1. ABSTRACT
Single-molecule fluorescence resonance energy transfer (smFRET) has emerged as a powerful tool for mechanistic investigations of increasingly complex biochemical systems. Recently, we and others have successfully used smFRET to directly investigate the role of structural dynamics in the function and regulation of the cellular protein synthesis machinery. A significant challenge to these experiments, and to analogous experiments in similarly complex cellular machineries, is the need for specific and efficient fluorescent labeling of the biochemical system at locations that are both mechanistically informative and minimally perturbative to the biological activity. Here we describe the development of a highly-purified, fluorescently-labeled in vitro translation system that we have successfully designed for smFRET studies of protein synthesis. The general approaches we outline should be amenable to single-molecule fluorescence studies of other complex biochemical systems.
2. INTRODUCTION
Rapid and accurate translation of messenger RNA (mRNA) into the encoded protein product comprises a vital step in gene expression within all living cells. The central component of translation is the ribosome, a two-subunit, ribonucleoprotein-based molecular machine (Fig. 1A) which translocates along an mRNA template and synthesizes a polypeptide chain through the repetitive, mRNA-directed binding and incorporation of aminoacyl-transfer RNA (aa-tRNA) substrates (Fig. 1B). Throughout translation, a number of essential protein factors, termed initiation (IF), elongation (EF), release (RF) and ribosome recycling (RRF) factors interact with the ribosome, catalyzing many of the individual steps of translation and helping to ensure the overall speed and accuracy of protein synthesis (Liljas, 2004; Wilson et al., 2002).
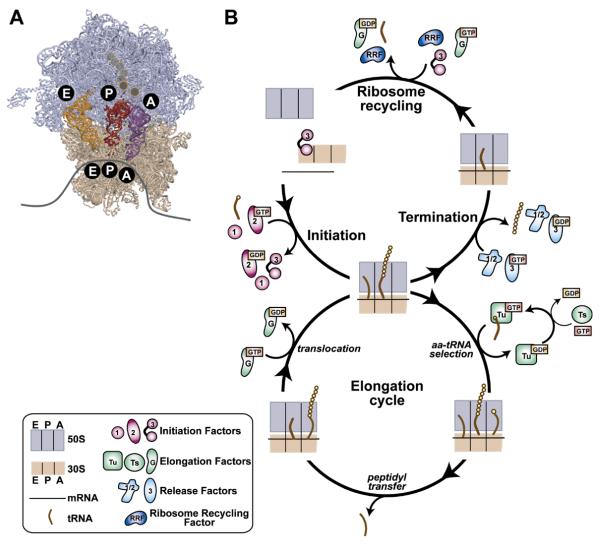
Figure 1.
Ribosome structure and protein synthesis. (A) X-ray crystallographic structure of the ribosome and its tRNA substrates (Selmer et al., 2006). The 50S ribosomal subunit is shown in lavender and the 30S ribosomal subunit in tan. The mRNA (cartooned as a gray curve) binds to the 30S subunit where the sequence of mRNA codons specifies the amino acid sequence of the protein to be synthesized. There are three tRNA binding sites on the ribosome specific for aa-tRNA (purple tRNA, A site), peptidyl-tRNA (red tRNA, P site), and deacylated-tRNA (orange tRNA, E site). (B) Cartoon representation of the translation cycle. During the initiation stage of translation, assembly of the 70S initiation complex from the 30S and 50S subunits, mRNA, and initiator tRNA, fMet-tRNAfMet, is mediated by IF1, 2 and 3. During the elongation stage of translation, the 70S ribosomal complex undergoes multiple rounds through the elongation cycle, with each cycle involving EF- Tu-catalyzed incorporation of the mRNA-encoded aa-tRNA, ribosome-catalyzed peptidyl transfer, and EF-G-catalyzed translocation of the mRNA-tRNA complex by one codon. Translocation of a stop codon into the A site triggers the termination stage of translation, during which RF1 or 2 hydrolyzes the newly-synthesized polypeptide chain followed by RF3-catalyzed dissociation of RF1/2. Finally, the post-termination ribosomal complex is disassembled during the ribosome recycling stage of translation by the action of RRF, EF-G, and IF3.
Recently, single-molecule fluorescence resonance energy transfer (smFRET) (Ha, 2001; Roy et al., 2008) has emerged as a powerful tool in mechanistic studies of protein synthesis (Frank and Gonzalez Jr., 2010; Marshall et al., 2008a). By combining the ability to monitor single molecules with a time-resolved, biophysical signal that is exquisitely sensitive to conformational changes, smFRET complements static structural and ensemble biochemical/biophysical studies by revealing the conformational trajectories of individual molecules in real time. Thus, smFRET studies often provide mechanistically-important dynamic data that are unavailable from static X-ray crystallographic and cryogenic electron microscopic structures and are obscured by the signal averaging inherent to biochemical/biophysical studies of asynchronous molecular ensembles.
smFRET studies of the mechanism through which the translating ribosome selects the correct, mRNA-encoded (i.e. cognate) aa-tRNA while discriminating against nearly-correct (i.e. near-cognate) aa-tRNAs (aa-tRNA selection step in Fig. 1B) provide one example of the type of mechanistic detail that is uniquely accessible to this approach (Blanchard et al., 2004a; Gonzalez et al., 2007; Lee et al., 2007). These smFRET studies revealed that incoming aa-tRNAs, delivered as a ternary complex with EF-Tu and GTP, sample a short-lived intermediate configuration on the ribosome that is decisive in discriminating cognate from near-cognate aa-tRNAs. This intermediate configuration of the ternary complex represents a critical branchpoint during aa-tRNA selection, at which the ribosome selectively permits a cognate ternary complex to progress forward in the reaction pathway but rapidly dissociates near-cognate ternary complexes. In this example, the asynchronous nature of ternary complex binding events amongst the ensemble of ribosomes, combined with the energetically unstable and short-lived nature of this ribosome-bound ternary complex configuration, yields an intermediate that is rarely populated and non-accumulating. As a result, this critical intermediate during aa-tRNA selection had gone unobserved and uncharacterized in ensemble biochemical/biophysical (Daviter et al., 2006) and static structural (Li et al., 2008; Ogle and Ramakrishnan, 2005; Schuette et al., 2009; Stark et al., 2002; Valle et al., 2003; Villa et al., 2009) studies. Additional examples of the contributions that smFRET studies have made to our mechanistic understanding of protein synthesis have been recently reviewed (Frank and Gonzalez Jr., 2010; Marshall et al., 2008a).
