Abstract
Objective
The diagnosis of multiple sclerosis (MS) presently relies on radiographic assessments of imperfect specificity. Recent data using T2* methodology for the detection of the “central vessel sign” (CVS) in MS lesions suggests this novel MRI technique may distinguish MS from other disorders. Our aim was to determine if evaluation for CVS on 3T FLAIR* MRI differentiates MS from migraine.
Methods
Patients with MS or migraine and a prior brain MRI demonstrating at least two hyperintense lesions ≥3 mm were recruited. Exclusion criteria included any additional comorbidity known to cause brain MRI abnormalities. 3T MRI was performed in each participant with administration of gadopentetate dimeglumine, and FLAIR* images were generated in postprocessing. The total number of discrete ovoid lesions ≥3 mm were counted on FLAIR, per participant, and subsequently evaluated for presence of CVS on FLAIR*. An exploratory method evaluating for CVS in a maximum of 12 lesions per subject was also completed.
Results
Ten participants with MS and 10 with migraine completed the study. The median percentage (quartiles) of lesions in MS participants with CVS was 84 (79, 94) compared to 22 (15, 54) in migraine (P = 0.008). In a subanalysis by brain region, in the subcortical and deep white matter, the median percentage (quartiles) of lesions in MS participants with CVS was 88 (81, 100) compared to 19 (11, 54) in migraine (P = 0.004). This difference was not identified in juxtacortical, periventricular, or infratentorial regions.
Interpretation
Identification of CVS using FLAIR* on 3T MRI helps differentiate MS from migraine, particularly in the subcortical and deep white matter.
Introduction
There remains no single highly accurate diagnostic test for multiple sclerosis (MS). At present, therefore, the diagnosis of MS relies on the combined interpretation of clinical, laboratory, and radiographic assessments.1 MS diagnosis can be challenging, particularly since a number of syndromes and diseases may mimic its clinical and/or radiographic appearance.2, 3 In spite of the refinement of diagnostic criteria over the last several decades, misdiagnosis of MS has remained a significant problem,4, 5, 6, 7, 8 with the potential to result in clinical and psychosocial sequelae as well as substantial unnecessary cost to healthcare systems.4, 9
The development of novel imaging techniques may improve the ability to distinguish MS from other disorders. One such method may be detection of the “central vessel sign” (CVS).10 It has been known from histopathological studies at autopsy that most MS lesions are centered around veins,11 and only recently have a variety of imaging techniques using susceptibility‐weighted imaging on ultrahigh‐field 7‐tesla (T) research magnets demonstrated this relationship in vivo.12, 13, 14, 15, 16, 17 A small number of studies have also evaluated the ability of 3T scanners, which are used routinely in clinical practice, to detect a “central vessel” (CV) in MS lesions.18, 19, 20, 21 These studies have used a variety of methods to image vessels and have included small numbers of participants, yet several suggest that detection of CVS may discriminate MS from alternative diagnoses on 3T.18, 22, 23, 24 FLAIR*, which combines T2‐FLAIR with high‐resolution contrast‐enhanced T2*‐weighted imaging, is a novel MRI technique that has provided high‐quality imaging of central vessels (CVs) in MS lesions at 3T.20
Given the promise of CVS demonstrated by a number of small studies using various techniques, further data are needed to evaluate the optimal methods on 3T MRI to determine the ability of CVS to distinguish MS from syndromes of overlapping radiographic appearance. In this study, we compared FLAIR* MRI in migraine and MS, on the premise that evaluation of this methodology in a constrained population of patients without MS who share a single diagnosis and presumed underlying pathophysiology for white matter abnormalities, and in patients with MS without additional comorbidities to account for their white matter abnormalities, would allow optimal assessment of the degree to which CVS by FLAIR* can aid in the diagnosis. Clinical symptoms and radiographic findings in migraine can be mistaken for MS,4, 25 although the proposed pathophysiology of MRI findings in migraine26 likely differs from MS. Thus, we hypothesized that the white matter lesions in patients with migraine would demonstrate fewer CVs when compared with MS lesions. The sub‐aim of our study was to determine if the frequency of CVS in lesions within specific brain regions would best distinguish MS from migraine.
