Abstract
Extracellular matrix synthesis and remodelling are driven by increased activity of transforming growth factor beta 1 (TGF-β1). In tendon tissue repair, increased activity of TGF-β1 leads to progressive fibrosis. Decorin (DCN) and interleukin 10 (IL-10) antagonise pathological collagen synthesis by exerting a neutralising effect via downregulation of TGF-β1. Herein, we report that the delivery of DCN and IL-10 transgenes from a collagen hydrogel system supresses the constitutive expression of TGF-β1 and a range of pro-fibrotic extracellular matrix genes.
Tendons and ligaments represent the most common musculoskeletal injuries. Of the estimated 100 million musculoskeletal injuries occurring annually worldwide, 30–50% are tendon and ligament related, with an associated healthcare expenditure in excess of US$ 140 billions1,2,3. Unfortunately contemporary therapeutic strategies, including the gold standard autologous graft-based surgical repair, often lead to fibrotic healing with formation of adhesions, contractures and scars4,5. As a result, the healed tissue is structurally and mechanically weaker than the original tendon tissue with an increased propensity for re-injury.
The pathologic hallmark of tendon fibrosis is underlined by changes in the composition of interstitial extracellular matrix (ECM)6,7,8,9,10. This is associated with the failure of timely termination of key molecular processes in the normal healing cascades, presumably due to continuing presence of physical and biochemical triggers and regulators11. Transforming growth factor beta 1 (TGF-β1) is a pleiotropic growth factor that plays a central role in orchestrating fibroblast mitogenesis, proliferation and an array of molecular responses to injuries12. However, excessive or prolonged production of TGF-β1 has been directly linked to the accumulation and disorderliness of fibrillogenesis in wound healing and several fibrotic tissue diseases, including tendons13. Indeed, in vitro studies have demonstrated that tenocytes and mesenchymal in origin cells grown in the presence of TGF-β1 acquired a more fibroblastic phenotype with increased expression of collagen type I, collagen type III, fibronectin, elastin, laminin and proteoglycans13,14. Consistently, in vivo studies in tendons and other tissues have revealed that administration of TGF-β1 promptly triggers a range of pro-fibrotic tissue reactions12,15, whilst neutralisation effectively abrogates the development of the fibrotic response and in some cases also induces dissolution of already formed fibrotic tissues15. Therefore, the suppression or control of TGF-β1 and its pro-fibrotic activities has been identified as candidate prophylactic and therapeutic strategies in addressing fibrotic wound healing.
Proteoglycans play an important role in stabilising collagen fibres and docking of growth factors required for normal tendon function10, whilst pro-inflammatory and anti-inflammatory cytokines have regenerative effect in tendon healing16,17. Specifically to fibrosis, decorin (DCN), a predominant proteoglycan constituent of tendon ECM, has been shown to bind and inhibit the production of TGF-β1, thus modulating a number of TGF-β1 related ECM metabolic activities, including fibrillogenesis and collagen fibril organisation18,19,20. In a similar manner, the anti-inflammatory cytokine interleukin 10 (IL-10) has been shown to protect against TGF-β1 induced scar formation and to promote remodelling and wound healing21,22,23,24,25.
Given that the development of fibrosis in tendon is orchestrated through a battery of signalling molecules secreted by multiple cell types, multi-modal therapeutic interventions could potentially overcome the drawbacks of single molecule therapeutics26,27,28,29,30,31,32,33. Herein, it is hypothesised that a multi-modal and sustained delivery of DCN and IL-10 encoding plasmid DNA via a collagen type I reservoir will downregulate TGF-β1 and associated ECM components in primary human tendon fibroblast culture.
Results
Assessment of pDCN, pIL-10 and pDCN+IL-10 immobilisation and release
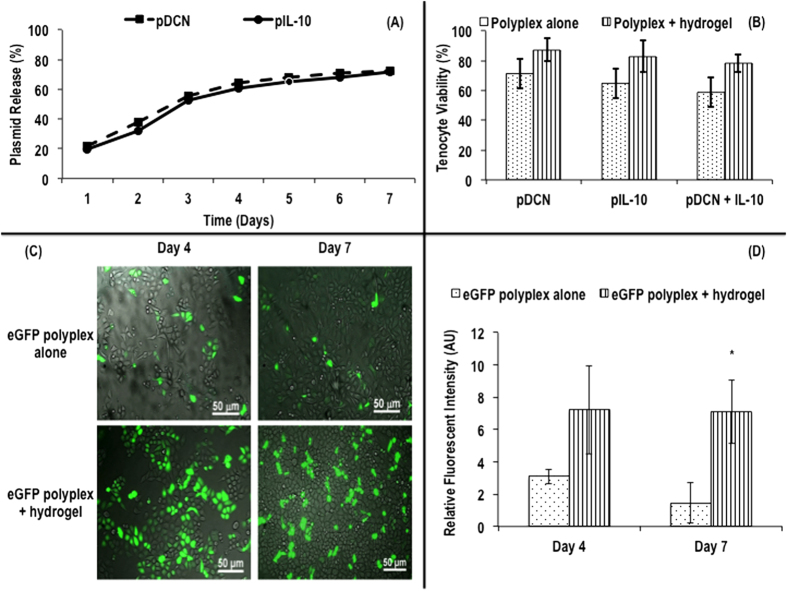
pDCN, pIL-10 and pDCN+IL-10 were embedded within the atelocollagen hydrogel to determine the plasmid release profile over a 7 day period. Within the first 24 hours, a 20% elution of immobilised pDNA into the PBS incubation buffer was observed. The total DNA elute increased to over 70% on day 4 and continued to rise gradually reaching 74% on day 7 irrespective of the specific pDNA immobilised (Fig. 1A).
Figure 1.
