ischemic core was rCBF ≤ 30% within delay time ≥ 3 s (AUC = 0.758; 95% CI, 0.757-0.760, mean magnitude of volume difference = 10.8 ml). In conclusion, delay time ≥ 3 s and rCBF ≤ 30% within delay time ≥ 3 s are the optimal thresholds for penumbra and core, respectively. These results may allow the application of the mismatch on CTP to reperfusion therapy.
Stroke is the leading cause of death in China. However, only 1.6% of stroke patients receive tissue plasminogen activator (rtPA) therapy in China1 despite proven effectiveness in reducing disability. By identifying the tissue at risk (penumbra) and the tissue that is irreversibly injured (ischemic core) for those patients who arrive at hospital outside the time window or with unknown onset time, perfusion imaging may increase the rate of thrombolytic therapy2. The presence of a “mismatch” between a small core and large penumbra may indicate the potential for such patients to benefit from off-label reperfusion therapies.
Recent studies focused on computed tomography perfusion (CTP) to select eligible patients for reperfusion therapy3,4,5, with the advantage of fast imaging6. However, consensus on the optimal CTP thresholds for penumbra and core has not been reached7,8,9,10,11. More recently, whole brain CTP has become available, allowing doctors to evaluate the perfusion status throughout the entire brain. Studies have shown that the diagnostic performance of whole brain CTP is considerably different from previous non-whole brain CTP, which does not cover the entire territory of middle cerebral artery (MCA) and could entirely miss lesions in anterior cerebral artery (ACA) territory7,8,11. It is also unclear whether perfusion thresholds are the same when derived from CTP with different coverage of brain voxels. Moreover, most prior studies singly used either receiver operating characteristic (ROC) analysis or volume validation to determine the thresholds9,12. It may be necessary to perform both methods to derive precise results since they reflect either spatial or volumetric accuracy. In addition, for the Chinese population, only one small study has examined optimal perfusion thresholds13. It remains unknown whether the optimal thresholds in the Asian population, with a high incidence of intracranial atherosclerosis, are similar to other populations. We sought to define the specific thresholds of core and penumbra in ischemic stroke based on whole brain CTP, by performing both ROC analysis and volumetric consistency testing. We hypothesized that the infarct area at 24 hours in patients with major reperfusion after thrombolysis could be used to define ischemic core using baseline CTP. The infarct area at 24 hours in patients without reperfusion was used to define the extent of penumbra on baseline CTP. The optimal threshold for core and penumbra was derived from baseline CTP parameters by dual-verification of voxel- and volume-base analysis in our consecutively treated thrombolysis patients.
Materials and Methods
Ethics statement
Written informed consent was obtained from each patient or an appropriate family member. The human ethics committee of The Second Affiliated Hospital of Zhejiang University approved the protocol of this study. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Patients
We reviewed 457 consecutive patients with acute ischemic stroke within 6 hours of onset between July 2011 and March 2015. All received baseline imaging evaluation and then were treated with intravenous rtPA. The inclusion criteria: 1) stroke onset ≤6 hours; 2) patients with National Institute of Health Stroke Scale (NIHSS) scores ≥ 4; 3) underwent CTP before intravenous rtPA, and CTP or MRP at 24 hours after thrombolysis; 4) confirmed as anterior-circulation ischemic stroke. Exclusion criteria: 1) baseline hypoperfusion (Tmax ≥ 6 s) volume ≤ 10 ml14; 2) patients with unknown symptom onset time; 3) hemorrhagic transformation that hindered the evaluation of imaging; 4) image quality that was insufficient for analysis.
