Abstract
Objective
A mild traumatic brain injury (mTBI), or concussion, has known neuropsychological sequelae, and neuroimaging shows disturbed brain connectivity during the resting state. We hypothesized that task‐based functional connectivity measures, using magnetoencephalography (MEG), would better link the neurobiological underpinnings of cognitive deficits to specific brain damage.
Methods
We used a mental flexibility task in the MEG and compared brain connectivity between adults with and without mTBI.
Results
Affected individuals showed significant reductions in connectivity. When challenged with a more difficult task, these individuals were not able to “boost” their connectivity, and as such, showed deterioration in performance.
Interpretation
We discuss these findings in the context of limitations in cognitive reserve as a consequence of a mTBI.
Introduction
A mild traumatic brain injury (mTBI) is defined as an insult to the head that disrupts normal brain function as manifested by a change in mental status or consciousness for less than 30 min or a score of >13 on the Glasgow Coma Scale (GCS).1, 2 While the majority of individuals with an mTBI recover fully, it is well established that a significant proportion continue to suffer with physical, emotional, and cognitive symptoms. Of these symptom clusters, cognitive complaints can include, for example, difficulties with concentration, attention, memory confusion, and slowness in thinking.3, 4, 5, 6, 7 While these symptoms can be subtle, their persistent nature has garnered both clinical and research attention focused on elucidating the etiology, pathophysiology, and mechanism of these complaints.
It is known that one of the most common pathologies seen in a severe brain injury is damage to white matter tracts via diffuse axonal injury8, 9, 10 and microstructural alterations of axons in both gray and white matter.11, 12, 13 It is thought that these kinds of injury result in a disconnection within and between brain areas that is manifested as a loss or reduction of cognitive function.14, 15, 16 Functional connectivity studies acquired in the functional magnetic resonance imaging (fMRI) using a resting state paradigm in patients with mTBI report abnormalities in brain networks that include visual processing, limbic, motor, and cognitive functions,17 default mode function,18 default mode regulation,19 and aberrant connectivity in thalamo‐cortical networks that correlated with neurocognitive function and clinical symptomatology.20
More recently, magnetoencephalography (MEG) neuroimaging has been applied to the study of mTBI. In contrast to fMRI, which measures hemodynamic change as a surrogate for brain activity, MEG captures the magnetic fields generated by neuronal conduction and is thus a direct measure of brain activity.21, 22 Furthermore, MEG measures of functional connectivity are comparable to fMRI, but include the additional dimensions of time and oscillatory frequency that are present in neurophysiological data.23 Using a resting state protocol, it has been shown that the location of slow waves in the delta (1–4 Hz) frequency range corresponded to sites of brain injury24, 25 and differentiated individuals with mTBI from controls.26 Functional connectivity metrics acquired from resting state MEG recordings demonstrated decreases in functional connectivity even in mTBI27 and correlated with cognitive recovery and neuropsychological assessments in acquired brain injury.28 Given the changes in functional connectivity achieved with resting state protocols, we postulated that using a task‐based functional connectivity analysis, with a task sensitive to cognitive deficits in mTBI, would be even more likely to capture brain changes induced by mTBI.
Mental flexibility is a core feature of cognitive executive functions and underlies the ability to integrate new information and to appropriately and accordingly adapt one's behavior. Mental inflexibility is one of the cognitive complaints experienced in mTBI14 and manifests as a tendency to perseverate or “become stuck.” The neural underpinnings of mental flexibility have been well studied using the Wisconsin Card Sort Task,29 and areas in prefrontal, frontal, and posterior cortical regions have been implicated using both fMRI30, 31, 32, 33, 34, 35, 36 and MEG.37, 38, 39 Our group has designed a simpler task aimed at probing the core “shifting” aspect of mental flexibility and we have optimized this for MEG.40
Using MEG source localization analysis, we41 demonstrated that, compared to matched controls, individuals with mTBI showed significantly delayed reaction times with a disorganized sequence of brain activations when completing a mental flexibility task. This approach identified that core brain regions were activated in an unexpected order in mTBI, and raised the possibility that connectivity between these brain regions was likely disrupted. With recent advancements in task‐based functional connectivity analyses for MEG, and our understanding that mTBI disrupts microstructural integrity that most likely manifests as impairments in functional connectivity, we applied functional connectivity analyses to this data set to understand the impact of a mTBI on mental flexibility.