Complexed pDCN and pIL-10 release from hydrogel in PBS over seven days (A). No significant difference (p > 0.05) in % human tenocyte viability was observed after transfection with complexed plasmids (pDCN, pIL-10, pDCN+IL-10) or plasmids (pDCN, pIL-10, pDCN+IL-10) in collagen hydrogel (B). The use of atelocollagen gel embedment of pDNA-polymer complexes to deliver eGFP showed improved green florescent protein expression at both 4 and 7 days post transfection, when compared with conventional direct pDNA-polymer complexes exposure to cells (C). Relative fluorescent intensity of eGFP after transfection revealed that transgene overexpression was extended for a period of 7 days (D). Note: *indicates p < 0.001.
The viability of the tenocytes was assessed in culture after the addition of pDNA polyplexes (e.g. pDCN, pIL-10 and pDCN+IL-10) with or without the collagen hydrogel. No statistical significance between the groups was observed (Fig. 1B).
To further characterise the influence of collagen hydrogel as a reservoir for prolonged pDNA release, an enhanced green fluorescent protein (eGFP) reporter gene was complexed and loaded in collagen hydrogel and delivered to human tenocytes. Fluorescence microscopy (Fig. 1C) and complementary relative fluorescent intensity analysis (Fig. 1D) confirmed that transgene overexpression was extended for a period of 7 days.
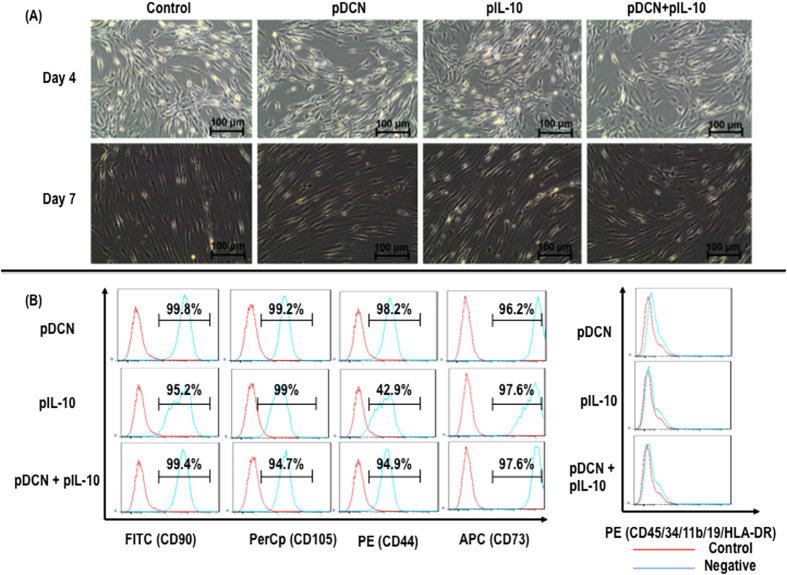
Transfer of pDCN, pIL-10 and pDCN+IL-10 did not affect tenocyte morphology (Fig. 2A) and did not affect surface marker expression (Fig. 2B).
Figure 2.
pDCN, pIL-10 and pDCN+IL-10 did not change tenocyte morphology (A). Flow cytometry analysis indicated that pDCN, pIL-10 and pDCN+IL-10 did not affect CD90, CD105, CD44 and CD73 expression (B).
Assessment of pDCN, pIL-10 and pDCN+IL-10 on DCN and IL-10 expression in human tenocyte culture
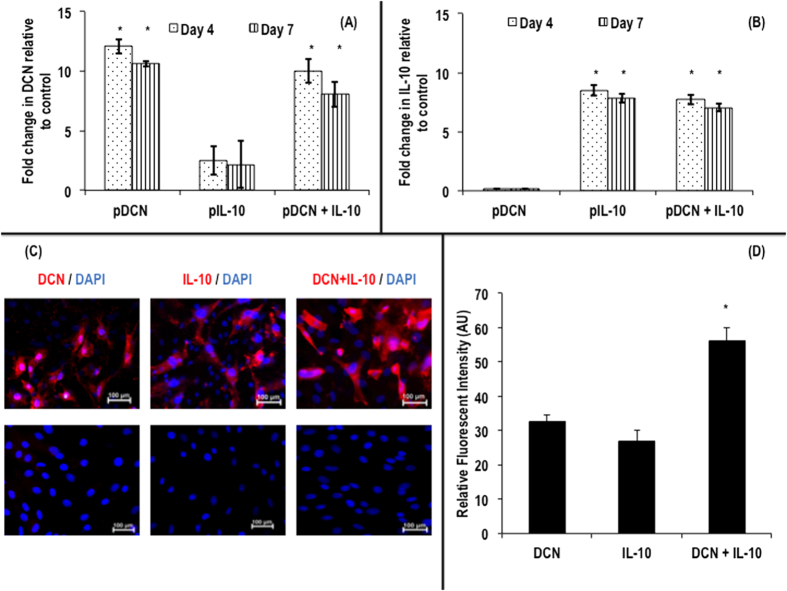
Compared to baseline expression (expression levels in non-transfected tenocytes), at day 4 and at day 7, a 12.1- and a 10.7- fold increase in gene expression of DCN was observed respectively, when human tenocytes were treated with pDCN (Fig. 3A). At day 4 and at day 7, a 2.5- and a 2.2- fold increase in gene expression of DCN was observed respectively, when human tenocytes were treated with pIL-10 (Fig. 3A). At day 4 and at day 7, a 10.0- and an 8.1- fold increase in gene expression of DCN was observed respectively, when human tenocytes were treated with pDCN+IL-10 (Fig. 3A).
Figure 3.