One of the most significant challenges to smFRET studies of complex biochemical systems such as the cellular protein synthesis machinery is the labeling of system components with the donor and acceptor fluorophores that are required to generate the smFRET signal. Fluorescent labeling for smFRET studies must be: (1) efficient, such that a large population of the observed molecules contain both a donor and an acceptor fluorophore; (2) specific, such that any heterogeneity detected over the entire population of observed molecules reflects the conformational heterogeneity of the molecular ensemble rather than heterogeneity in the positions of the donor or acceptor fluorophores; (3) mechanistically informative, such that the conformational change of interest yields a distance change between the donor and acceptor pair that generates a detectable change in FRET value; (4) minimally perturbative, such that the presence of the donor or acceptor fluorophore does not block or significantly interfere with the biochemical reaction under investigation.
Here, we describe a highly-purified in vitro translation system which, in combination with a series of standard biochemical assays, has allowed us to develop and validate numerous fluorescence labeling strategies for smFRET studies of protein synthesis. We present a general strategy for the design of fluorophore labeling positions, and describe the procedures used to generate site-specifically labeled ribosomes, translation factors, and tRNA constructs. These fluorescently-labeled translation components can then be tested using the biochemical assays described below in order to assess their compatibility with our in vitro translation system and, thus, their suitability for use in smFRET experiments. Many of the protocols we describe here are adaptations or modifications of protocols previously developed by numerous groups working on structural and mechanistic studies of protein synthesis. Thus, throughout this article we only briefly describe and provide references for those protocols that are used essentially as previously reported and describe in detail only those protocols that we have significantly modified or developed de novo. It is our hope that the general approaches we outline here will be applicable to smFRET investigations of other complex biochemical systems such as DNA replication, transcription, and pre-mRNA splicing.
3. A HIGHLY-PURIFIED, ESCHERICHIA COLI-BASED IN VITRO TRANSLATION SYSTEM
3.1. Tris-Polymix Buffer system
The Tris-Polymix Buffer used in our experiments is primarily based on the polymix buffer originally described by (Jelenc and Kurland, 1979) and further elaborated upon by (Pavlov and Ehrenberg, 1996; Wagner et al., 1982). We further optimized this polymix buffer by testing the protein synthesis activity of purified ribosomes (see below) within a partially-purified, fractionated in vitro translation system as described by (Chambliss et al., 1983). The mRNA template used for these buffer optimization experiments was an in vitro transcribed mRNA (McKenna et al., 2007; Milligan et al., 1987; Wyatt et al., 1991) encoding a C-terminal truncated variant of gene product 32 from bacteriophage T4, where the UUC codon encoding phenylalanine at position 225 was mutated to a UAA stop codon (hereafter referred to as T4gp321-224 mRNA). In these experiments, the yield and rate of T4gp321-224 synthesis was monitored by analyzing [35S]-methionine-labeled translation products by SDS-PAGE (Gallagher, 2006). The optimal buffer conditions, which were adopted for all of our biochemical and single-molecule experiments, are 50 mM Tris-acetate (Tris-HOAc) (pH25°C=7.5), 100 mM KCl, 3.5–15 mM Mg(OAc)2 (exact concentration depends on the nature of the experiment), 5 mM NH4OAc, 0.5 mM Ca(OAc)2, 6 mM β-mercaptoethanol (BME), 5 mM putrescine-HCl, and 1 mM spermidine-free base (Blanchard et al., 2004b).
3.2. Preparation and purification of ribosomes and ribosomal subunits
Highly-active, tightly-coupled E. coli 70S ribosomes are purified by preparative sucrose density gradient ultracentrifugation of S30 cleared lysates of E. coli strain MRE600 using a combination of the protocols reported by (Blanchard et al., 2004b; Powers and Noller, 1991) and (Robertson and Wintermeyer, 1981). The use of strain MRE600, which lacks the gene encoding the ribosomal RNA (rRNA)-active RNase I (Cammack and Wade, 1965), helps ensure the integrity of 70S ribosomes during purification. 70S ribosomes are distinguished by their sedimentation as intact 70S ribosomes, rather than as dissociated small (30S) and large (50S) ribosomal subunits, when centrifuged through sucrose density gradients containing a specified, low Mg2+ concentration (Hapke and Noll, 1976). The specific concentration of Mg2+ used to define tightly-coupled 70S ribosomes varies depending on the E. coli strain used (5.25 mM for MRE600 (Robertson and Wintermeyer, 1981)). Highly-active 30S and 50S subunits can be obtained by dissociating purified, tightly-coupled 70S ribosomes into their constituent 30S and 50S subunits via dialysis against buffer containing 1 mM Mg2+ and subsequently purifying the 30S and 50S subunits by preparative sucrose density gradient ultracentrifugation in buffer containing 1 mM Mg2+ (Powers and Noller, 1991; Recht et al., 1999).