Patients and Methods
Participants
Thirty‐two adult males participated in the study and were previously described.41 These consisted of 16 men diagnosed with mTBI (mean: 31.0 ± 7.5 SD years) and 16 controls (mean: 27.7 ± 5.3 SD years). Inclusion criteria for the mTBI were a mild injury to the brain sustained within the last 2 months, with or without symptoms, loss of consciousness for <30 min, posttraumatic amnesia for <24 h, alterations of consciousness (dazed, confused) for <24 h, GCS score ≥13 in the first 24 h postinjury, no history of previous concussion, and normal computed tomography (CT) scan of the head at admission. Individuals with mTBI were civilians injured in motor vehicle and sports accidents and recruited, in the acute phase, from the Emergency Department at Sunnybrook Health Sciences Centre. Control individuals were recruited from the community, and exclusion criteria included any previous head injury or any history of neurological, psychological, or psychiatric disease. The MEG study was conducted in the Neuromagnetic Lab at the Hospital for Sick Children. Institutional ethics approvals were received from both Sunnybrook Health Sciences Centre and the Hospital for Sick Children, and all participants gave written informed consent.
All participants completed a short battery of assessments, which included the Wechsler Abbreviated Scale of Intelligence,42, the Patient Health Questionnaire,43 and the Symptom Checklist and Symptom Severity Score.44
Task and MEG data acquisition
Subjects completed a test of mental flexibility called the intraextra dimensional set shift (IED) (Cambridge Neuropsychological Test Automated Battery [CANTAB(C)], Cambridge Cognition), which required subjects to match stimuli on color or shape. On each trial, subjects were presented with two candidate images and a probe image where one of the candidates always matched the probe by shape or color. Participants experienced a few trials where the match dimension remained the same, for example, the color “red.” Then, a set‐switch would require matching along a different parameter, for example, the color “blue” or the shape “circle.” If the switch was within the same dimension, that is, color to color, or shape to shape, this was termed an “easy” or “intradimensional” switch. If the switch was between dimensions, that is, color to shape, or shape to color, this is a more difficult shift, called an “extradimensional” shift. Participants received training on the task outside of the scanner, and were only tested in the MEG when they had reached a high level of competence.
Participants pressed a button to indicate which candidate image matched the probe. A total of 370 probes were presented with 50 intradimensional and 50 extradimensional shifts. Stimuli were presented using Presentation software (Neurobehavioral Systems, Inc., Berkeley, CA) via a back projection screen placed 78 cm from the subjects' eyes. The stimuli were foveal and subtended 13° of arc (6.5° on either side of the midline). The task was self‐paced and each probe was presented until a response was recorded, to a maximum of 4 sec. Stimulus onset asynchrony was randomly jittered between 0.8 and 1.2 sec. The entire task required a maximum of 30 min if 4 sec was taken for each response; however, participants completed the task easily and the average testing time was under 10 min.
Participants were tested supine in a whole‐head 151‐channel MEG (CTF Omega, MISL, Coquitlam, Canada). MEG data were recorded continuously with a 600‐Hz sampling rate, DC‐100 band‐pass, and third‐order spatial gradient noise cancelation. Data were processed offline. Head movement was monitored and all runs had <5 mm head movement. After completion of MEG testing, a structural MRI (T1‐weighted, 3D sagittal MPRAGE, TR/TE/TI/FA = 2300/2.96/900/9, GRAPPA = 2; FOV/Res = 192 × 240 × 256, 1.0 mm isotropic voxels) was obtained on a 3T scanner (Magnetom Tim Trio, Siemens AG, Erlangen, Germany) with a 12‐channel head coil.
Three fiducial coils were placed on each subject's nasion, and left and right preauricular points to allow tracking of the subject's head position within the MEG. After MEG testing, these coils were replaced with vitamin E capsules, visible on MRI, to allow coregistration of MEG data with structural MRI.
MEG connectivity analysis
A multisphere head model was created for each participant using their individual MRI.45 MEG data were downsampled to 667 Hz, and broadband (1–150 Hz) time series were reconstructed using a vector beamformer,46 from sources located at 90 cortical and subcortical seed points specified in the automated anatomical labeling atlas.47 SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2) was used to unwarp the coordinates for the seed points from standard MNI space to corresponding locations in each subject's individual head space.