Quantitative real-time PCR analysis of DCN (A) and IL-10 (B) gene expression in cultured human tenocytes after transfection with pDCN, pIL-10 and pDCN+IL-10. pDCN and pDCN+IL-10 at both time points (day 4 and 7) resulted in significant increase (p < 0.001) in DCN gene expression (A). pIL-10 and pDCN+IL-10 at both time points (day 4 and 7) resulted in significant increase (p < 0.001) in IL-10 gene expression (B). (C) Supplementary immunocytochemistry analysis further confirmed increased DCN and IL-10 protein expression in transfected cells (up row) at day 7 (C). Relative fluorescent intensity confirmed increased DCN and IL-10 protein expression in transfected cells at day 7, with DCN+IL-10 exhibiting the highest (p < 0.001) increase (D). Note: *indicates p < 0.001.
Compared to baseline expression (expression levels in non-transfected tenocytes), at day 4 and at day 7, a 0.1- and a 0.2- fold increase in gene expression of IL-10 was observed respectively, when human tenocytes were treated with pDCN (Fig. 3B). At day 4 and at day 7, an 8.5- and a 7.8- fold increase in gene expression of IL-10 was observed respectively, when human tenocytes were treated with pIL-10 (Fig. 3B). At day 4 and at day 7, a 7.7- and a 7.1- fold increase in gene expression of IL-10 was observed respectively, when human tenocytes were treated with pDCN+IL-10 (Fig. 3B).
Immunocytochemical staining (Fig. 3C) and complementary relative fluorescent intensity analysis (Fig. 3D) further confirmed increased DCN and IL-10 protein expression in transfected cells at day 7.
Assessment of pDCN, pIL-10 and pDCN+IL-10 on TGF-β1 expression of human tenocytes
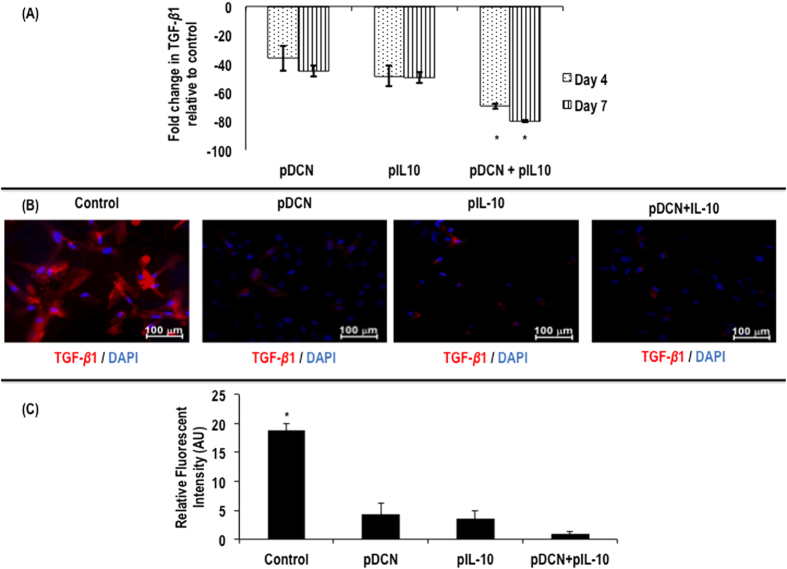
Expression of TGF-β1 gene was reduced at day 4 by ~36.00% and at day 7 by ~45.10 in pDCN and by ~48.50% at day 4 and ~49.60% at day 7 in pIL-10 transfected human tenocytes (Fig. 4A). Combined treatment with pDCN+IL-10 led to pronounced reduction in TGF-β1 gene expression, with ~69.40% and ~79.70% relative to non-transfected cells at day 4 and 7 respectively (Fig. 4A). At day 7, immunofluorescent staining (Fig. 4B) and complementary relative fluorescent intensity analysis (Fig. 4C) confirmed the downregulation of TGF-β1.
Figure 4.
Quantitative real-time PCR analysis of TGF-β1 gene expression in human tenocytes following transfection with pDCN, pIL-10 and pDCN+IL-10 polyplexes delivered using a collagen hydrogel system indicate that pDCN+IL-10 is more effective (p < 0.001) in suppressing TGF-β1 gene expression (A). Supplementary immunocytochemistry analysis for TGF-β1 further confirmed the increased efficacy of pDCN+IL-10 over pDCN and pIL-10 in supressing TGF-β1 expression (B). Relative fluorescent intensity analysis confirmed the effectiveness of pDCN, pIL-10 and pDCN+pIL-10 in significantly (p < 0.001) supressing TGF-β1 expression (C). Note: *indicates p < 0.001.
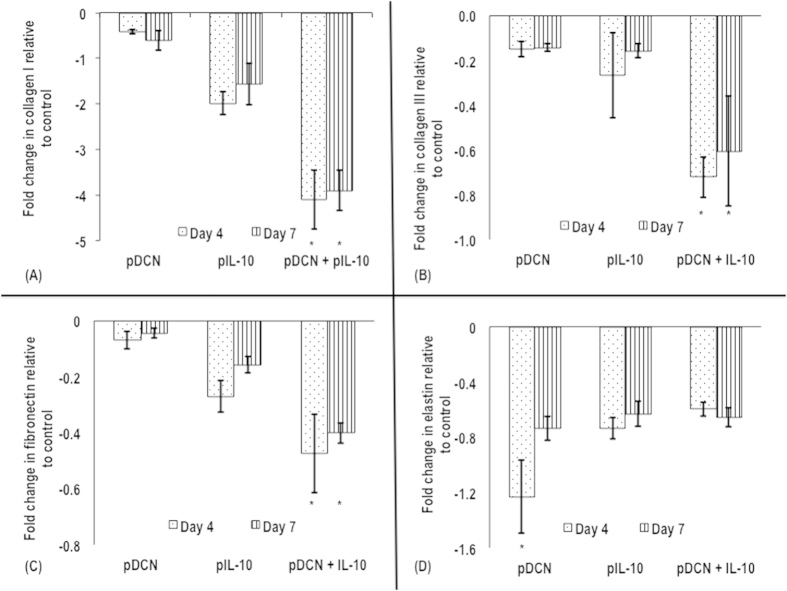
Assessment of pDCN, pIL-10 and pDCN+IL-10 on collagen type I, collagen type III, fibronectin and elastin gene expression in human tenocyte culture
pDCN and pIL-10 decreased expression of collagen type I (Fig. 5A), collagen type III (Fig. 5B), fibronectin (Fig. 5C) and elastin (Fig. 5D) genes in transfected tenocytes, when compared to untreated cells (control group) at both 4 and 7 days. pDCN+IL-10 not only suppressed collagen type I (Fig. 5A), collagen type III (Fig. 5B) and fibronectin (Fig. 5C), when compared to untreated cells (control group), but also it was by at least 2-fold more effective than pDCN or pIL-10 alone treatments at both time points (days 4 and 7). However, such inhibitory effect was not seen in the gene expression of elastin (Fig. 5D).