3.3. Preparation of mRNAs
The mRNAs used for biochemical and smFRET studies in our laboratory are either chemically-synthesized (Dharmacon, Inc.) or in vitro transcribed using well-established protocols (McKenna et al., 2007; Milligan et al., 1987; Wyatt et al., 1991). Chemically-synthesized mRNAs are purified by the manufacturer using high-performance liquid chromatography and are resuspended in mRNA Buffer (10 mM Tris-HOAc (pH25°C=7.5), 10 mM KCl, and 0.1 mM EDTA) prior to use. In vitro transcription reactions are quenched by addition of 0.1× reaction volume of 500 mM EDTA, and the mRNA product is extensively buffer exchanged into mRNA Buffer and concentrated using a molecular weight cut off (MWCO)=10,000 centrifugal filtration device (Amicon Ultra, Millipore).
The mRNAs used in all of our studies are variants of the T4gp321-224 mRNA (Section 3.1) and are based on the following general sequence construct: 5'- [GG]CAACCUAAAACUUACACAGGGCCCUAAGGAAAUAAAAAUG(XYZ)n-3', where nucleotides that facilitate in vitro transcription are bracketed, nucleotides that serve as a target sequence for hybridizing a complementary, 3′-biotinylated DNA oligonucleotide (Integrated DNA Technologies; 5'-TGTGTAAGTTTTAGGTTGATTTG-Biotin-3') to enable surface-immobilization for smFRET studies (Zhuang et al., 2000) are underlined, the core Shine-Dalgarno ribosome binding site is underlined in bold, the AUG start codon encoding initiator fMet-tRNAfMet is underlined in italics, and the number of codons that are appended to the end of the general construct, which is variable depending on the study, are denoted by (XYZ)n.
3.4. Preparation and purification of fMet-tRNAfMet, Phe-tRNAPhe, and Lys-tRNALys
Overexpression vectors for E. coli methionyl tRNA synthetase and E. coli formylmethionyl-tRNA formyltransferase were provided by Prof. Sylvain Blanquet (CNRS-Ecole Polytechnique, Palaiseau Cedex, France), for phenylalanyl tRNA synthetase by Prof. David Tirrell (California Institute of Technology, Pasadena, CA, USA), and for lysyl tRNA synthetase by Prof. Takuya Ueda (University of Tokyo, Japan). Methionyl tRNA synthetase was prepared as reported in (Fourmy et al., 1991), formylmethionyl-tRNA formyltransferase as reported in (Schmitt et al., 1999), and phenylalanyl tRNA synthetase and lysyl tRNA synthetase as reported in (Shimizu et al., 2001).
The formyl donor substrate for formylmethionyl-tRNA formyltransferase, 10-formyltetrahydrofolate, is chemically prepared starting from the calcium salt of folinic acid (Acros Organics) as previously described (Dubnoff et al., 1971). Aminoacylation and formylation of tRNAfMet (Sigma or MP Biomedicals) is achieved simultaneously by incubating 20 μM tRNAfMet with 25 mM Tris-HCl (pH37°C=7.5), 7 mM MgCl2, 150 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol (DTT), 2.5 mM ATP, 300 μM 10-formyltetrahydrofolate, 80 μM methionine, 0.02 μM methionyl tRNA synthetase and 0.2 μM formylmethionyl-tRNA formyltransferase for 10 min at 37 °C. Aminoacylation of tRNAPhe (Sigma) is achieved by incubating 15 μM tRNAPhe (Sigma) with 200 mM Tris-HCl (pH37°C=7.5), 15 mM MgCl2, 25 mM KCl, 2 mM BME, 5 mM ATP, 10 mM phosphoenolpyruvate (PEP), 30 U ml−1 pyruvate kinase, 55 μM phenylalanine, and 0.75 μM phenylalanyl tRNA synthetase for 10 min at 37 °C. Aminoacylation of tRNALys (Sigma) is achieved by incubating 20 μM tRNALys (Sigma) with 50 mM Tris-HCl (pH37°C=7.5), 7 mM MgCl2, 150 mM KCl, 0.1 mM EDTA, 1 mM DTT, 2.5 mM ATP, 80 μM lysine, and 1.1 μM lysyl tRNA synthetase for 10 min at 37 °C.
All formylation and/or aminoacylation reactions are quenched by addition of 0.1× reaction volume of 3 M NaOAc (pH=5.2), extracted twice with 1× reaction volume of phenol, and extracted twice with 1× reaction volume of chloroform. tRNAs are then ethanol precipitated by addition of 3× reaction volume of −20 °C ethanol and incubation for a minimum of 1 h at −80 °C, followed by centrifugation for 15 min at 18,000×g at 4 °C. Pellets are resuspended in ice cold 10 mM KOAc (pH=5.0), passed through a Micro Bio-Spin 6 gel filtration spin column (Bio-Rad) equilibrated against ice cold 10 mM KOAc (pH=5.0), rapidly aliquoted, flash-frozen in liquid nitrogen, and stored at −80 °C. One aliquot is used to measure the final tRNA concentration using ultraviolet absorbance at 260 nm; the extinction coefficient at 260 nm for a particular species of purified tRNA can be estimated based on the amino acid acceptor activity of 1 A260 Unit of the purified tRNA, a value which is typically provided by the supplier. One A260 Unit is the amount of tRNA per 1 ml that yields an absorbance of 1 in a 1 cm light path cuvette at 260 nm.