Methods
Patients over the age of 18, with a confirmed diagnosis of MS or migraine, and with a prior brain MRI with ≥2 hyperintense lesions ≥3 mm in diameter, were recruited for the study. For the MS cohort, an existing diagnosis, made by a neurologist with subspecialty training in MS, was required, together with fulfillment of current MS diagnostic criteria.1 For the migraine cohort, the diagnosis was required to have been made by a neurologist during a prior clinical evaluation. Exclusion criteria for both cohorts included additional comorbidities associated with brain MRI abnormalities, including but not limited to hypertension, diabetes mellitus, vitamin deficiencies, prior infections of the central nervous system, neoplasm, seizure disorder, history of head injury, rheumatologic disease, or history of tobacco use. MS patients with a history of migraine were also excluded. Exclusion criteria also included contraindication to MRI, known pregnancy, and concurrent breastfeeding. Informed consent was obtained following approval from the University of Vermont Institutional Review Board prior to the start of the study.
T2‐weighted FLAIR (1 mm isotropic) and T2*‐weighted multishot echo‐planar imaging (0.55 mm isotropic voxels) data were acquired on a 3T Philips dStream MRI with 32‐channel head coil (Table 1). A single‐dose of gadopentetate dimeglumine (Magnevist) was injected manually, and acquisition of the T2* sequence followed immediately. FLAIR images were coregistered and resampled to the space of the T2*‐weighted images using SPM8. The product of the voxelwise signal intensities, FLAIR*, was imported to a clinical image viewer to allow multiplanar reformatting.
Table 1.
MRI data acquisition parameters
| T2 FLAIR | T2*‐weighted mutlishot EPI | |
|---|---|---|
| Acquisition | TSE | GE‐EPI |
| Orientation | Sag 3D | Sag 3D |
| TE (ms) | 369 | 29 |
| TR (ms) | 4800 | 54 |
| TI (ms) | 1600 | – |
| Flip angle (deg) | 90 | 10 |
| Number of echoes | 178 | 15 |
| Field of view (mm) | 240 × 240 × 180 | 240 × 240 × 185 |
| Resolution (mm) | 1.0 × 1.0 × 1.0 | 0.55 × 0.55 × 0.55 |
| SENSE factor (AP) | 3.0 | 2.0 |
| SENSE factor (RL) | 2.0 | 2.0 |
| Number of averages | 2 | 2 |
| Acquisition time (min:sec) | 5:50 | 4:15 |
TE, echo time; TR, repetition time; TI, inversion time; SENSE, sensitivity encoding; EPI, echo‐planar imaging; TSE, turbo spin‐echo; GE, gradient echo; AP, anteroposterior; RL, right‐left; Sag, sagittal.
A trained neurologist, experienced in MS neuroimaging, but unaware of the diagnosis in each case, reviewed coded MRI scans in random order, counted the total number and location of discrete lesions ≥3 mm at longest diameter, and determined if a CV was present in each lesion on FLAIR*. A “discrete” lesion was defined as a lesion with distinct borders surrounded by normal appearing white matter, which may also contact the cortex or ventricular surface at one edge. Lesions of morphologies that were not ovoid or spherical in appearance, or that were confluent, were not counted for analysis. A CV was defined as a single vessel within a lesion with approximately equal distance to the lesion edges on all sides in at least one plane. The Wilcoxon rank‐sum test was used to compare, between cohorts, the total number of lesions and percentage of those lesions with CVS. Lesions were further subcategorized to juxtacortical (discrete lesions with one edge in contact with the cortex), subcortical and deep white matter, periventricular (discrete lesions with one edge in contact with a ventricle), and infratentorial, and the percentages of lesions with CVs in these regions were also compared as above.
An exploratory method of CV counting (“select three”) was also completed to determine if an assessment of a limited number of lesions for CV might distinguish MS from migraine. On FLAIR images, a different trained neurologist, also unaware of diagnosis, reviewed de‐identified MRIs in random order and selected up to three discrete ovoid lesions, ≥3 mm in diameter, from each of the following regions: juxtacortical, subcortical and deep white matter (grouped together), periventricular, and infratentorial. Lesions were selected at random. Once ≤3 lesions were selected per region on FLAIR, corresponding FLAIR* sequences were reviewed to determine if CVS was present in each lesion. This method was repeated 3 weeks later following re‐coding and repeat randomization of the presentation order. Logistic regression was used for each region separately, combining the two trials of “select three” to predict the probability of an MRI being from an MS patient based on the number of selected lesions and the number of lesions that contained CVs. The logistic regression model could not be applied in regions showing no central veins in one of the cohorts; in these cases, Fisher's exact test was used. Stepwise logistic regression was used to develop a prediction equation for the probability of the MRI being from an MS patient, using number of lesions and number of lesions with CVs, in each of the four brain regions.