Figure 5.
Quantitative real-time PCR analysis of collagen type I (A), collagen type III (B), fibronectin (C) and elastin (D) as a function of pDCN, pIL-10 and pDCN+IL-10 polyplexes and time in culture (4 and 7 days). pDCN+IL-10 significantly (p < 0.001) suppressed collagen type I (A), collagen type III (B) and fibronectin (C) by at least 2-fold more than single pDCN or pIL-10 treatments. Similar inhibitory effect was not seen in the gene expression of elastin (D). Note: *indicates p < 0.001.
Discussion
Disproportionate synthesis and deposition of ECM in response to trauma and persistent inflammation lead to tissue fibrosis and consequent loss of tissue integrity, pain, inelastic scars, intercepting fibrillar regeneration and interrupted functional tenogenesis34. TGF-β1 is known to be one of the key mediators in driving fibrosis through canonical (e.g. SMAD 2 and 3) and non-canonical (e.g. MAPK) pathways35. In vitro studies have demonstrated that tenocytes and mesenchymal progenitors grown in the presence of TGF-β1 acquired a more fibroblastic phenotype, with increased expression of collagen type I, collagen type III, fibronectin, elastin, laminin and proteoglycans13,14. Consistently, in vivo studies in tendons and other tissues, have revealed that administration of TGF-β1 proteins promptly triggers a range of pro-fibrotic tissue reactions12,15. Several vector-based gene therapy studies have targeted TGF-β1 or its mediators, in order to knockdown scar formation and promote tenogenesis36,37,38. However, clinical translation of vector-based gene therapy products pose major safety and toxicology concerns17.
In the present study, we have developed a collagen-based hydrogel for delivery pDCN, pIL-10 or pDCN+IL-10 as a means to modulate expression of TGF-β1 and associated ECM mediators of tissue fibrosis. The rationale of using a collagen-based scaffold is based on the fact that collagen serves as bioactive depot and acts as a natural entrapment for binding of growth factors and proteoglycans, promoting tissue ingrowth39. We recognise that the high binding affinity of biglycan and fibromodulin to TGF-β has been previously harnessed to sequester TGF-β signalling activities in vitro40,41. Indeed, whereas DCN and biglycan only sequesters the active form of TGF-β, fibromodulin is able to bind to even the latent form of TGF-β, resulting in a more comprehensive inhibition. In theory, fibromodulin gene therapy could represent a more robust control of TGF-β mediated fibroplasia in vivo. However, given the multiplicity roles of this growth factor in wound healing, excessive inhibition of TGF-β could be deleterious to the innate process of wound healing. Thus, herein DCN and IL-10 were chosen, as their anti-TGF-β1 efficacy has been well established in the literature (e.g. DCN42,43,44,45, IL-1021,46,47,48).
Sustained release of the plasmids was observed over 7 days period, without any negative effects on tenocyte viability, morphology and surface marker expression. Although polyplexes can induce cytotoxicity due to molecular weight, charge and degradation products49, similar to our results, loading polyplexes within a collagen hydrogel prevents polyplex-induced cytotoxicity50,51,52.
Delivery of pDCN and pIL-10 did not significantly elevate transcription of IL-10 and DCN respectively, whilst delivery of pDCN+IL-10 induced comparable levels of DCN and IL-10 expression, illustrating no competitive or inhibitory mode of action. When the efficacy of pDCN and pIL-10 against TGF-β1 was assessed, both were found to be proportionately effective, however pDCN+IL-10 was more effective than the individual plasmids. The co-expression of pDCN+IL-10 against TGF-β1 was also verified with downregulation in collagen type I, collagen type III and fibronectin gene expression. Previous studies have demonstrated that ECM genes are regulated by TGF-β activity53 and the magnitude of difference depends on the cell type54,55,56. With respect to elastin expression, the most profound difference was observed in pDCN. DCN has been shown to be involved in early stages of elastino-genesis57,58. Particularly, action of DCN via TGF-β sequestration destabilises ECM by accelerating tissue destruction59,60. On the other hand, IL-10 in presence of TGF-β is known to up-regulate elastin gene expression61. Hence, we believe that simultaneous delivery of pDCN+IL-10 modulates the opposing functions of DCN, leading to lower elastin gene expression at transcriptional level. These observations, not only illustrate the independent mode of action of the plasmids, but also corroborate previous publications, where co-delivery of therapeutics/biologics was demonstrated to be superior to mono-domain approaches62,63,64,65,66,67.
Although virus-based direct transfer of gene sequences with remarkable efficiency has been demonstrated, safety concerns including the risk of cytotoxicity, immunogenicity and teratogenicity have perennially limited the clinical efficacy of viruses as vectors in gene therapy68. Despite the rather expansive preclinical data generated on adenovirus based gene therapies, inflammatory reaction to adenovirus vectors accounted for the first human fatality in gene therapy69,70. Given that integration of viruses into human genome preferentially occurs at transcriptionally active regions, concerns of mutagenesis leading to malignancies remains clinically relevant with viral vector strategies68. Therefore, non-viral vector platforms71,72,73,74, such as the one described in the present study, promise to address these safety concerns.