Imaging protocol
CTP was performed on a dual-source 64-slice CT scanner (SOMATOM Definition Flash; Siemens, Forchheim, Germany), including non-enhanced CT head scan (120 kV, 320 mA, contiguous 5 mm axial slices), and volume perfusion CT (VPCT) (100 mm in the z-axis, 4 seconds delay after start of contrast medium injection, 74.5 seconds total imaging duration, 80 kV, 120 mA, effective dose = 3.68 mSv, slice thickness 10 mm, collimation 32 × 1.2 mm). VPCT consisted of 26 consecutive spiral acquisitions of brain. All 26 scans were divided into 4 parts: 1) 2 scans with 3 s cycle time; 2) 15 scans with 1.5 s cycle time; 3) 4 scans with 3 s cycle time; 4) 5 scans with 6 s cycle time. A 60-mL bolus of contrast medium (Iopamidol; Braccosine, Shanghai, China) was used at a flow rate of 6 ml/s, followed by a 20 mL saline chaser at 6 ml/s. 4D CTA images were reconstructed from VPCT in axial, coronal and sagittal planes with 20-mm-thick MIPS.
MRI on a 3.0T system (Sigma Excite HD, General Electric, Milwaukee, USA) equipped with an 8-channel phased array head coil. Foam pads were inserted into the space between the subject’s head and the MRI head coil to minimize head motion. The MRI protocol included diffusion weighted imaging (DWI), enhanced 3D multi-echo GRE T2*-weighted angiography (ESWAN), and PWI. DWI was performed with a spin echo-planar sequence (field of view = 240 mm, slice thickness = 1 mm, number of slices = 18, slice gap = 1 mm, acquisition matrix = 160 × 160). PWI was performed with gradient echo-planar imaging (field of view = 240 mm, repetitive time = 1500 ms, echo time = 30 ms, acquisition matrix = 128 × 128. Repetitive scanning times = 50. gadolinium dose = 15 ml, contrast speed = 4–5 ml/s, duration = l min 15 s).
Image analysis
CTP and MRI were analyzed by commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Australia). The software automatically generated delay time, absolute cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) maps using delay- and dispersion-corrected singular value deconvolution (dd-SVD)15. The relative CBF (rCBF), relative CBV (rCBV), relative MTT (rMTT) were calculated based on the automatically derived normal values.
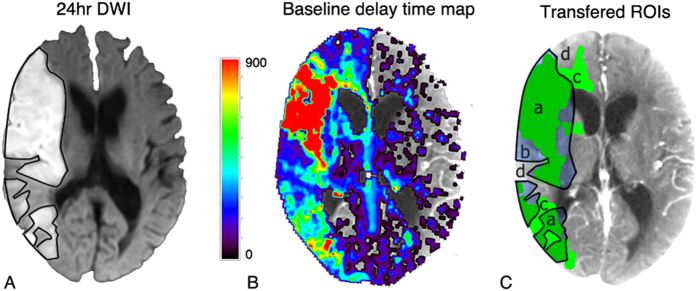
Non-contrast CT or DWI at 24 hours was co-registered and re-sliced to reach the maximum correspondence with baseline CTP (using MIStar). Infarct lesions on co-registered and re-sliced image were manually delineated by an experienced neurologist (5 years experience) using the MIStar ROI tool. These ROIs were drawn without reference to other images except for baseline non-contrast CT to exclude old infarct, and then transferred to baseline CT perfusion maps for voxel-based analysis (Fig. 1). A range of thresholds were investigated (Table 1). (Supplemental material)
Figure 1. An example of image processing for voxel-based analysis.
Images have been co-registered and re-sliced. (A) Black lines on 24 hr DWI represent the ROIs of the 24 hr infarct lesion. (B) baseline delay time map. MIStar automatically marked set threshold as green region (delay time ≥ 3 s was shown as green regions in C). (C) 24 hr infarction ROIs were saved and co-registered to baseline CT. Then, every threshold was marked and analyzed. a, areas thought to be “true positive”; b, areas of “false negative”; c, areas of “false positive”; d, areas of “true negative”.