Results
Ten participants with MS and 10 participants with migraine completed the study. The MS cohort was comprised of nine women and one man with relapsing remitting MS with mean age 44 (standard deviation: 16). The migraine cohort was comprised of 10 women, age 47 (13) (P = 0.65). MS and migraine cohorts did not differ in the number of lesions per case, but CVs were present in more lesions in the MS group (Table 2 and Fig. 1). The median % (quartiles) of CVs was 84 (79, 94) in MS and 22 (15, 54) in migraine (P = 0.008).
Table 2.
Total number of lesions and percentage with central vessel in multiple sclerosis (MS) and migraine
| MS | Migraine | P‐value | |
|---|---|---|---|
| Lesions (total) | 236 | 182 | |
| Mean per participant | 24 | 18 | 0.38 |
| Median per participant | 21 | 13 | 0.20 |
| Lesions with CV (total) | 191 | 78 | |
| Mean% lesions w/CV per participant | 80% | 34% | <0.001 |
| Median% lesions w/CV per participant | 84% | 22% | 0.008 |
CV: central vessel.
Figure 1.
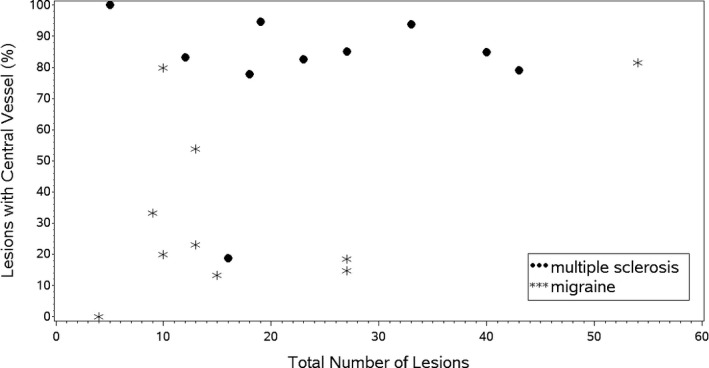
Total number of lesions compared to the percentage of lesions with central vessels in participants with multiple sclerosis and migraine.
All 20 participants had lesions in the subcortical and deep white matter. There was no between‐group difference in the number of lesions per participant in this region. However, MS participants had a higher percentage of CVs in deep white matter lesions. Figure 2 demonstrates a typical lesion with CV in the subcortical and deep white matter region in MS and a typical lesion without CV in the subcortical and deep white matter region in migraine. Table 3 presents summary analysis for all four regions.
Figure 2.

Examples of 3T FLAIR* imaging demonstrating the “central vessel sign” in multiple sclerosis (A) and absence of the “central vessel sign” in migraine (B).
Table 3.
Lesions and percentage with central vessel in multiple sclerosis (MS) and migraine by region
| Brain region | Median # lesions | P‐value | Median % with CV | P‐value | ||
|---|---|---|---|---|---|---|
| MS | Migraine | MS | Migraine | |||
| Juxtacortical | 4 | 0.5 | 0.18 | 85% | 50% | 0.39 |
| Subcortical and DWM | 14 | 13 | 0.88 | 88% | 19% | 0.004 |
| Periventricular | 3 | 0 | 0.002 | 76% | 100% | 0.23 |
| Infratentorial | 1 | 0 | 0.006 | 100% | 50% | 0.09 |
CV, central vessel; DWM, deep white matter.
Lesions meeting inclusion criteria were not identified in every participant in the three remaining regions. Juxtacortical lesions were identified in 8/10 MS participants and 5/10 migraine participants. Periventricular lesions were identified in 10 MS participants and two migraine participants. Infratentorial lesions were identified in nine MS participants and two migraine participants. Median lesion count differed between MS and migraine in the periventricular and infratentorial regions, but median percentage of CV‐positive lesions did not differ by region. (Note that participants without lesions in a given region could not be included in this analysis.)
For the exploratory method of CV counting (“select three”), logistic regression for the probability of an MRI being from MS based on the total number of subcortical and deep white matter lesions was not significant (P = 0.21). However, the probability of an MRI being from MS was related to the number of subcortical/deep white matter CVs (odds ratio 5.21, 95% CI 1.73–15.75, P = 0.003). Thus, for every increase of one CV (from 0 to 3) in the subcortical and deep white matter, the odds of the MRI being from MS increased by five fold.