Conclusions
In the present study, pDCN+IL-10 delivered through a collagen hydrogel system enhanced suppressive effect on TGF-β1 expression and related ECM genes (collagen type I, collagen type III and fibronectin) in human tenocyte culture. These findings have important therapeutic implications in fibrosis and further corroborate the notion for cocktail treatments.
Materials and Methods
Cell culture
Primary human tenocytes were purchased from Cambridge Biosciences (Cambridge, UK). Primers were purchased from Eurofins MWG GmbH (Ebersberg, Germany). TransIT®-LT1 transfection reagent was purchased from Mirus Bio (Madison, WI, USA). Polyclonal antibodies (raised in rabbit) against human TGF-β, DCN and IL-10 were purchased from Abcam (Abcam Inc., Cambridge, MA, USA). All media and media supplements were obtained from Gibco/Invitrogen (Bio-sciences, Dublin, Ireland). Laboratory consumables were obtained from Sigma-Aldrich (Arklow, Ireland), unless otherwise stated.
Plasmid polyplex formation and gene transfection
TransIT®-LT1 transfection reagent was complexed with plasmid DNA (eGFP, DCN or IL-10 as appropriate) following manufacturer’s instructions. Human IL-10 cDNA clone inserted in plasmid DNA (pCMV6-XL5, Cat. No. SC300099) and human DCN cDNA clone inserted in plasmid DNA (pCMV6-AC, Cat. No. SC320831) were purchased from OriGene Technologies (Rockville, MD, USA). Briefly, TransIT®-LT1 transfection reagent was mixed with pDNA at a variety of TransIT®-LT1 transfection reagent amino–DNA phosphate charge (N:P) ratios (1:1, 2:1, 5:1, 10:1 etc.) in serum-free media and were allowed to form complexes for 20 minutes. Human tenocytes were cultured in DMEM supplemented with 10% foetal bovine serum (FBS; Invitrogen), 1% pen-strep (Invitrogen) and 0.2% L-Glutamine (Invitrogen, Bio-sciences, Dublin, Ireland) at 37 °C in a humidified atmosphere of 5% CO2. Tenocytes were plated on 6-well plates in the presence of atelocollagen gels containing pDNAs complexes encoding IL-10 and/or DCN. Cellular metabolic activity was analysed on days 4 and 7 using the alamarBlue® assay, as per manufacturer’s protocol (Life Technologies™, Bio Sciences, Dublin, Ireland).
Atelocollagen gel embedment of pDNA-polymer complexes
Freeze-dried type I atelocollagen, extracted from bovine Achilles tendon as has been described previously75, was sterilised in ethanol and reconstituted in diluted acetic acid (1:1000 acetic acid in double distilled water) at a concentration of 0.15%. Eight volumes of the 0.15% collagen solution were mixed with 2 volumes of pDNA-polymer complexes (previously determined ratio) in serum-free culture media and 0.34 M NaOH. The solution was kept in ice to avoid premature self-assembly. Self-assembly occurred at 37 °C.
Plasmid DNA release
The release of the pDNA polyplexes from the atelocollagen hydrogel was characterised using the PicoGreen™ assay Kit (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocols. Briefly, the hydrogels containing pDNA polyplexes were prepared as described above in a 6-well plate. The loaded gels were left standing for 3 hours at room temperature to allow for complete gelation. Subsequently, gels were transferred to 6-well plates. Differences in the release profiles of two different pDNAs (IL-10 and DCN) from the gel were investigated. To this, phosphate buffered saline (PBS) was added to the wells and incubated at 37 °C (pH 7.0). At each time point, the wash media was removed and replaced with an equal volume. At the end of the experiment, the DNA content of the wash media was quantified using the PicoGreen™ assay and the cumulative release of DNA from the gels was calculated following comparison with a standard curve.
RNA extraction
Extraction of RNA from the human tenocytes exposed to gels with or without pDNA was performed according to the manufacturer’s protocols. Briefly, cells were detached from 6-well plates using trypsin treatment. 1 ml of TRI Reagent® (Applera Ireland, Dublin, Ireland) was added to each sample and incubated for 5 minutes at room temperature. Chloroform phase separation was performed and total RNA was purified using an RNeasy® kit (Qiagen), following protocols recommended by the manufacturer. The total quantity and purity of RNA was evaluated with an ultraviolet spectrophotometer (NanoDrop ND-1000 Spectrophotometer; NanoDrop Technologies, Wilmington, USA).
Reverse transcription polymerase chain reaction (RT-PCR)
Reverse transcription (RT) reactions were carried out and monitored with the TaqMan® Real-time Gene Expression Mastermix (Applied Biosystems, Foster City, CA, USA) and an ABI 7000 sequence detection system (Applied Biosystems®). Details of specific primers are provided in Table 1. The primers were designed and their specificity checked via primer-BLAST (www.ncbi.nlm.nih.gov). Gene transcription was inferred from calibration samples and normalised in relation to transcription of the housekeeping gene glyceraldehydes-3-phosphate dehydrogenase (GAPDH). The 2ΔΔ−Ct method was used to calculate relative expression for each gene.