The time series data were filtered into theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), and gamma (30–80 Hz) frequency ranges and the time series of instantaneous phase values were computed for each epoch, frequency, and subject using the Hilbert transform. The phase lag index was used to determine the task‐dependent connectivity dynamics for each frequency band.48 This produced, for each subject and frequency, a 90x90 adjacency matrix at each time point, which was then grand‐averaged across all subjects and source pairs to produce a time series plot of average network connectivity for each condition and group. This plot was used to determine time windows, where task‐dependent inter‐regional phase locking differed between groups or conditions, and thus merited further investigation.
To characterize task‐dependent changes in inter‐regional phase locking for a specific frequency band and condition, the 90x90 adjacency matrix for that frequency band and condition was averaged across all time points in a time window of interest. Baseline adjacency matrices were obtained by averaging an identical number of data points in the pre‐stimulus interval, and connectivity differences between active and baseline windows were evaluated using the network‐based statistic toolbox.49 This toolbox applied a univariate statistical threshold to identify the size of the largest interconnected component. A permutation approach was applied which shuffled group membership 5000 times to create a surrogate dataset for the null distribution. The “real” observed data were compared to the null distribution to obtain a statistical significance. As the same original univariate threshold was applied to both surrogate and real data, protection against false positives due to multiple comparisons is provided at any threshold.49, 50 The results were visualized using BrainNet Viewer51 and we plotted significant connections at a threshold of P < 0.05. For regions identified in this manner, graph theoretical analysis was used to derive network topologies which characterize their involvement in large‐scale task‐dependent brain networks (see Bullmore and Sporns52). Graph properties for these regions were calculated using the brain connectivity toolbox.53 To evaluate network topologies for these regions, we chose the graph theoretical measure of node strength. Strength reflects how functionally connected a given region is to other regions in the analyzed network.54, 55
Results
Participant demographics and clinical symptomatology
The control and mTBI groups were not significantly different on age (controls: 27.7 ± 5.3 years; mTBI: 31.0 ± 7.5 years; n.s.) and IQ (controls: 114.7 ± 8.31; mTBI: 106.7 ± 12.6; n.s.). However, significant differences were observed on the Patient Health Questionnaire (controls: 3.1 ± 5.4; mTBI: 9.0 ± 6.9; P < 0.01), the Symptom Checklist (controls: 2.3 ± 3.9; mTBI: 8.1 ± 5.9; P < 0.001), and the Symptom Severity Measure (controls: 5.7 ± 15.5; mTBI: 18.1 ± 18.4; P < 0.05), with the mTBI group more severely affected in all cases.
Reaction time and accuracy
Figure 1 contains the reaction time and accuracy results for each group in both the easy (ID) and difficult (ED) conditions. Two‐by‐two mixed factorial analyses of variance (ANOVAs) were performed with group as the between‐variable and condition as the repeated measures within variable for reaction time and accuracy separately. For reaction time (Fig. 1A), a main effect of condition (F[1, 13] = 11.0, P < 0.002) was found with the extradimensional, harder shift being slower. For accuracy (Fig. 1B), while both groups performed very well, a significant difference was found for condition (F[1, 30] = 5.72, P < 0.02). Post hoc testing revealed a significant difference between the ED (84.2 ± 4.2%) and ID (90.8 ± 4.5%) conditions for the mTBI group, although the interaction was not significant on the full factorial ANOVA.
Figure 1.
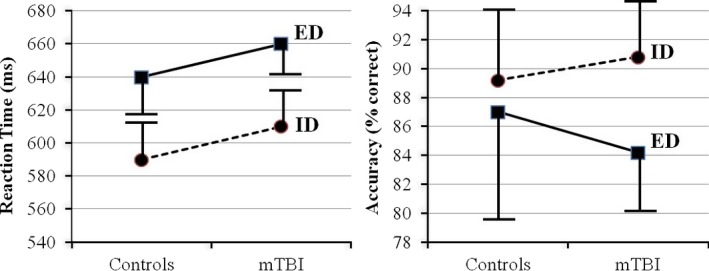
Mean and standard deviations for (A) reaction time and (B) accuracy for each group and condition.
Changes in connectivity during set shifting
Figure 2A shows task‐related changes in overall functional connectivity across time by frequency band, task difficulty, and group. Controls are shown in green and the mTBI group is shown in blue. Distinct peaks are seen in the theta, alpha, and beta bands that increased immediately after stimulus presentation and peaked at approximately 0.2 sec. The only significant differences between groups were for the difficult condition in the alpha band where the mTBI group showed lower connectivity in the 150–250 msec and 250–350 msec time windows. Subsequent analyses focus on these two time windows in the extradimensional condition.