Using the “select three” method, no infratentorial or periventricular lesions with CVS were identified in the migraine cohort, and Fisher's exact test was used to compare the presence of any lesions with CV in these regions between cohorts (P = 0.008 for each region). The number of juxtacortical lesions with CV in each cohort was not cohort‐dependent (P = 0.19).
Stepwise logistic regression modeled the probability of an MRI being from MS based on the total lesion number and the number of lesions with CVS in each of the four regions identified using the “select three” method. Once the number of subcortical and deep white matter lesions with CVs entered the model, no other measure achieved statistical significance at the P = 0.05 level to enter the model.
Discussion
Our results demonstrate that quantification of CVS using 3T FLAIR* MRI may differentiate MS from migraine. This perivenous configuration of MS lesions has been recently demonstrated using T2*‐weighted ultrahigh‐field 7T MRI,13, 15 and in studies that have included non‐MS populations, the quantification of CVs has predicted a diagnosis of MS.12, 14, 17, 27, 28, 29 Several studies reported detection of CVs in lesions of MS patients more frequently than other populations, including patients with Susac syndrome29 and neuromyelitis optica spectrum disorder,17 and controls with high risk for vascular disease.28 A number of studies have suggested that a finding of >40% of lesions with CVS may predict MS.12, 14 Several of these studies have also suggested that MS cohorts had more CVs in lesions specifically located in the “subcortical” and “deep” locations compared to other regions.14, 27, 28
A limited number of medical centers have access to 7T magnets, and demonstrating the ability of T2*‐weighted imaging to differentiate MS on 3T MRI would allow wider research in this area and more general clinical application. Our study is one of only several that have demonstrated the feasibility of detection of CVS on 3T MRI in MS,19, 20, 30, 31 and only a few studies have investigated whether the CVS distinguishes MS from other populations at 3T.16, 18 Lummel et al.16 found no difference in the total number of CVs in 15 MS participants and 15 non‐MS participants with microangiopathic white matter lesions. Kau et al. prospectively studied 14 individuals, identifying a CV in 84% of lesions in participants subsequently diagnosed with MS and 11% in those who were not diagnosed with MS. They reported sensitivity of 84%, specificity of 89%, positive predictive value of 94%, negative predictive value of 73%, and accuracy of 86%, as well as good inter‐rater agreement.18 Preliminary data using 3T T2* imaging techniques presented at recent scientific meetings have also demonstrated more lesions with CVS in MS23 and have suggested that detection of CVs in approximately 40–45% of MRI lesions might be predictive of the MS diagnosis.22, 23, 24
Comparison of the results of these limited studies using 3T MRI is challenging due to their use of varying magnetic field strengths, methods for T2*‐weighted imaging, and criteria for participant and lesion inclusion. However, our data identifying a median percentage of lesions with CV in participants with MS of 84%, compared to 22% in migraine, supports data suggesting an approximate cutoff of >40% may aid in predicting of the MS diagnosis. The ability to differentiate diagnoses in our small cohort supports the notion that FLAIR* 3T MRI is feasible for the evaluation of CVs and warrants further study. Our results in subcortical and deep white matter lesions also support prior data from the 7T studies mentioned above, suggesting that this may be the region of highest yield for assessing the diagnostic value of the CVS. The reason for this finding may be that the perivascular topography is easier to discern on the sagittal plane due to the predominant right‐left orientation of the vessels in the subcortical and deep white matter.
There remains a need to develop consensus criteria for imaging methodology for further study of the CVS in MS. We propose further investigation on 3T MRI using methods employed in this study, as FLAIR* may be more sensitive than prior approaches due to its ability to produce high‐resolution, isotropic voxels with adequate T2* weighting, its use of a multichannel coil, and the addition of a gadolinium‐based contrast agent to enhance the susceptibility contrast within veins.20, 32, 33 Although use of a 32‐channel coil may have yielded better images in this study, prior studies using FLAIR* have been performed with standard SENSE‐8 head coils that are widely used at imaging centers.20, 32
Most prior studies counted all lesions and subsequently evaluated the percentage with CVs. While this method is an important first step in establishing the potential significance of FLAIR*, the assessment of every lesion for CVS is impractical for clinical application, and there remains a need to develop and test the less time‐consuming algorithms. More limited quantification of the presence of CVs, particularly given the relationship between number of CVs in subcortical and deep white matter lesions demonstrated using our exploratory “select three” method, suggests that further development of such an algorithm, with an eye toward applicability in the radiological reading room, should be tested in both MS and non‐MS populations.