Table 1. Set of primers used for real-time quantitative PCR.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Collagen I | 5′ TGACCTCAAGATGTGCCACT 3′ | 5′ ACCAGACATGCCTCTTGTCC 3′ |
| Collagen III | 5′ GCTGGCATCAAAGGACATCG 3′ | 5′ TGTTACCTCGAGGCCCTGGT 3′ |
| Fibronectin | 5′ CGCAGCTTCGAGATCAGTGC 3′ | 5′ TCGACGGGATCACACTTCCA 3′ |
| Elastin | 5′ CTGGCGTGCCTGGGGCAATTCCTG 3′ | 5′ TTGCGGCTAGGGTCTCCGAGGTC 3′ |
| TGF-β1 | 5′ TGAAGTGGTCTTTTGACG 3′ | 5′ GTTGGTTGTAGAGGGCAAGG 3′ |
| DCN | 5′ CGCCTCATCTGAGGGAGCTT 3′ | 5′ TACTGGACCGGGTTGCTGAA 3′ |
| IL-10 | 5′ AGAACCT GAAGACCCTCAGGC 3′ | 5′ CCACGGCCTTGCTCTTGTT 3′ |
| GAPDH | 5′ CCATGAGAAGTATGACAACAGCC 3′ | 5′ CCTTCCACGATACCAAAGTTG 3′ |
Immunofluorescence assay
Human tendon cells were seeded onto sterilised microscope slide coverslips at a density of 104/mL and cultured in a 6-well plate as described above. At predetermined time points, the cells on coverslips were washed in PBS and fixed in 2% paraformaldehyde-PBS for 20 minutes, permeabilised in 0.5% Triton X-100 in PBS and blocked in 1% bovine serum albumin for 30 minutes at room temperature. Cells were subsequently incubated overnight with appropriate primary antibody (1:200), followed by PBS washing and detection with TRITC-conjugated secondary antibody (1:300). The nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). Quantitative evaluation of relative fluorescence intensity was conducted on digital fluorescent images of cells captured using an Olympus IX81 microscope at 10× and 20× magnifications.
Flow cytometry
Flow cytometry analysis was used for representative tendon-derived cell surface markers2,76,77. The cells were detached and washed twice in PBS. The cells were re-suspended in FACS buffer (PBS, 2% FBS and 0.1% NaN3). Approximately, 2.5 × 105 cells were incubated with anti-human primary monoclonal antibodies CD90, CD105, CD44, CD73, CD45, CD34, CD11b, CD19, and HLA-DR (Miltenyi Biotec, Bergisch-Gladbach, Germany). Data were acquired by BD FACS Canto (BD Biosciences, San Jose, CA) FACS Calibur flow cytometer and analysed by FlowJo software (TreeStar Inc., OR, USA).
Statistical analysis
Results are expressed as mean ± standard deviation and analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests were used to determine statistical significance. Data were considered statistically significant at p < 0.05. In all studies, the minimum sample size was 4.
Additional Information
How to cite this article: Abbah, S. A. et al. Co-transfection of decorin and interleukin-10 modulates pro-fibrotic extracellular matrix gene expression in human tenocyte culture. Sci. Rep. 6, 20922; doi: 10.1038/srep20922 (2016).
Acknowledgments
This work was supported by: the EU FP7/2007–2013, Marie Curie, Industry-Academia Partnerships and Pathways (IAPP) award, part of the People programme, Tendon Regeneration Project (Grant Agreement Number: 251385) to DZ; the EU FP7/2007–2013, NMP award, Green Nano Mesh Project (Grant Agreement Number: 263289) to DZ; the Health Research Board, Health Research Awards Programme (Grant Agreement Number: HRA_POR/2011/84) to DZ; the Science Foundation Ireland and the European Regional Development Fund (Grant Agreement Numbers: 09/SRC/B1794 and 13/RC/2073) to AP. The authors acknowledge the Flow Cytometry Core Facility funded by NUI Galway and the Irish Government’s Programme for Research in Third Level Institutions (Cycle 5) and the National Development Plan 2007–2013.
Footnotes
Author Contributions S.A.A., D.T. and S.B. conducted the experimental work. T.O’.B., A.P. and D.I.Z. supervised the work.
References
- Lomas A. et al. The past, present and future in scaffold-based tendon treatments. Adv Drug Deliv Rev. 84, 257–277 (2015). [DOI] [PubMed] [Google Scholar]
- Spanoudes K., Gaspar D., Pandit A. & Zeugolis D. The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends Biotechnol. 32, 474–482 (2014). [DOI] [PubMed] [Google Scholar]
- Ljungqvist A. et al. International Olympic Committee consensus statement: Molecular basis of connective tissue and muscle injuries in sport. Clin Sports Med. 27, 231–239 (2008). [DOI] [PubMed] [Google Scholar]
- Gaspar D., Spanoudes K., Holladay C., Pandit A. & Zeugolis D. Progress in cell-based therapies for tendon repair. Adv Drug Deliv Rev. 84, 240–256 (2015). [DOI] [PubMed] [Google Scholar]
- Abbah S., Spanoudes K., O’Brien T., Pandit A. & Zeugolis D. Assessment of stem cell carriers for tendon tissue engineering in pre-clinical models. Stem Cell Res Ther. 5, 38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samiric T. et al. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix Biol. 28, 230–236 (2009). [DOI] [PubMed] [Google Scholar]
- Attia M. et al. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. Br J Sports Med. 48, 469–475 (2014). [DOI] [PubMed] [Google Scholar]
- Galatz L., Gerstenfeld L., Heber-Katz E. & Rodeo S. Tendon regeneration and scar formation: The concept of scarless healing. J Orthop Res. 33, 823–831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I., Heaton A. & McCullagh K. Cell morphology and collagen types in equine tendon scar. Res Vet Sci. 28, 302–310 (1980). [PubMed] [Google Scholar]
- Ryan C. et al. Glycosaminoglycans in tendon physiology, pathophysiology, and therapy. Bioconj Chem. 26, 1237–1251 (2015). [DOI] [PubMed] [Google Scholar]
- Parkinson J. et al. Change in proteoglycan metabolism is a characteristic of human patellar tendinopathy. Arthr Rheum. 62, 3028–3035 (2010). [DOI] [PubMed] [Google Scholar]
- Cutroneo K. TGF-beta-induced fibrosis and SMAD signaling: Oligo decoys as natural therapeutics for inhibition of tissue fibrosis and scarring. Wound Repair Regen. 15, S54–S60 (2007). [DOI] [PubMed] [Google Scholar]