Table 1. Range and increments for parameters to investigate the threshold.
| Parameters | Range | Increment |
|---|---|---|
| Delay time (s) | 1~10 | 1 |
| rCBF (%)* | 20~55(60~80) | 5(10) |
| CBF (ml/100 g/min) | 2~20 | 2 |
| rCBV (%) | 20~80 | 10 |
| CBV (ml/100 g) | 0.5~5.0 | 0.5 |
| rMTT (%) | 125~250 | 25 |
| MTT (s) | 5~15 | 2 |
*Increment of 5% was applied in the range of 20~55% where the threshold is likely to be based on previous studies.
CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transient time.
Patient groups
Since Tmax ≥ 4~6 s has been demonstrated to be comparable between CTP and MRP16,17,18,19,20, in defining hypoperfused regions, we used it to calculate the reperfusion rate: Reperfusion rate = 1 – [24 h Tmax ≥ 6 s volume / baseline Tmax ≥ 6 s volume]. We defined 3 patient groups: minimal-reperfusion ( ≤ 20% reperfusion), partial reperfusion (20~80% reperfusion), and major reperfusion ( ≥ 80% reperfusion)8,21. The minimal-reperfusion group were used to define the extent of penumbra, as the corresponding infarct area at 24 h represented the sum of baseline penumbra and core. The major reperfusion group were used to define the extent of core, as the infarct area at 24 h should correspond to baseline ischemic core.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 (SPSS Inc, Chicago, USA), R software (R Development Core Team, R: A Language Environment for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0; http://www.R-project.org, 2011).
Voxel-based analysis
In voxel-based analysis, voxels that fell within both infarct area at 24 hours and baseline hypoperfusion lesion (delineated by the thresholds in Table 1) were considered as “true positive”; voxels not within infarct area but in baseline hypoperfusion lesion as “false positive”; voxels within infarct area but not in baseline hypoperfusion lesion as “false negative”; and voxels not within both lesions were “true negative”. (Figure 1) ROC curve analysis was then performed and the optimal threshold was determined by Youden’s index. To reduce the false-positive regions from leukoaraiosis in analysis of ischemic core20,22, only voxels within penumbra was analyzed, once the penumbral threshold was identified.
Volume-based analysis
Volume-based analysis was conducted by volumetric agreement between baseline threshold-based hypoperfusion lesion and infarct area at 24 hours with Bland-Altman and correlation analysis. The optimal threshold should have the best volumetric agreement, determined by the smallest mean magnitude (absolute) difference in lesion volume, see supplemental material).
Results
Baseline clinical data
In total, 154 patients treated with intravenous rtPA had both baseline CTP and perfusion imaging at 24 hours, of whom 129 had anterior-circulation ischemic stroke. Twenty-six patients were excluded due to three reasons: baseline hypoperfusion region ≤10 ml (n = 16), hemorrhagic transformation that hindered the evaluation (n = 6), and poor image quality (n = 4).
Ultimately 103 patients were included in the analysis, of whom 27 (26.2%) patients had minimal-reperfusion and 55 (53.4%) had major reperfusion. Twenty-one (20.4%) patients with partial reperfusion were not analyzed further. At 24 hours, CTP was performed in 44 patients and MRP in 59 patients. Clinical data is listed in Table 2.
Table 2. Baseline clinical data.
| Minimal-reperfusion group (n = 27) | Major reperfusion group (n = 55) | P value | |
|---|---|---|---|
| Female, % | 8 (29.6) | 30 (54.5) | 0.033 |
| Median age, y (range) | 74 (46, 91) | 70 (21, 88) | 0.877 |
| Median baseline NIHSS (range) | 13 (5, 27) | 11 (0, 32) | 0.272 |
| 24 hr Infarct volume, ml (x ± s) | 109.1 ± 76.6 | 35.5 ± 34.4 | < 0.001 |
| DNT, min (x ± s) | 72 ± 29 | 74 ± 39 | 0.92 |
| ONT, min (x ± s) | 253 ± 118 | 208 ± 76 | 0.047 |
NIHSS, National Institutes of Health Stroke Scale; DNT, door to needle time; ONT, onset to needle time.