Our study had a number of limitations. Strict exclusion criteria were designed to limit possible confounders. Therefore, our data cannot at present be generalized beyond the current population, requiring further evaluation of our methods in participants with MS and other diagnoses with multiple comorbidities that might generate white matter abnormalities. Although epidemiological data suggest that both MS and migraine are more common in women and in approximately similar proportions,34, 35, 36, 37, 38 only one man was included in the study due to recruitment challenges, and this may limit the generalizability of our results. It is interesting to note that several participants with migraine had a high number of CVs, perhaps suggesting that the pathophysiology of these lesions may be more heterogeneous than presumed. Given this and our one MS participant with 18% CVs, a cutoff of 40% may not prove to have high specificity for MS once larger studies are completed. As such, it is possible that algorithms using CVS may be found to be most useful in combination with current MS diagnostic criteria. A third limitation is that lesions meeting our inclusion criteria were not identified in all participants in the juxtacortical, periventricular, and infratentorial regions, and were disproportionately more frequent in the MS cohort; this limits conclusions that may be drawn on the value of detection of CVs for differentiating MS from migraine in these regions. While all subjects with migraine were diagnosed by a neurologist and no further explanation for their MRI abnormalities was identified, given the lack of a specific biomarker for migraine or MS, we cannot exclude the possibility that some of these subjects had presymptomatic MS, or what has been termed “radiologically isolated syndrome.”39 Lastly, our methodology is currently limited by the need for offline postprocessing to create FLAIR* images, but the steps required to generate these images are straightforward and implementable in standard postprocessing packages offered by scanner manufacturers.
In summary, identification of CVS using FLAIR* imaging on 3T MRI showed promise for the differentiation of MS from migraine in our initial study of 20 subjects. Further evaluation of algorithms that include limited quantification of CVs in larger prospective cohort studies may lead to clinically practical methods to supplement current MS diagnostic criteria.
Conflict of Interest
Dr. Reich reports grants from Vertex Pharmaceuticals, outside the submitted work; In addition, has a patent PCT/US2012/067997 pending, and a patent PCT/US2013/033334 pending.
Acknowledgments
Angela Applebee, Jay Gonyea, Scott Hipko, Robert Shapiro, Martha Lubet.
References
- 1. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller DH, Weinshenker BG, Filippi M, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 2008;14:1157–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Charil A, Yousry TA, Rovaris M, et al. MRI and the diagnosis of multiple sclerosis: expanding the concept of “no better explanation”. Lancet Neurol 2006;5:841–852. [DOI] [PubMed] [Google Scholar]
- 4. Solomon AJ, Klein EP, Bourdette D. “Undiagnosing” multiple sclerosis: the challenge of misdiagnosis in MS. Neurology 2012;78:1986–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomon AJ, Weinshenker BG. Misdiagnosis of multiple sclerosis: frequency, causes, effects, and prevention. Curr Neurol Neurosci Rep 2013;13:1–7. [DOI] [PubMed] [Google Scholar]
- 6. Herndon RMBB. Misdiagnosis of multiple sclerosis. Semin Neurol 1985;5:94–98. [Google Scholar]
- 7. Rudick RA, Schiffer RB, Schwetz KM, Herndon RM. Multiple sclerosis. The problem of incorrect diagnosis. Arch Neurol 1986;43:578–583. [DOI] [PubMed] [Google Scholar]
- 8. Rudick RA, Miller AE. Multiple sclerosis or multiple possibilities: the continuing problem of misdiagnosis. Neurology 2012;78:1904–1906. [DOI] [PubMed] [Google Scholar]
- 9. Solomon AJ, Klein E. Disclosing a misdiagnosis of multiple sclerosis: do no harm? Continuum (Minneapolis, Minn) 2013;19:1087–1091. [DOI] [PubMed] [Google Scholar]
- 10. Quinn MP, Kremenchutzky M, Menon RS. Venocentric lesions: an MRI marker of MS? Front Neurol 2013;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fog T. On the vessel‐plaque relationships in the brain in multiple sclerosis. Acta Neurol Scand Suppl 1964;40(Suppl 10):9–15. [PubMed] [Google Scholar]
- 12. Mistry N, Dixon J, Tallantyre E, et al. Central veins in brain lesions visualized with high‐field magnetic resonance imaging: a pathologically specific diagnostic biomarker for inflammatory demyelination in the brain. JAMA Neurol 2013;70:623–628. [DOI] [PubMed] [Google Scholar]