- Farhat Y. et al. Gene expression analysis of the pleiotropic effects of TGF-β1 in an in vitro model of flexor tendon healing. PLoS One. 7, e51411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon N., Ward R., McGrew G. & Last J. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 58, 772–777 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Thunder R., Most D., Longaker M. & Lineaweaver W. Studies in flexor tendon wound healing: Neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 105, 148–155 (2000). [DOI] [PubMed] [Google Scholar]
- Ackermann P., Domeij-Arverud E., Leclerc P., Amoudrouz P. & Nader G. Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc. 21, 1801–1806 (2013). [DOI] [PubMed] [Google Scholar]
- Schulze-Tanzil G. et al. The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: New insights. Scand J Med Sci Sports. 21, 337–351 (2011). [DOI] [PubMed] [Google Scholar]
- Reed C. & Iozzo R. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 19, 249–255 (2002). [DOI] [PubMed] [Google Scholar]
- Järveläinen H. et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 14, 443–452 (2006). [DOI] [PubMed] [Google Scholar]
- Ferdous Z., Wei V., Iozzo R., Höök M. & Grande-Allen K. Decorin-transforming growth factor- interaction regulates matrix organization and mechanical characteristics of three-dimensional collagen matrices. J Biol Chem. 282, 35887–35898 (2007). [DOI] [PubMed] [Google Scholar]
- Shi J. et al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts. PLoS One. 9, e98228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J. et al. Protection against TGF-β1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. Arch Dermatol Res. 305, 341–352 (2013). [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Eckes B. & Krieg T. Effect of interleukin-10 on the gene expression of type I collagen, fibronectin, and decorin in human skin fibroblasts: Differential regulation by transforming growth factor-beta and monocyte chemoattractant protein-1. Biochem Biophys Res Commun. 281, 200–205 (2001). [DOI] [PubMed] [Google Scholar]
- Reitamo S., Remitz A., Tamai K. & Uitto J. Interleukin-10 modulates type I collagen and matrix metalloprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 94, 2489–2492 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occleston N., O’Kane S., Goldspink N. & Ferguson M. New therapeutics for the prevention and reduction of scarring. Drug Discov Today. 13, 973–981 (2008). [DOI] [PubMed] [Google Scholar]
- Browne S. & Pandit A. Multi-modal delivery of therapeutics using biomaterial scaffolds. J Mater Chem B. 2, 6692–6707 (2014). [DOI] [PubMed] [Google Scholar]
- Dube A. et al. Multimodal nanoparticles that provide immunomodulation and intracellular drug delivery for infectious diseases. Nanomedicine. 10, 831–838 (2014). [DOI] [PubMed] [Google Scholar]
- Kim C., Ghosh P. & Rotello V. Multimodal drug delivery using gold nanoparticles. Nanoscale. 1, 61–67 (2009). [DOI] [PubMed] [Google Scholar]
- O’Rorke S., Keeney M. & Pandit A. Non-viral polyplexes: Scaffold mediated delivery for gene therapy. Prog Polym Sci. 35, 441–458 (2010). [Google Scholar]
- Foti S., Samulski R. & McCown T. Delivering multiple gene products in the brain from a single adeno-associated virus vector. Gene Ther. 16, 1314–1319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajeesh S. et al. Efficient intracellular delivery and multiple-target gene silencing triggered by tripodal RNA based nanoparticles: A promising approach in liver-specific RNAi delivery. J Contr Rel. 196, 28–36 (2014). [DOI] [PubMed] [Google Scholar]
- Liu J., Xu L., Li Y. & Ma J. Temporally controlled multiple-gene delivery in scaffolds: A promising strategy to enhance bone regeneration. Med Hypoth. 76, 173–175 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. A magnetic nanoparticle-based multiple-gene delivery system for transfection of porcine kidney cells. PLoS One. 9, e102886 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva D., Müller S., Majewski M. & Evans C. Biologics for tendon repair. Adv Drug Deliv Rev. 84, 222–239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlers D. et al. TGF-beta and fibrosis in different organs - Molecular pathway imprints. Biochim Biophys Acta. 1792, 746–756 (2009). [DOI] [PubMed] [Google Scholar]
- Ricchetti E. et al. Effect of interleukin-10 overexpression on the properties of healing tendon in a murine patellar tendon model. J Hand Surg Am. 33, 1843–1852 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N. et al. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene Ther. 5, 1165–1170 (1998). [DOI] [PubMed] [Google Scholar]
- Basile P. et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for GDF5 gene delivery. Mol Ther. 16, 466–473 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Turnbull J. & Guimond S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 209, 139–151 (2011). [DOI] [PubMed] [Google Scholar]
- Hildebrand A. et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 302, 527–534 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M., Ota M. & Rifkin D. Matrix control of transforming growth factor-β function. J Biochem. 152, 321–329 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S. et al. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 54, 1205–1216 (1997). [DOI] [PubMed] [Google Scholar]
- Shimizukawa M. et al. Intratracheal gene transfer of decorin reduces subpleural fibroproliferation induced by bleomycin. Am J Physiol Lung Cell Mol Physiol. 284, L526–L532 (2003). [DOI] [PubMed] [Google Scholar]
- Baghy K., Iozzo R. & Kovalszky I. Decorin-TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem. 60, 262–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Sime P., Bringas P., Gauldie J. & Warburton D. Adenovirus-mediated decorin gene transfer prevents TGF-beta-induced inhibition of lung morphogenesis. Am J Physiol. 277, L412–L422 (1999). [DOI] [PubMed] [Google Scholar]