Defining ischemic penumbra
Of 27 patients with minimal-reperfusion, 22 were ultimately included after 5 patients were excluded due to brain herniation (n = 3) and new infarct (n = 2) at 24 h that hampered the calculation of final infarct volume. The optimal thresholds for each parameter and the Bland-Altman plot are listed in Table 3 and Figure 2. ROC analysis demonstrated that the optimal parameter was delay time (AUC = 0.813; 95% CI, 0.812-0.814) with the threshold of delay time ≥ 3 s (Youden’s Index = 0.49, sensitivity = 0.75, specificity = 0.74). Delay time ≥ 3 s also had the best agreement and correlation in volume-based analysis (mean magnitude of volume difference = 29.1 ml).
Table 3. Accuracy of optimal thresholds for penumbra (minimal-reperfusion group).
| Threshold | Youden’s Index | Sensitivity | Specificity | Mean magnitude (ml) | r | P value |
|---|---|---|---|---|---|---|
| Delay time ≥ 2 s | 0.45 | 0.83 | 0.61 | 44.9 | 0.849 | < 0.001 |
| Delay time ≥ 3 s | 0.49 | 0.75 | 0.74 | 29.1 | 0.900 | < 0.001 |
| Delay time ≥ 4 s | 0.49 | 0.66 | 0.83 | 30.5 | 0.879 | < 0.001 |
| rMTT ≥ 150% | 0.45 | 0.73 | 0.72 | 49.4 | 0.631 | 0.002 |
| MTT ≥ 9 s | 0.43 | 0.74 | 0.68 | 69.0 | 0.584 | 0.004 |
| rCBF ≤ 50% | 0.38 | 0.64 | 0.74 | 41.8 | 0.732 | 0.001 |
| CBF ≤ 10 ml/100 g/min | 0.36 | 0.57 | 0.79 | 50.1 | 0.691 | < 0.001 |
| rCBV ≤ 60% | 0.18 | 0.44 | 0.74 | 61.8 | 0.400 | 0.101 |
| CBV ≤ 1.5 ml/100 g | 0.17 | 0.44 | 0.73 | 62.0 | 0.300 | 0.121 |
More data in supplemental material.
CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transient time.
Figure 2. Volumetric agreement of baseline delay time ≥ 3 s and 24 h infarct in non-reperfusers (penumbral threshold) and the core threshold using rCBF ≤ 30% within delay time ≥ 3 s versus 24 h infarct lesion in those with major reperfusion.
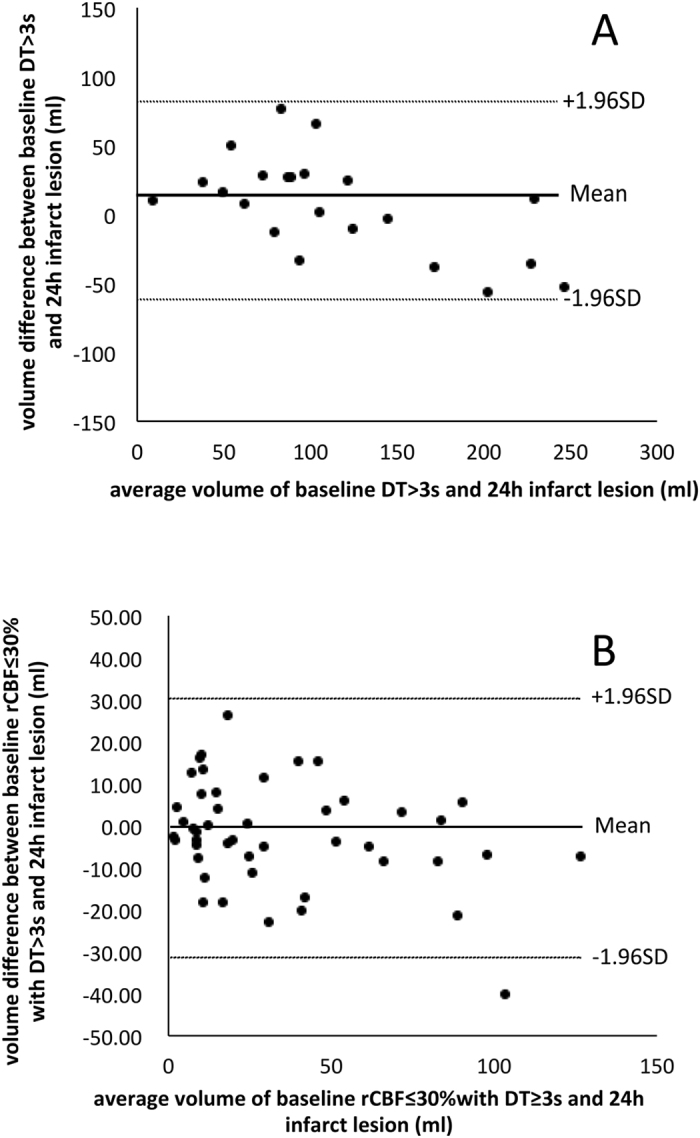
Solid lines represent mean difference (bias) and dashed lines for 95% limits of agreement. (A) Bland-Altman plot between baseline delay time ≥ 3 s and 24 h infarct lesion. (B) Bland-Altman plot between baseline rCBF ≤ 30% within delay time ≥ 3 s and 24 h infarct lesion.