tRNAfMet aminoacylation/formylation yields are assessed by hydrophobic interaction chromatography (HIC) on a TSKgel Phenyl-5PW column (8.0 mm (ID) × 7.5 cm (L)) (Tosoh Bioscience) operating at 4 °C using a previously described protocol (Schmitt et al., 1999). An aliquot from the aminoacylation/formylation reaction (~0.05 nmol of tRNA) is diluted 10-fold into ice cold tRNA HIC Buffer A (1.7 M NH4SO4, 10 mM NH4OAc (pH=6.3); note that the pH of the stock NH4OAc solution, rather than of the final tRNA HIC Buffer A, should be adjusted to 6.3), injected onto the Phenyl-5PW column pre-equilibrated against tRNA HIC Buffer A, and eluted using a linear gradient of 0–100% tRNA HIC Buffer B (10 mM NH4OAc (pH=6.3), 10% CH3OH; note that the pH of the stock NH4OAc solution, rather than of the final tRNA HIC Buffer B, should be adjusted to 6.3) over 25 column volumes. Due to the increasing hydrophobicity of deacylated tRNAfMet, Met-tRNAfMet, and fMet-tRNAfMet, these species elute from the Phenyl-5PW column at ~15.5%, ~18.5%, and ~24% tRNA HIC Buffer B, respectively, providing an effective means of assessing the yields of the aminoacylation/formylation reactions. This same protocol can be used to assess the yields of tRNAPhe and tRNALys aminoacylation reactions. In line with its increased hydrophobicity, Phe-tRNAPhe exhibits an increased retention volume relative to deacylated tRNAPhe, whereas the positively charged Nε of Lys-tRNALys generates a decreased retention volume relative to deacylated tRNALys. Based on this assessment, we are routinely able to achieve >90% aminoacylation/formylation of tRNAfMet, >90% aminoacylation of tRNAPhe, and ~60% aminoacylation of tRNALys.
3.5. Preparation and purification of translation factors
Genes encoding the ten canonical translation factors: IF1, 2 (γ isoform), and 3, EF-Tu, Ts, and G, RF1, 2, and 3, and RRF (Fig. 1B) were PCR amplified from E. coli K12 genomic DNA prepared as described (Wilson, 1988) or purchased from the American Type Culture Collection (ATCC #10798D-5). The PCR primers targeting each factor gene introduce appropriate restriction sites for cloning into the pProEX-HTb plasmid expression vector system (Invitrogen). Translation factor genes cloned into pProEX-HTb are placed under the control of an isopropyl β-D-1-thiogalactopyranoside (IPTG)-inducible pTrc promoter. In addition, the pProEX-HTb vector introduces a six-histidine (6xHis) affinity tag followed by a highly-specific tobacco etch virus (TEV) protease cleavage site at the amino terminus of the expressed factor. The 6xHis tag allows affinity purification of each factor using Ni2+-nitrilotriacetic acid (Ni2+-NTA) resin (Qiagen), and the TEV protease cleavage site allows subsequent removal of the 6xHis tag from the purified factor. Due to the sequence recognition and cleavage requirements of TEV protease as well as limitations in the restriction enzymes which can be used to clone the individual factor genes into pProEX-HTb, the N-terminus of each purified factor includes 1–5 additional, non-wild-type amino acids which precede the wild-type amino acid sequence. Thus, the N-terminal ends of each of our specific clones are: G-A-M1 (IF1), G-A-Q-D-D-M1 (IF2γ), G-A-M-A-K2 (IF3), G-A-M-G-S2 (EF-Tu), G-A-M1 (EF-Ts), G-A-M-G-S-A2 (EF-G), G-A-M1 (RF1), G-A-M1 (RF2), G-A-M1 (RF3), and G-A-M1 (RRF), where the underlined amino acid and sequence position denote the beginning of the wild-type gene sequence.
We have developed a general translation factor purification strategy based on standard Ni2+-NTA affinity purification procedures (Hoffmann and Roeder, 1991), which can be applied to all ten translation factors. For several factors this general strategy must be slightly modified to meet special conditions or expanded to include additional chromatographic steps in order to achieve high purity. Thus, in this paragraph we describe our general strategy and in the paragraphs that follow we describe special considerations specific to several factors. Each factor is overexpressed in BL21(DE3) cells in 1–2 L Terrific Broth (Difco) (Elbing and Brent, 2002) supplemented with 100 μg ml−1 α-carboxybenzylpenicillin (Sigma) (Raleigh et al., 2002). IPTG is added to a final concentration of 1 mM when the cell cultures reach an optical density of 0.8–1.0 at 600 nm. Overexpressing cells are grown for an additional 2–4 h at 37 °C (IFs, RFs, RRF) or overnight at 30 °C (EFs) and subsequently harvested by centrifugation at 5,000×g for 15 min at 4 °C. All subsequent steps are performed at 4 °C. The resulting cell pellet is resuspended into TF Buffer A (20 mM Tris-HCl (pH4°C=7.5), 300 mM NaCl, 10 mM imidazole, 0.2 mM phenylmethanesulphonyl fluoride (PMSF), and 2 mM BME) and lysed by passing through a French Press at an internal cell pressure of 1,200 psi. The resulting lysate is cleared by centrifugation at 20,000×g for 30 min. The cleared lysate is added to 2–3 ml Ni2+-NTA resin that has been pre-equilibrated with 5 column volumes of TF Buffer A, and the mixture is slowly stirred in a disposable polypropylene tube (BD Biosciences) for 30 min in order to allow binding of the 6xHis-tagged factor to the Ni2+-NTA resin. The Ni2+-NTA resin is then poured into a disposable polypropylene column (Pierce) and washed with 10 column volumes of TF Buffer B (TF Buffer A containing 30 mM imidazole). Bound 6xHis-tagged factor is eluted with 4 column volumes of TF Buffer C (Buffer A containing 500 mM NaCl and 250 mM imidazole) and collected over 4–10 fractions.