Figure 2.
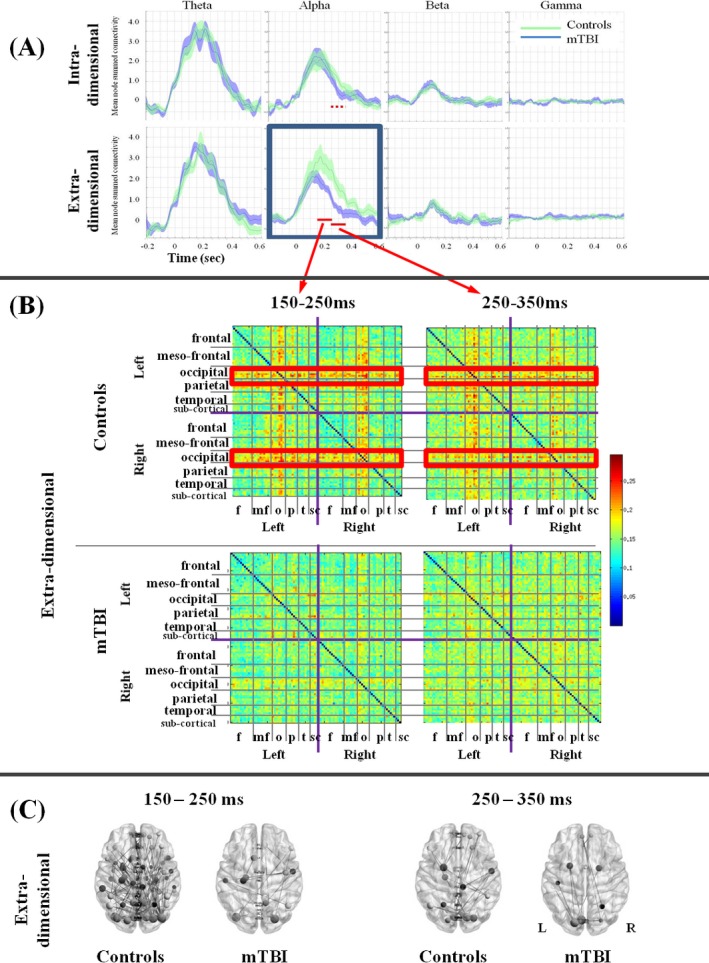
(A) Plots of whole‐brain connectivity changes over time in each frequency band, for the easy (intra‐) and hard (extradimensional) conditions, in the control and mild traumatic brain injury (mTBI) groups. The red bars indicate time windows where significant differences are seen between groups. (B) Adjacency matrices for the two time windows in the extradimensional condition for the two groups. Clear connections are seen in the left and right occipital regions with most other regions in both ipsilateral and contralateral cortex in the controls, but not in the mTBI group. (C) For the two time windows in the extradimensional condition, plots of connectivity strength are shown for the control and mTBI groups.
Figure 2B shows the adjacency matrices for the two groups, in the difficult (extradimensional) condition, for the two time windows of interest. In the control group, there are clear, strong, sustained connections between left and right occipital cortices with all brain other regions, both contra‐ and ipsilateral. This is not seen for the mTBI group. Figure 2C plots node strength connectivity (P < 0.05, corrected) in the alpha band for each group (suprathreshold t‐value = 4.0 for the first time window and t=3.0 for the second time window) on the ED condition for the two time windows of interest. The control group shows a number of long‐range connections, while the connections in the mTBI group are not well observed.
Discussion
In this study, we used a set‐shifting task, a measure of mental flexibility, in a group of control adults and adults with mTBI to assess differences in task‐based functional connectivity between groups. mTBI, by definition, is a mild condition where most individuals show few to no long‐term cognitive sequelae, although some individuals continue to complain of intermittent, remitting, and variable cognitive deficits. The results of our study are consistent with clinical reports where cognitive deficits are small but clear.