Defining ischemic core
Among 55 patients with major reperfusion, 47 were included after exclusion of 8 patients where the infarct lesion on non-contrast CT at 24 hours was too indistinct and small to define. The performance of other parameters and Bland-Altman plot of rCBF are detailed in Table 4 and Figure 2. ROC analysis indicated that the optimal parameter was rCBF (AUC = 0.758; 95% CI, 0.757-0.760) with an optimal threshold of rCBF ≤ 30% within area of delay time ≥ 3 s (Youden’s Index = 0.40, sensitivity = 0.64, specificity = 0.76). The volumetric analysis also revealed that rCBF ≤ 30% was the optimal threshold for ischemic core (mean magnitude of volume difference = 10.8 ml).
Table 4. Accuracy of optimal thresholds for ischemic core (major reperfusion group).
| Threshold (within delay time ≥ 3 s) | Youden’s Index | Sensitivity | Specificity | Mean magnitude (ml) | r | P value |
|---|---|---|---|---|---|---|
| rCBF ≤ 30% | 0.40 | 0.64 | 0.76 | 10.8 | 0.881 | < 0.001 |
| CBF ≤ 6 ml/100 g/min | 0.36 | 0.55 | 0.81 | 19.4 | 0.716 | < 0.001 |
| rCBV ≤ 50% | 0.27 | 0.49 | 0.78 | 24.7 | 0.530 | < 0.001 |
| CBV ≤ 1 ml/100 g | 0.26 | 0.40 | 0.86 | 22.5 | 0.566 | < 0.001 |
| Delay time ≥ 7 s | 0.28 | 0.57 | 0.71 | 17.6 | 0.752 | < 0.001 |
| rMTT ≥ 200% | 0.20 | 0.53 | 0.66 | 18.4 | 0.743 | < 0.001 |
| MTT ≥ 13 s | 0.20 | 0.41 | 0.79 | 27.0 | 0.348 | 0.017 |
More data in supplemental material.
CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transient time.
Discussion
In this study, by performing both ROC analysis and volumetric consistency testing, we confirmed that delay time ≥ 3 s was the optimal threshold for ischemic penumbra and rCBF ≤ 30% within the area of delay time ≥ 3 s was the optimal threshold for ischemic core. To the best of our knowledge, this is the first calculation of specific thresholds for ischemic core and penumbra using whole brain CTP in the Chinese population.