Factor-containing fractions are identified and initial purity assessed by SDS-PAGE and Coomassie staining (Sasse and Gallagher, 2009). Factor-containing fractions are combined, 0.05 mg 6xHis-tagged TEV protease (Promega) is added per 1 mg of factor (as measured by the Bradford assay (Simonian and Smith, 2006)), and the reaction mixture is dialyzed against TF Buffer D (20 mM Tris-HCl (pH4°C=7.5), 200 mM NaCl, 0.1% Triton-X, and 2 mM BME). The cleavage reaction is monitored by the change in molecular weight of the cleaved versus uncleaved factor using SDS-PAGE and Coomassie staining. Depending on the activity of the TEV protease and on the specific factor being purified, cleavage may require 12–48 h to go to completion. After TEV cleavage is complete, cleaved factor is separated from uncleaved factor, cleaved 6xHis-tag fragments, and the 6xHis-tagged TEV protease by adding the cleavage reaction to 2–3 ml Ni2+-NTA resin pre-equilibrated against TF Buffer D supplemented with 30 mM imidazole. If the volume of the cleavage reaction is significantly increased during the dialysis/cleavage procedure, the cleavage reaction may be concentrated prior to mixing with the Ni2+-NTA resin using a centrifugal filtration device (Millipore) with an appropriate molecular weight cutoff. The cleavage reaction/Ni2+-NTA resin mixture is slowly stirred in a disposable polypropylene tube for 1 h, poured into a disposable polypropylene column, and the flow-through containing the cleaved, purified factor is collected. The column is washed with 2 column volumes of TF Buffer D supplemented with 30 mM imidazole to collect any remaining cleaved, purified factor. The cleaved, purified factor is then buffer exchanged into 2× TF Buffer E (20 mM Tris-HOAc (pH4°C=7.5), 100 mM KCl, 10 mM BME) and concentrated using a centrifugal filtration device, diluted to 1× TF Buffer E by addition of 100% glycerol, and stored at −20 °C. Final concentrations of all translation factors are typically determined using the Bradford assay, with the exception of IF2γ and EF-G (see below). Approximate final protein yields are 0.5 mg per L culture for IF1, 8 mg L−1 for IF2γ, 1 mg L−1 for IF3, 10–20 mg L−1 for EF-Tu, 25–50 mg L−1 for EF-Ts, 40 mg L−1 for EF-G, 1.5 mg L−1 for RF1/2, 50 mg L−1 for RF3, and 10 mg L−1 for RRF.
3.5.1. Special considerations for IF1
IF1 Buffer A (10 mM Tris-HCl (pH4°C=7.5), 60 mM NH4Cl, 10 mM MgCl2, 5 mM BME, 0.1 mM PMSF, and 10 mM imidazole) replaces TF Buffer A. Cells are lysed by three passes through a French Press at an internal cell pressure of 1,200 psi. After batch binding of 6xHis-tagged IF1 to the Ni2+-NTA resin and transfer to a disposable polypropylene column, the resin is washed with 10 column volumes of IF1 Buffer B (IF1 Buffer A lacking NH4Cl and containing 30 mM imidazole) to remove non-specifically bound proteins. Bound, 6xHis-tagged IF1 is eluted with IF1 Buffer C (IF1 Buffer B containing 250 mM imidazole). 6xHis-tagged IF1 containing fractions are identified using a Tris-tricine gradient gel (10–20%) (Gallagher, 2006) with Coomassie staining. Fractions containing 6xHis-tagged IF1 are pooled and dialyzed against TF Buffer D overnight. TEV cleavage proceeds as described in the general protocol above, and the cleavage reaction is monitored by Tris-tricine gradient gel (10–20%) with Coomassie staining. Removal of the cleaved 6xHis-tag fragments and the 6xHis-tagged TEV protease is achieved by mixing the cleavage reaction with Ni2+-NTA resin that has been pre-equilibrated against TF Buffer D supplemented with 30 mM imidazole. The flow-through and wash containing cleaved, purified IF1 is passed through a HiLoad 16/60 Superdex 75 prep grade (GE Biosciences) gel filtration column using TF Buffer E as a column pre-equilibration and running buffer. IF1 elutes at a retention volume of ~87 ml. The fractions containing IF1 are pooled, buffer exchanged into 2× TF Buffer E and concentrated using a centrifugal filtration device, diluted to 1× TF Buffer E by addition of 100% glycerol, and stored at −20 °C.
3.5.2. Special considerations for IF2γ
TF Buffer A is supplemented with 0.22 U ml−1 DNase I (New England BioLabs). Prior to addition of TEV protease, the 6xHis-tagged IF2γ eluted from the Ni2+-NTA resin is diluted to a final concentration of ~0.25 mg ml−1 using IF2γ Buffer D (50 mM Tris-HCl (pH4°C=7.5), 50 mM KCl, 0.1% Triton-X, and 2 mM BME), before dialyzing against TF Buffer D. Prior to mixing the cleavage reaction with the Ni2+-NTA resin, the cleaved IF2γ is concentrated using a MWCO=10,000 centrifugal filtration device. Removal of the cleaved 6xHis-tag fragments and the 6xHis-tagged TEV protease is achieved by mixing the cleavage reaction with Ni2+-NTA resin that has been pre-equilibrated against TF Buffer D. The flow-through and two washes of 1 column volume each are collected, and the cleaved IF2γ is loaded onto a HiTrap SP HP cation exchange column (5 ml column volume) (GE Biosciences) pre-equilibrated against IF2γ Buffer IEX1 (40 mM Tris-HCl (pH4°C=7.5), 30 mM NaCl, 40 mM NH4Cl, 5 mM MgCl2, 2 mM BME). The column is washed with 5–10 column volumes of IF2γ Buffer IEX1 and IF2γ is eluted with a linear gradient of 0–75% IF2γ Buffer IEX2 (IF2γ Buffer IEX1 containing 750 mM NaCl) over 30 column volumes (Ayman Antoun et al., 2004). IF2γ elutes at ~33% IF2γ Buffer IEX2. Fractions containing purified IF2γ are pooled, buffer exchanged into 2× IF2γ Buffer E (20 mM Tris-HOAc (pH4°C=7.5), 100 mM KCl, 20 mM Mg(OAc)2, 10 mM BME) and concentrated using a centrifugal filtration device, diluted to 1× IF2γ Buffer E by addition of 100% glycerol, and stored at −20°C. The final concentration of IF2γ is measured using ultraviolet absorbance at 280 nm and a molar extinction coefficient of 27,390 M−1 cm−1, calculated using the ProtParam tool on the ExPASy Proteomics Server (http://ca.expasy.org/tools/protparam.html), which bases its calculation on protein amino acid composition in conjunction with the molar extinction coefficients of tyrosine, tryptophan, and cystine.