- van Vlasselaer P., Borremans B., van Gorp U., Dasch J. & de Waal-Malefyt R. Interleukin 10 inhibits transforming growth factor-beta (TGF-beta) synthesis required for osteogenic commitment of mouse bone marrow cells. J Cell Biol. 124, 569–577 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadullah K., Sterry W. & Volk H. Interleukin-10 therapy – Review of a new approach. Pharmacol Rev. 55, 241–269 (2003). [DOI] [PubMed] [Google Scholar]
- Halliday G. & Le S. Transforming growth factor-beta produced by progressor tumors inhibits, while IL-10 produced by regressor tumors enhances, Langerhans cell migration from skin. Int Immunol. 13, 1147–1154 (2001). [DOI] [PubMed] [Google Scholar]
- Lv H., Zhang S., Wang B., Cui S. & Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Contr Rel. 114, 100–109 (2006). [DOI] [PubMed] [Google Scholar]
- Browne S., Fontana G., Rodriguez B. & Pandit A. A protective extracellular matrix-based gene delivery reservoir fabricated by electrostatic charge manipulation. Mol Pharmac. 9, 3099–3106 (2012). [DOI] [PubMed] [Google Scholar]
- Holladay C. et al. A matrix reservoir for improved control of non-viral gene delivery. J Contr Rel. 136, 220–225 (2009). [DOI] [PubMed] [Google Scholar]
- Newland B. et al. The neurotoxicity of gene vectors and its amelioration by packaging with collagen hollow spheres. Biomaterials. 34, 2130–2141 (2013). [DOI] [PubMed] [Google Scholar]
- Ranganathan P. et al. Expression profiling of genes regulated by TGF-beta: Differential regulation in normal and tumour cells. BMC Genomics. 8, 98 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Age-dependent alterations of decorin glycosaminoglycans in human skin. Sci Rep. 3, 2422 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni E. et al. Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respir Res. 5, 24 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida-Hibino S. et al. The effect of TGF-beta1 on differential gene expression profiles in human corneal epithelium studied by cDNA expression array. Invest Ophthalmol Vis Sci. 42, 1691–1697 (2001). [PubMed] [Google Scholar]
- Reinboth B., Finnis M., Gibson M., Sandberg L. & Cleary E. Developmental expression of dermatan sulfate proteoglycans in the elastic bovine nuchal ligament. Matrix Biol. 19, 149–162 (2000). [DOI] [PubMed] [Google Scholar]
- Reinboth B., Hanssen E., Cleary E. & Gibson M. Molecular interactions of biglycan and decorin with elastic fiber components: Biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J Biol Chem. 277, 3950–3957 (2002). [DOI] [PubMed] [Google Scholar]
- Neill T., Schaefer L. & Iozzo R. Decorin: A guardian from the matrix. Am J Pathol. 181, 380–387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K. et al. Possible dual role of decorin in abdominal aortic aneurysm. PLoS One. 10, e0120689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitamo S., Remitz A., Tamai K., Ledo I. & Uitto J. Interleukin 10 up-regulates elastin gene expression in vivo and in vitro at the transcriptional level. Biochem J. 302, 331–333 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang J., Wang B., Shen Y. & Ouahab A. Co-delivery of siRNA and hypericin into cancer cells by hyaluronic acid modified PLGA-PEI nanoparticles. Drug Dev Ind Pharm. (In Press). [DOI] [PubMed]
- Lee S. et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J Contr Rel. (In Press). [DOI] [PubMed]
- Dash B. et al. An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials. 65, 126–139 (2015). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Dual silencing of Bcl-2 and Survivin by HSV-1 vector shows better antitumor efficacy in higher PKR phosphorylation tumor cells in vitro and in vivo. Cancer Gene Ther. 22, 380–386 (2015). [DOI] [PubMed] [Google Scholar]
- Hong S. et al. Dual expression of shAkt1 and Pdcd4 suppresses lung tumorigenesis in K-rasLA1 mice. Anticancer Res. 35, 2015–2019 (2015). [PubMed] [Google Scholar]
- Mirdailami O. et al. Controlled release of rhEGF and rhbFGF from electrospun scaffolds for skin regeneration. J Biomed Mater Res A. 103, 3374–3385 (2015). [DOI] [PubMed] [Google Scholar]
- Glover D., Lipps H. & Jans D. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 6, 299–310 (2005). [DOI] [PubMed] [Google Scholar]
- Hollon T. Researchers and regulators reflect on first gene therapy death. Am J Ophthalmol. 129, 701 (2000). [DOI] [PubMed] [Google Scholar]
- Hollon T. Researchers and regulators reflect on first gene therapy death. Nat Med. 6, 6 (2000). [DOI] [PubMed] [Google Scholar]
- Li L., Wei Y. & Gong C. Polymeric nanocarriers for non-viral gene delivery. J Biomed Nanotechnol. 11, 739–770 (2015). [DOI] [PubMed] [Google Scholar]
- Ramamoorth M. & Narvekar A. Non viral vectors in gene therapy - An overview. J Clin Diagn Res. 91, 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 15, 541–555 (2014). [DOI] [PubMed] [Google Scholar]
- Wang W., Li W., Ma N. & Steinhoff G. Non-viral gene delivery methods. Curr Pharm Biotechnol. 14, 46–60 (2013). [PubMed] [Google Scholar]
- Zeugolis D., Paul R. & Attenburrow G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: Animal species and collagen extraction method. J Biomed Mater Res A. 86, 892–904 (2008). [DOI] [PubMed] [Google Scholar]
- Crockett R., Centrella M., McCarthy T. & Grant-Thomson J. Effects of cyclic strain on rat tail tenocytes. Mol Biol Rep. 37, 2629–2634 (2010). [DOI] [PubMed] [Google Scholar]
- Lui P. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ - Update and future development. Stem Cell Res Ther. 6, 106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]