Based on our results, delay time ≥ 3 s was found to be the optimal threshold for penumbra, with a high Youden’s Index and strong volumetric agreement. This result is slightly different from a previous study in Caucasian patients, which revealed that the optimal thresholds for penumbra were delay time ≥ 2 s based on non-whole brain CTP analysis8. In our study, delay time ≥ 2 s had a high sensitivity, but tended to overestimate penumbra volume. Delay time is a specific parameter generated by MIStar using ddSVD method. It is similar to Tmax but has correction of both delay and dispersion which may better reflect the pathophysiological process of the time for contrast to travel from selected AIF ROI to the tissue voxel8,23,24.
Delay time was also superior to other parameters for defining penumbra, in contrast to some previous studies which used rMTT9. Very limited studies of Chinese stroke patients suggested that rMTT ≥ 150% was the optimal threshold for penumbra13,25. Our data also indicated that rMTT ≥ 150% reasonably predicted penumbra, but we found that the recommended threshold varied with different post-processing algorithms26. Moreover, unlike delay time, MTT is not delay- and dispersion- corrected. This could explain the large mean magnitude of volumetric difference using MTT/rMTT, indicating overestimation and instability in predicting penumbra. CBF was also suggested as the optimal threshold for penumbra previously27. However, in our study CBF was less reliable than delay time with low Youden’s Index and large mean magnitude of volume difference, mainly because the measurement of CBF was easily influenced by the presence of leukoaraiosis, and the CBF in grey and white matter was different. CBV/rCBV was not a good predictor of penumbra, as the auto-regulation in penumbra tissue recruits collaterals and dilates vessels, generally leading to normal or increased CBV value.
Our optimal threshold for ischemic core was rCBF ≤ 30% (constrained within the region of delay time ≥ 3 s). An unconstrained rCBF threshold tends to include “false-positive” regions of leukoaraiosis, reducing its accuracy in delineating ischemic core20,22. Some prior studies indicated that CBV or CBF plus CBV can define ischemic core9,10,27,28,29. However, rCBV and CBV had poorer performance in our study than rCBF, which was consistent with recent studies7,15, possibly because we used a wider range of rCBF thresholds (20~80%) than prior studies (i.e. 40–90%9) and different post-processing methods. A previous Chinese study suggested delay time ≥ 2 s was the optimal threshold for ischemic core13. However, we found that they used CT perfusion maps at 24 hours to determine the threshold of ischemic core, which could be inaccurate. We observed that in some cases, regions of hypoperfused tissue at baseline were irreversibly injured despite reperfusion at 24 hours. Therefore defining core using CTP at 24 hours may underestimate the extent of infarction.
Our study had three strengths. First, we used whole brain CTP. Previous non-whole brain CTP mainly covered voxels in basal ganglia and cortex (MCA territory), so its thresholds could only be validated under the same imaging protocol. With a wider scan range, the whole brain CTP covered more white matter and ACA territory and we then included patients with occlusion of ACA or M2 segment or M2 beyond. Therefore, our results could be more widely applied. Second, we used dd-SVD algorithm, which has theoretical advantages in adjusting for the effects of delay and dispersion seen with collateral flow15. It is widely accepted that different processing algorithms have a major impact on the results of optimal thresholds. Third, we used two independent and complementary statistical approaches, with both voxel- and volume-based analysis. ROC analysis can evaluate the spatial accuracy, but is strongly influenced by the chosen of reference region, while the Bland-Altman plot and correlation study assess the volumetric accuracy, but ignore the spatial correspondence. We believe the combination of these two methods is useful to cross-validate the optimal perfusion thresholds.
The limitations of this study include the small number of enrolled cases and retrospective analysis from a single center. Second, either CTP or MRP were used to define reperfusion. However, we chose Tmax, which has been demonstrated to have comparable values on both CTP and MRP19,20, to define the perfusion lesion, which may minimize the difference in hypoperfusion volume. Third, we used non-contrast CT or DWI at 24 hours, which may potentially introduce variability in infarct volume delineation. Therefore, we performed ROC analysis separately and found similar results in patients with non-contrast CT and MRP at 24 hours. (See supplemental material) Fourth, the use of reperfusion at 24 hours to divide groups may include patients who had late reperfusion within 24 hours. The exact time of reperfusion after intravenous thrombolysis is unknown. Therefore, any infarct growth between imaging and reperfusion would lead to an increase in the optimal rCBF threshold for core. Further studies in patients receiving thrombectomy may be more suitable to validate the optimal thresholds. Fifth, compared with MRI, CT scan involves ionising radiation. However, the rapid acquisition and availability of CT have cemented its role in acute ischemic stroke imaging protocols. Moreover, we did not investigate clinical outcome variation between imaging strategies, which would warrant further prospective study.