3.5.3. Special considerations for IF3
Purification of IF3 is identical to the procedure described above for IF1 up through collection of cleaved, purified factor from the second Ni2+-NTA column. At this point the cleaved IF3 is loaded onto a HiTrap SP HP cation exchange column (5 ml column volume) (GE Biosciences) pre-equilibrated against 5 column volumes of IF2γ Buffer IEX1. The column is washed with 3 column volumes of IF2γ Buffer IEX1 and IF3 is eluted with a linear gradient of 0–100% of IF2γ Buffer IEX2 over 20 column volumes. IF3 elutes at ~65% IF2γ Buffer IEX2. Fractions containing purified IF3 are pooled, buffer exchanged into 2× TF Buffer E and concentrated using a centrifugal filtration device, diluted to 1× TF Buffer E by addition of 100% glycerol, and stored at −20°C.
3.5.4. Special considerations for EF-Tu
TF Buffers A-E are supplemented with 0.2 mM GDP and 0.5 mM MgCl2. These supplements help to maintain the integrity of EF-Tu throughout the purification procedure and during storage at −20°C.
3.5.5 Special considerations for EF-G
The final concentration of EF-G is measured using ultraviolet absorbance at 280 nm and a molar extinction coefficient of 61,310 M−1 cm−1, calculated using the ProtParam tool on the ExPASy Proteomics Server (http://ca.expasy.org/tools/protparam.html) as described in Section 3.5.2.
3.5.6. Special considerations for RF1 and 2
RF1 and 2 (RF1/2) are post-translationally modified through methylation at residue Q235 (RF1) or Q252 (RF2) by an N5-glutamine methyltransferase encoded by the PrmC gene, and defects in the efficiency of translation termination have been clearly correlated with incomplete modification (Dincbas-Renqvist V et al., 2000; Heurgue-Hamard et al., 2002; Mora et al., 2007). Therefore, to prepare fully-modified RF1/2, we have co-transformed BL21(DE3) strains for RF1/2 overexpression with a plasmid-encoded copy of the PrmC gene, and RF1/2 are cooverexpressed together with their methyltransferase.
4. BIOCHEMICAL ASSAYS
4.1. Initiation assays
During translation initiation, IF1, 2, and 3 promote the formation of a 30S initiation complex that contains initiator fMet-tRNAfMet and the correct AUG start codon at the 30S P site. Docking of the 50S subunit onto the 30S initiation complex is then catalyzed by IF2 in its GTP-bound form, an event that stimulates GTP hydrolysis by IF2. Subsequent dissociation of the IFs yields a 70S initiation complex that is competent for formation of the first peptide bond (Fig. 1B). We typically use the standard assays described below to test the biochemical activities of initiation components.
4.1.1. Primer-extension inhibition assay
The activities of ribosomes, fMet-tRNAfMet, and IFs in initiation are tested using a well-established primer-extension inhibition, or “toeprinting,” assay (Hartz et al., 1989; Hartz et al., 1988). Briefly, initiation reactions are carried out on an mRNA that has been pre-annealed with a 5'[32P]-labeled DNA primer. Subsequent reverse transcription of the primer-annealed, initiated mRNA is strongly blocked when the reverse transcriptase encounters an mRNA-bound ribosome, thereby producing a 5'[32P]-labeled cDNA of defined length, or “toeprint.” Analysis of the cDNA products on a 9% sequencing PAGE gel (Slatko and Albright, 1992) therefore reports the position of the ribosome on the mRNA with single-nucleotide resolution. Three distinct toeprinting assays, described below, are used to test the individual activities of IF1, IF2γ, and IF3.
All toeprinting assays are performed using T4gp321-224 mRNA (Section 3.1) pre-annealed with a 5'[32P]-labeled DNA primer of sequence TATTGCCATTCAGTTTAG (Integrated DNA Technologies). The Primer Labeling Reaction is performed by mixing 70 pmol DNA primer, 42 pmol [γ-32P]ATP (6,000 Ci mmol−1, Perkin Elmer), and 14 Units T4 polynucleotide kinase (New England Biolabs) in a final reaction volume of 30 μl, prepared in 1× T4 polynucleotide kinase buffer (New England Biolabs) and incubating for 30 min at 37 °C. The labeling reaction is subsequently incubated for 10 min at 75 °C to inactivate the T4 polynucleotide kinase and unincorporated [γ-32P]ATP is removed using a G25 Sephadex gel filtration spin column (GE Healthcare). The Primer Annealing Reaction is performed by mixing 4 μl of the Primer Labeling Reaction with 100 pmol of T4gp321-224 in a final reaction volume of 40 μl, prepared in 25 mM Tris-HOAc (pH25°C=7.0), incubating in a dry block heater for 1.5 min at 90 °C, and slowly cooling to room temperature by transferring the dry block from the heater to the bench top.
The IF2γ assay tests the ability of IF2γ to direct the selection of fMet-tRNAfMet over elongator tRNA during initiation. The T4gp321-224 mRNA's AUG start codon, encoding tRNAfMet, is followed by a UUU triplet at the second codon position, encoding tRNAPhe. Binding of fMet-tRNAfMet to the AUG start codon at the 30S P site generates a toeprint at a position that is 15 nucleotides 3' to the A nucleotide of the AUG start codon (i.e. a +15 toeprint), whereas binding of tRNAPhe to the UUU codon at the 30S P site generates a toeprint at a position that is 18 nucleotides 3' to the A nucleotide of the AUG start codon (i.e. a +18 toeprint). Thus, selection of fMet-tRNAfMet over tRNAPhe using the T4gp321-224 mRNA can be easily observed by monitoring the intensity of the +15 toeprint relative to the intensity of the +18 toeprint. Each Initiation Reaction is performed in three steps:
A mixture of 10 pmol 30S subunits, 100 pmol IF2γ, and 16 nmol GTP is incubated for 10 min at 37 °C.