Our behavioral results showed that our task manipulation affected the groups differently. On reaction time, it is interesting to note that while both groups took longer to complete the more difficult extradimensional condition, there was no significant difference between the groups. However, while both groups perform comparably on accuracy for the intradimensional condition, the accuracy of the mTBI group decreases with the harder condition. Although this interaction is not statistically significant, inspection of Figure 1 would suggest that the mTBI group manages to maintain reaction time by trading off accuracy.
Our behavioral results fit with our connectivity data which showed specific reductions in the ability to recruit coordinated activity among brain regions to support task performance, only in the difficult condition. Examination of Figure 2A shows that the summed level of connectivity is not increased between the easy and hard conditions for the mTBI group, whereas the control group showed a “boost” of connectivity in the alpha band when challenged by the more difficult task; this probably allowed them to maintain accuracy or performance. For the mTBI group, with the easy task, the brain is driven at capacity, and there is no cognitive reserve to provide the additional “boost” that is needed for more difficult tasks. In this case, the result is a deterioration in accuracy.
When we examined the brain data by frequency bands, we found significant differences in connectivity only in the alpha band, and Figure 2B shows that the “boost” in alpha connectivity was between occipital regions to all other regions. There is evidence that alpha phase dynamics play a direct role in visual attention, and connectivity in the alpha band is required for higher order cognitive processing and consciousness (for a review, see Palva and Palva56, 57). Furthermore, an fMRI study of top‐down allocation of visual attention found that hemodynamic activations in healthy controls showed robust patterns of task‐related activations in bilateral dorsolateral prefrontal cortices and bilateral visual streams.19 Our findings suggest that the mTBI group were unable to boost long‐range alpha connectivity between occipital and frontal regions, possibly affecting their ability to appropriately allocate visual attention. This raises the possibility that the cognitive deficits reported in mTBI may not be due to specific damage to a specific brain region or neuronal mechanism, but the cognitive deficit culminates out of nonspecific visual attentional problems whereby an inadequate ability to allocate attention results in an inability to increase alpha connectivity to a level that maintains performance. In other words, an inability to boost alpha connectivity could result in an inability to activate the “global neuronal workspace” to allow further cognitive processing.56
To our knowledge, this article is the first to apply task‐based functional connectivity analyses to MEG data in mTBI. Our results demonstrate the feasibility of such an approach, support the hypothesis that there are cognitive consequences with even a mild brain injury, and suggest that a traumatic brain injury inflicts fundamental damage to the visual attention system which has a cascade effect on downstream processes. These findings have implications for how we think about future developments for intervention and rehabilitation for individuals with an mTBI. Rather than pursuing treatments focused on changing specific behavioral symptoms, perhaps rehabilitation that improves visual inattention may target the foundational deficit in this condition, and result in global cognitive improvements. It would be important, as a next step, to elucidate how the damage incurred by a mTBI translates into deficits in alpha connectivity and how this links to subsequent reductions in cognitive and behavioral performance.
Author Contributions
E. W. P., L. dC., and M. J. T. were involved in the conception and design of the study. L. dC. recruited the clinical participants and acquired the clinical data. B. T. D. and S. M. D. implemented and oversaw analysis of the connectivity data. All authors were involved in data interpretation and integration. E. W. P. prepared the manuscript. All authors edited the manuscript and approved the final version.
Conflicts of Interest
None declared.
Acknowledgments
The authors thank Matt J. MacDonald, Marc Lalancette, Amanda Robertson, and Allison Bethune for their assistance with data analysis, data acquisition, and patient recruiting. This work was supported by a Defence Research and Development Canada contract (# W7719‐135182/001/TOR) to E. W. P. and M. J. T.