Summary
Based on whole brain CTP and dual-verification of voxel- and volume-based analysis, we firstly confirmed that delay time ≥ 3 s and rCBF ≤ 30% constrained within the region of delay time ≥ 3 s were the optimal thresholds for ischemic penumbra and core. Since reperfusion or non-reperfusion was used to verify these thresholds, we believe these results may allow the application of the “mismatch” to reperfusion therapy in clinical trials and practice.
Additional Information
How to cite this article: Yu, Y. et al. Defining Core and Penumbra in Ischemic Stroke: A Voxel- and Volume-Based Analysis of Whole Brain CT Perfusion. Sci. Rep. 6, 20932; doi: 10.1038/srep20932 (2016).
Supplementary Material
Acknowledgments
This study is a collaborative research with International Stroke Perfusion Imaging Registry (INSPIRE) program. Cases included and MIStar software are belong to INSPIRE program. MIStar was provided by Professor Mark Parsons, Australia. This work was supported by the Science Technology Department of Zhejiang Province (2013C03043-3) and the National Natural Science Foundation of China (81171095 & 81471170).
Footnotes
Author Contributions Y.Y. designed the study, interpreted the data, and drafted the manuscript. Y.Y., Q.H. and Q.C., K.Y. performed statistical analysis. X.D. performed imaging scan. S.Z. helped design the study. S.Z., S.Y., B.C. and M.L. contributed to the interpretation of the data and revision of the manuscript. M.P. and S.W. introduced INSPIRE program. M.P. provided MIStar software. All authors have read and approved the final version of the manuscript and its conclusions.
References
- Wang Y. et al. Using recombinant tissue plasminogen activator to treat acute ischemic stroke in China: analysis of the results from the Chinese National Stroke Registry (CNSR). Stroke; a journal of cerebral circulation 42, 1658–1664 (2011). [DOI] [PubMed] [Google Scholar]
- Janjua N. Use of neuroimaging to guide the treatment of patients beyond the 8-hour time window. Neurology 79, S95–99 (2012). [DOI] [PubMed] [Google Scholar]
- Campbell B. C. et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 372, 1009–1018 (2015). [DOI] [PubMed] [Google Scholar]
- Parsons M. et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. The New England journal of medicine 366, 1099–1107, (2012). [DOI] [PubMed] [Google Scholar]
- Campbell B. C. V. et al. CT perfusion improves diagnostic accuracy and confidence in acute ischaemic stroke. J Neurol Neurosur Ps 84, 613–618 (2013). [DOI] [PubMed] [Google Scholar]
- Parsons M. W. Perfusion CT: is it clinically useful ? International journal of stroke: official journal of the International Stroke Society 3, 41–50 (2008). [DOI] [PubMed] [Google Scholar]
- Campbell B. C. V. et al. Cerebral Blood Flow Is the Optimal CT Perfusion Parameter for Assessing Infarct Core. Stroke; a journal of cerebral circulation 42, 3435–U3180 (2011). [DOI] [PubMed] [Google Scholar]
- Bivard A., Spratt N., Levi C. & Parsons M. Perfusion computer tomography: imaging and clinical validation in acute ischaemic stroke. Brain: a journal of neurology 134, 3408–3416 (2011). [DOI] [PubMed] [Google Scholar]
- Wintermark M. et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke; a journal of cerebral circulation 37, 979–985 (2006). [DOI] [PubMed] [Google Scholar]
- Dani K. A. et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Annals of neurology 70, 384–401 (2011). [DOI] [PubMed] [Google Scholar]
- Bivard A. et al. Defining acute ischemic stroke tissue pathophysiology with whole brain CT perfusion. Journal of neuroradiology. Journal de neuroradiologie 41, 307–315 (2014). [DOI] [PubMed] [Google Scholar]