2 μl of the Primer Annealing Reaction is added to the reaction, followed by an additional 10 min incubation at 37 °C.
16 pmol each of fMet-tRNAfMet and tRNAPhe, prepared as an equimolar mixture, are added to the reaction, followed by an additional 10 min incubation at 37 °C.
The final reaction volume is 20 μl, prepared in Tris-Polymix Buffer (3 mM Mg2+). Initiation Reactions are placed on ice until ready for use in primer extension reactions.
Each Primer Extension Reaction is performed by mixing 5 μl of an Initiation Reaction with 30 nmol ATP, 12.5 nmol each of dATP, dGTP, dCTP, and dTTP, and 6 Units AMV reverse transcriptase (Promega) in a final reaction volume of 25 μl, prepared in Tris-Polymix Buffer (10 mM Mg2+), and incubating for 15 min at 37 °C. Primer Extension Reactions are extracted twice with 1× reaction volume of phenol and twice with 1× reaction volume of chloroform. cDNA products are ethanol precipitated by mixing Primer Extension Reactions with 0.1× reaction volume of 3 M Na(OAc) (pH=5.5) and 3× reaction volume of 100% ethanol, followed by incubation for 10 min at room temperature and centrifugation at 18,000×g for 10 min. The resulting cDNA pellets are washed once with 70% ethanol. The cDNA pellets are scintillation counted and ~5,000–10,000 counts per minute (cpm) are loaded into each lane of a 9% sequencing PAGE gel (40 cm × 20 cm, 0.2–0.4 mm thickness), which is run at a constant power of 55 W in 1× TBE (Tris/borate/EDTA) electrophoresis buffer (Moore, 2000). The gel is then dried and phosphorimaged using a STORM PhosphorImager (GE Healthcare).
Five control Primer Extension Reactions are typically performed with all toeprinting assays. The first four control Primer Extension Reactions are performed by mixing 3.5 μl of diluted Primer Annealing Reaction (diluted 2.5-fold with Tris-Polymix Buffer (10 mM Mg2+)), 50 nmol ATP, 20 nmol each of dATP, dGTP, dCTP, and dTTP, 10 nmol of either dideoxy ATP, GTP, CTP, or TTP, and 10 Units AMV reverse transcriptase in a final volume of 40 μl, prepared in Tris-Polymix Buffer (10 mM Mg2+), and incubating for 30 min at 37 °C. These mRNA sequencing reactions allow the +15 and +18 toeprint positions to be located within the T4gp321-224 mRNA. The fifth control Primer Extension Reaction is performed as described in the previous paragraph, but in the absence of added Initiation Reaction and incubated for only 15 min at 37 °C in order to detect intrinsic sites of reverse transcriptase stops caused by local secondary structures within the mRNA. The intensities of the bands corresponding to the +15 and +18 cDNA products in this control reaction are used to background correct the intensities of all +15 and +18 toeprints.
Four reactions are typically performed to test the activity of IF2γ. The first and second reactions are performed in the absence of IF2γ but in the presence of either fMet-tRNAfMet or tRNAPhe, in order to demonstrate that both tRNAs can actively bind to the 30S P site and generate strong +15 and +18 toeprints, respectively. The third and fourth reactions are run in the absence or presence of IF2γ and equimolar amounts of fMet-tRNAfMet and tRNAPhe. In the absence of IF2γ, one observes +15 and +18 toeprints of equal intensity, consistent with the inability of the mRNA-bound 30S subunit to discriminate between fMet-tRNAfMet and tRNAPhe in the absence of IF2γ. In the presence of IF2γ, however, one observes a very strong +15 toeprint and a missing or very weak +18 toeprint, demonstrating the ability of IF2γ to direct the selection of fMet-tRNAfMet over tRNAPhe.
The IF1 assay tests the ability of IF1 to enhance the formation of a correctly-initiated 70S initiation complex in the presence of IF2 and IF3 (Hartz et al., 1989). Each Initiation Reaction is prepared in four steps:
A mixture of 12 pmol each of 30S and 50S subunits are incubated for 10 min at 37°C.
12 pmol IF3, 48 pmol IF2γ, and 48 pmol IF1 are added to the reaction, followed by an additional 10 min incubation at 37 °C.
2.4 μl of Primer Annealing Reaction is added to the reaction, followed by an additional 10 min incubation at 37 °C.
35 pmol each of fMet-tRNAfMet and tRNAPhe, prepared as an equimolar mixture, are added to the reaction, followed by an additional 10 min incubation at 37 °C.
The final reaction volume is 26 μl, prepared in Tris-Polymix Buffer (5 mM Mg2+). Primer Extension Reactions and all subsequent steps are performed as in the IF2γ assay. Reactions in the absence and presence of IF1 are typically performed. An ~3-fold increase in the intensity of the +15 toeprint is observed in the presence versus the absence of IF1, demonstrating IF1's ability to enhance the formation of a correctly-initiated 70S initiation complex.
The IF3 assay demonstrates the ability of IF3 to regulate fMet-tRNAfMet selection on 30S subunits. Initiation Reactions are prepared in two steps:
A mixture of 2 pmol 30S subunits, 2 μl of diluted Primer Annealing Reaction (diluted 5-fold into Tris-Polymix Buffer (5 mM Mg2+)), 20 pmol tRNAfMet, and 200 pmol tRNAPhe are incubated for 10 min at 37°C.