- 13. Gaitan MI, Maggi P, Wohler J, et al. Perivenular brain lesions in a primate multiple sclerosis model at 7‐tesla magnetic resonance imaging. Mult Scler 2013;20:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra‐high‐field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011;76:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kollia K, Maderwald S, Putzki N, et al. First clinical study on ultra‐high‐field MR imaging in patients with multiple sclerosis: comparison of 1.5T and 7T. AJNR. Am J Neuroradiol 2009;30:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lummel N, Boeckh‐Behrens T, Schoepf V, et al. Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology 2011;53:311–317. [DOI] [PubMed] [Google Scholar]
- 17. Sinnecker T, Dorr J, Pfueller CF, et al. Distinct lesion morphology at 7‐T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology 2012;79:708–714. [DOI] [PubMed] [Google Scholar]
- 18. Kau T, Taschwer M, Deutschmann H, et al. The “central vein sign”: is there a place for susceptibility weighted imaging in possible multiple sclerosis? Eur Radiol 2013;23:1956–62. [DOI] [PubMed] [Google Scholar]
- 19. Luo J, Yablonskiy DA, Hildebolt CF, et al. Gradient echo magnetic resonance imaging correlates with clinical measures and allows visualization of veins within multiple sclerosis lesions. Mult Scler 2013;20:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sati P, George IC, Shea CD, et al. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012;265:926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grabner G, Dal‐Bianco A, Schernthaner M, et al. 2011. Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. J Magn Reson Imaging 33:543–549. [DOI] [PubMed] [Google Scholar]
- 22. Campion T, Smith P, Turner B, et al. FLAIR* for the non‐invasive histological diagnosis of multiple sclerosis. Abstract S29003 Presented at the 2015 American Academy of Neurology Annual Meeting. 2015.
- 23. Mistry N, Dixon JE, Tallantyre EC, et al. 3 Tesla T2*‐weighted brain MRI distinguishes multiple sclerosis from incidental white matter microangiopathic lesions. Mult Scler 2013b;19(11 Suppl):8–597.24151639 [Google Scholar]
- 24. George I, Sati P, Absinta M, et al. FLAIR* MRI improves diagnostic accuracy in multiple sclerosis. Abstract S29002 Presented at the American Academy of Neurology 2015 Annual Meeting.
- 25. Liu S, Kullnat J, Bourdette D, et al. Prevalence of brain magnetic resonance imaging meeting Barkhof and McDonald criteria for dissemination in space among headache patients. Mult Scler 2013;19:1101–5. [DOI] [PubMed] [Google Scholar]
- 26. Takano T, Tian GF, Peng W, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci 2007;10:754–762. [DOI] [PubMed] [Google Scholar]
- 27. Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh‐field MR (7 T) imaging of brain lesions in neuromyelitis optica. Multiple Sclerosis International 2013;2013:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilsdonk ID, Wattjes MP, Lopez‐Soriano A, et al. Improved differentiation between MS and vascular brain lesions using FLAIR* at 7 Tesla. Eur Radiol 2013;24:841–9. [DOI] [PubMed] [Google Scholar]
- 29. Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler 2012;18:1592–1599. [DOI] [PubMed] [Google Scholar]
- 30. Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol 2009;44:491–494. [DOI] [PubMed] [Google Scholar]
- 31. Dixon JE, Simpson A, Mistry N, et al. Optimisation of T(2)*‐weighted MRI for the detection of small veins in multiple sclerosis at 3 T and 7 T. Eur J Radiol 2013;82:719–727. [DOI] [PubMed] [Google Scholar]
- 32. Sati P, Thomasson DM, Li N, et al. Rapid, high‐resolution, whole‐brain, susceptibility‐based MRI of multiple sclerosis. Mult Scler 2014;20:1464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samaraweera A, Clarke M, Mougin O, et al. A comparison of FLAIR* and T2*‐weighted imaging in detecting white matter lesions and central veins in patients with MS and ischaemic lesions at 3T. Abstract 4361 presented at The International Society for Magnetic Resonance in Medicine, Toronto Canada, May 2015.
- 34. Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–349. [DOI] [PubMed] [Google Scholar]
- 35. Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population‐based studies. Neurology 1994;44(6 Suppl 4):S17–S23. [PubMed] [Google Scholar]
- 36. Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol 2006;5:932–936. [DOI] [PubMed] [Google Scholar]
- 37. Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koch‐Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010;9:520–532. [DOI] [PubMed] [Google Scholar]
- 39. Lebrun C. The radiologically isolated syndrome. Rev Neurol (Paris) 2015;171:698–706. [DOI] [PubMed] [Google Scholar]