- Bivard A., McElduff P., Spratt N., Levi C. & Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovascular diseases 31, 238–245 (2011). [DOI] [PubMed] [Google Scholar]
- Pan J. W. et al. Value of Perfusion Computed Tomography in Acute Ischemic Stroke: Diagnosis of Infarct Core and Penumbra. J Comput Assist Tomo 37, 645–649 (2013). [DOI] [PubMed] [Google Scholar]
- Mlynash M. et al. Refining the definition of the malignant profile: insights from the DEFUSE-EPITHET pooled data set. Stroke; a journal of cerebral circulation 42, 1270–1275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivard A., Levi C., Spratt N. & Parsons M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 267, 543–550 (2013). [DOI] [PubMed] [Google Scholar]
- Olivot J. M. et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke; a journal of cerebral circulation 40, 469–475 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemmanam T. et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 75, 1040–1047 (2010). [DOI] [PubMed] [Google Scholar]
- Zaro-Weber O., Moeller-Hartmann W., Heiss W. D. & Sobesky J. Maps of Time to Maximum and Time to Peak for Mismatch Definition in Clinical Stroke Studies Validated With Positron Emission Tomography. Stroke; a journal of cerebral circulation 41, 2817–2821 (2010). [DOI] [PubMed] [Google Scholar]
- Lin L. T., Bivard A., Levi C. R. & Parsons M. W. Comparison of Computed Tomographic and Magnetic Resonance Perfusion Measurements in Acute Ischemic Stroke Back-to-Back Quantitative Analysis. Stroke; a journal of cerebral circulation 45, 1727- + (2014). [DOI] [PubMed] [Google Scholar]
- Campbell B. C. et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke; a journal of cerebral circulation 43, 2648–2653 (2012). [DOI] [PubMed] [Google Scholar]
- Miteff F. et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain: a journal of neurology 132, 2231–2238 (2009). [DOI] [PubMed] [Google Scholar]
- Yu Y. N., Ding X. F., Zhang S. & Lou M. Thresholds of CT perfusion in predicting ischemic penumbra and infarct core in patients with acute ischemic stroke. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Medical sciences 43, 7–13 (2014). [DOI] [PubMed] [Google Scholar]
- Yang Q., inventor; Apollo Medical Imaging Technology Pty Ltd, assignee. Method and system of obtaining improved data in perfusion measurements. US patent 8,942,451 B2. Jan 27, 2015.
- Yang Q., inventor; Apollo Medical Imaging Technology Pty Ltd, assignee. Method and system of obtaining improved data in perfusion measurements. US patent 8,855,985 B2. Oct 7, 2014.
- Bao D. Z. et al. 64-Slice spiral CT perfusion combined with vascular imaging of acute ischemic stroke for assessment of infarct core and penumbra. Exp Ther Med 6, 133–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- kamalian S. et al. CT Perfusion Mean Transit Time Maps Optimally Distinguish Benign Oligemia from True “At-Risk” Ischemic Penumbra, but Thresholds Vary by Postprocessing Technique. AJNR. American journal of neuroradiology 33, 545–549 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandera E. et al. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke; a journal of cerebral circulation 37, 1334–1339 (2006). [DOI] [PubMed] [Google Scholar]
- Murphy B. D. et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke; a journal of cerebral circulation 37, 1771–1777 (2006). [DOI] [PubMed] [Google Scholar]
- Heit J. J. & Wintermark M. Imaging selection for reperfusion therapy in acute ischemic stroke. Curr Treat Options Neurol 17, 332, doi: 10.1007/s11940-014-0332-3 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.