References
- 1. Ruff R. Two decades of advances in understanding of mild traumatic brain injury. J Head Trauma Rehabil 2005;20:5–18. [DOI] [PubMed] [Google Scholar]
- 2. Willer B, Leddy JJ. Management of concussion and post‐concussion syndrome. Curr Treat Options Neurol 2006;8:415–426. [DOI] [PubMed] [Google Scholar]
- 3. Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995;45:1253–1260. [DOI] [PubMed] [Google Scholar]
- 4. Bigler ED. Neuropsychology and clinical neuroscience of persistent post‐concussive syndrome. J Int Neuropsychol Soc 2008;14:1–22. [DOI] [PubMed] [Google Scholar]
- 5. Rutherford WH. Sequelae of concussion caused by minor head injuries. Lancet 1977;1:1–4. [DOI] [PubMed] [Google Scholar]
- 6. Rutherford WH, Merrett JD, McDonald JR. Symptoms at one year following concussion from minor head injuries. Injury 1979;10:225–230. [DOI] [PubMed] [Google Scholar]
- 7. Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry 2003;15:310–316. [DOI] [PubMed] [Google Scholar]
- 8. Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil 2003;18:307–316. [DOI] [PubMed] [Google Scholar]
- 9. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol 2013;246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shenton ME, Hamoda HM, Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012;6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Helmer KG, Pasternak O, Fredman E, et al. Hockey Concussion Education Project, Part 1. Susceptibility‐weighted imaging study in male and female ice hockey players over a single season. J Neurosurg 2014;120:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasternak O, Koerte IK, Bouix S, et al. Hockey Concussion Education Project, Part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free‐water MRI study. J Neurosurg 2014;120:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasaki T, Pasternak O, Mayinger M, et al. Hockey Concussion Education Project, Part 3. White matter microstructure in ice hockey players with a history of concussion: a diffusion tensor imaging study. J Neurosurg 2014;120:882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karr JE, Areshenkoff CN, Garcia‐Barrera MA. The neuropsychological outcomes of concussion: a systematic review of meta‐analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology 2014;28:321–336. [DOI] [PubMed] [Google Scholar]
- 15. Messé A, Caplain S, Pélégrini‐Issac M, et al. Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav 2012;6:283–292. [DOI] [PubMed] [Google Scholar]
- 16. Sharp DJ, Scott G, Leech R. Network dysfunction after traumatic brain in jury. Nat Rev Neurol 2014;10:156–166. [DOI] [PubMed] [Google Scholar]
- 17. Stevens MC, Lovejoy D, Kim J, et al. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain Imaging Behav 2012;6:293–318. [DOI] [PubMed] [Google Scholar]
- 18. Mayer AR, Mannell MV, Ling J, et al. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp 2011;32:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayer AR, Yang Z, Yeo RA, et al. A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain Imaging Behav 2012;6:343–354. [DOI] [PubMed] [Google Scholar]
- 20. Tang L, Ge Y, Sodickson DK, et al. Thalamic resting‐state functional networks: disruption in patients with mild traumatic brain injury. Radiology 2011;260:831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hämäläinen MS. Magnetoencephalography: a tool for functional brain imaging. Brain Topogr 1992;5:95–102. [DOI] [PubMed] [Google Scholar]
- 22. Hari R, Salmelin R. Magnetoencephalography: from SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. NeuroImage 2012;61:386–396. [DOI] [PubMed] [Google Scholar]
- 23. Brookes MJ, Woolrich M, Luckhoo H, et al. Investigating the electrophysiological basis of resting state networks using magnetoencephalography. Proc Natl Acad Sci USA 2011;108:16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang M‐X, Nichols S, Robb A, et al. An automatic MEG low‐frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non‐blast causes. NeuroImage 2012;61:1067–1082. [DOI] [PubMed] [Google Scholar]
- 25. Huang M‐X, Theilmann RJ, Robb A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma 2009;26:1213–1226. [DOI] [PubMed] [Google Scholar]
- 26. Lewine JD, Davis JT, Bigler ED, et al. Objective documentation of traumatic brain injury subsequent to mild head trauma: multimodal brain imaging with MEG, SPECT and MRI. J Head Trauma Rehabil 2007;22:141–155. [DOI] [PubMed] [Google Scholar]
- 27. Tarapore PE, Findlay AM, LaHue SC, et al. Resting state magnetoencephalography functional connectivity in traumatic brain injury. J Neurosurg 2013;118:1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castellanos NP, Paúl N, Ordóñez VE, et al. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain 2010;133:2365–2381. [DOI] [PubMed] [Google Scholar]
- 29. Milner B. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch Neurol 1963;9:90. [Google Scholar]
- 30. Konishi S, Kawazu M, Uchida I, et al. Contribution of working memory to transient activation in human inferior prefrontal cortex during performance of the Wisconsin Card Sorting Test. Cereb Cortex 1999;9:745–753. [DOI] [PubMed] [Google Scholar]
- 31. Lie CH, Specht K, Marshall JC, et al. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. NeuroImage 2006;30:1038–1049. [DOI] [PubMed] [Google Scholar]
- 32. Monchi O, Petrides M, Petre V, et al. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event‐related functional magnetic resonance imaging. J Neurosci 2001;21:7733–7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagahama Y, Okada T, Katsumi Y, et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage 1999;10:193–199. [DOI] [PubMed] [Google Scholar]
- 34. Nagahama Y, Okada T, Katsumi Y, et al. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex 2001;11:85–392. [DOI] [PubMed] [Google Scholar]
- 35. Rogers RD, Andrews TC, Grasby PM, et al. Contrasting cortical and subcortical activations produced by attentional‐set shifting and reversal learning in humans. J Cogn Neurosci 2000;12:142–162. [DOI] [PubMed] [Google Scholar]
- 36. Wilmsmeier A, Ohrmann P, Suslow T, et al. Neural correlates of set‐shifting: decomposing executive functions in schizophrenia. J Psychiatry Neurosci 2010;35:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henaff M, Bayle D, Krolak‐Salmon P, Fonlupt P. Cortical dynamics of a self driven choice: a MEG study during a card sorting task. Clin Neurophysiol 2010;121:508–515. [DOI] [PubMed] [Google Scholar]
- 38. Perianez JA, Maestu F, Barcelo F, et al. Spatiotemporal brain dynamics during preparatory set shifting: MEG evidence. NeuroImage 2004;21:687–695. [DOI] [PubMed] [Google Scholar]
- 39. Wang L, Kakigi R, Hoshiyama M. Neural activities during Wisconsin Card Sorting Test – MEG observation. Brain Res Cogn Brain Res 2001;12:19–31. [DOI] [PubMed] [Google Scholar]
- 40. Oh A, Vidal J, Taylor MJ, Pang EW. Neuromagnetic correlates of intra‐ and extra‐dimensional set‐shifting. Brain Cogn 2014;86:90–97. [DOI] [PubMed] [Google Scholar]
- 41. Da Costa L, Robertson A, Bethune A, et al. Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. J Neurol Neurosurg Psychiatry 2015;86:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Pearson Education, Inc., 1999. [Google Scholar]
- 43. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 44. McCrory P, Meeuwisse W, Johnston K, et al. Consensus statement on Concussion in Sport 3rd International Conference on Concussion in Sport held in Zurich, November 2008. Clin J Sport Med 2009;19:185–200. [DOI] [PubMed] [Google Scholar]
- 45. Lalancette M, Quraan M, Cheyne D. Evaluation of multiple‐sphere head models for MEG source localization. Phys Med Biol 2011;56:5621–5635. [DOI] [PubMed] [Google Scholar]
- 46. Quraan M, Cheyne D. Reconstruction of correlated brain activity with adaptive spatial filters in MEG. NeuroImage 2010;49:2387–2400. [DOI] [PubMed] [Google Scholar]
- 47. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 48. Stam CJ, Nolte GN, Daffertshofer A. Phase lag index: assessment of functional connectivity from multichannel EEG and MEG with diminished bias from common sources. Hum Brain Mapp 2007;28:1178–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zalesky A, Fortino A, Bullmore E. Network‐based statistic: identifying differences in brain networks. NeuroImage 2010;53:1197–1207. [DOI] [PubMed] [Google Scholar]
- 50. Zalesky A, Cocci L, Fortino A, et al. Connectivity differences in brain networks. NeuroImage 2012;60:1055–1062. [DOI] [PubMed] [Google Scholar]
- 51. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 2013;8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bullmore E, Sporns O. Complex brain networks; graph theoretical analysis of structural and functional systems. Nat Neurosci 2009;10:186–198. [DOI] [PubMed] [Google Scholar]
- 53. Rubinov M, Sporns O. Complex measures of brain connectivity: uses and interpretations. NeuroImage 2010;52:1059–1069. [DOI] [PubMed] [Google Scholar]
- 54. Onnela JP, Saramaki J, Kertesz J, Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys 2005;71:065103. [DOI] [PubMed] [Google Scholar]
- 55. Yu S, Huang D, Singer W, Nikolic D. A small world of neuronal synchrony. Cereb Cortex 2008;18:2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palva S, Palva JM. Discovering oscillatory interaction networks with M/EEG: challenges and breakthroughs. Trends Cogn Sci 2012;16:219–230. [DOI] [PubMed] [Google Scholar]
- 57. Palva S, Palva JM. New vistas for alpha‐frequency band oscillations. Trends Neurosci 2007;30:150–158. [DOI] [PubMed] [Google Scholar]