24 pmol IF3 is added to the reaction, followed by an additional 10 min incubation at 37 °C.
The final reaction volume is 20 μl, prepared in Tris-Polymix Buffer (5 mM Mg2+). Primer Extension Reactions and all subsequent steps are performed as in the IF2γ assay. Reactions in the absence and presence of IF3 are typically performed. In the absence of IF3, the 10-fold molar excess of tRNAPhe produces a strong +18 toeprint relative to the +15 toeprint. In the presence of IF3, a strong +15 toeprint, relative to the +18 toeprint, is observed despite the 10-fold molar excess of tRNAPhe; this result demonstrates the ability of IF3 to regulate the binding of tRNAs to the 30S P site (Hartz et al., 1989; Hartz et al., 1988; Maar et al., 2008).
4.1.2. GTP hydrolysis assay
Ribosome-dependent, multiple-turnover GTP hydrolysis by IF2γ is assayed using [α-32P]GTP and thin layer chromatography as described by (Brandi et al., 2004), with several modifications. The reaction is performed in three steps:
A GTP/[α-32P]GTP Mix is prepared by mixing 200 nmol of GTP and 2 pmol [α-32P]GTP (3,000 Ci mmol−1, PerkinElmer) in a final volume of 1 ml, prepared in Barnstead NANOpure (Thermo Scientific) purified water and adjusted to pH=7.0 with 1 M KOH.
A 70S/IF2γ Mix is prepared by mixing 6 pmol 70S ribosomes (or the equivalent amounts of 30S and 50S subunits) with 18 pmol IF2γ in a final volume of 13 μl, prepared in Tris-Polymix Buffer (5 mM Mg2+).
2 μl of the GTP/[α-32P]GTP Mix is added to 13 μl of the 70S/IF2γ Mix and the reaction is incubated for 10 min at 37 °C.
The reaction is quenched by addition of 5 μl 100 mM EDTA (pH=9.5), heated at 95 °C for 1 min, and centrifuged for 5 min at 18,000×g. 2 μl of the supernatant is spotted onto a PEI-F cellulose thin layer chromatography (TLC) plate (EMD Chemicals), and separation of [α-32P]GTP and [α-32P]GDP is achieved using 0.9 M guanidine HCl as solvent (Bochner and Ames, 1982; Liu et al., 1998). The TLC plates are dried, phosphorimaged, and the extent of GTP hydrolysis is quantified by calculating the percentage of [α-32P]GTP hydrolyzed to [α-32P]GDP. Reactions in the absence of 70S ribosomes, IF2γ, or both, typically exhibit a basal level of ~1% hydrolysis. Reactions in the presence of all reaction components exhibit ~30% hydrolysis. An analogous assay is available for testing the GTPase activity of EF-G (Mohr et al., 2002).
4.2. Elongation
During each elongation cycle, aa-tRNA, in a ternary complex with EF-Tu and GTP, is selected and incorporated into the A site. Peptidyl transfer from the P-site peptidyl-tRNA to the newly-incorporated A-site aa-tRNA results in deacylation of the P-site tRNA and formation of a peptidyl-tRNA at the A site that has been elongated by one amino acid. Following peptidyl transfer, EF-G promotes translocation of the mRNA-tRNA complex by precisely one codon (Fig. 1B). We typically use the standard assays described below to test the biochemical activities of elongation components.
4.2.1. Primer-extension inhibition assay
The toeprinting assay used to test initiation components (Section 4.1.1) (Hartz et al., 1989; Hartz et al., 1988) can be easily adapted for testing the activities of ribosomes, aa-tRNAs, and EFs in elongation (Fredrick and Noller, 2003; Joseph and Noller, 1998).
An Initiation Reaction is performed in three steps:
A mixture of 35 pmol 70S ribosomes (or equivalent amounts of 30S and 50S subunits), 45 pmol IF1, 45 pmol IF2γ, 45 pmol IF3, and 40 nmol GTP is incubated for 10 min at 37 °C.
6.4 μl Primer Annealing Reaction (see Section 4.1.1) is added to the reaction, followed by a 10 min incubation at 37 °C.
45 pmol fMet-tRNAfMet is added to the reaction, followed by a 10 min incubation at 37 °C.
The final Initiation Reaction volume is 20 μl, prepared in Tris-Polymix Buffer (3 mM Mg2+). The Initiation Reaction is then placed on ice until use.
A Phe-tRNAPhe Ternary Complex is formed in three steps:
A GTP Charging Mix is prepared by mixing 200 nmol GTP, 600 nmol phosphoenolpyruvate, and 0.25 Units pyruvate kinase in a final volume of 20 μl, prepared in TC Buffer (50 mM Tris-HOAc (pHRT=7.5), 100 mM KCl, 50 mM NH4OAc, 1 mM Ca(OAc)2, 0.1 mM EDTA, 5 mM Mg(OAc)2 and 6 mM BME).
An EF-Tu(GTP)/EF-Ts Mix is prepared by mixing 320 pmol of EF-Tu, 240 pmol of EF-Ts, and 2.2 μl GTP Charging Mix in a final volume of 20 μl, prepared in TC Buffer, and incubating for 3 min at 37 °C.
30 pmol Phe-tRNAPhe is added to 15 μl EF-Tu(GTP)/EF-Ts Mix in a final volume of 20 μl, prepared in TC Buffer, and the reaction is incubated for another 3 min at 37 °C.
Phe-tRNAPhe Ternary Complex is then placed on ice until use.
EF-G(GTP) is prepared by mixing 260 pmol EF-G with 2 μl GTP Charging Mix in a final reaction volume of 20 μl, prepared in Tris-Polymix Buffer (10 mM Mg2+) and incubating for 3 min at 37 °C. EF-G(GTP) is then placed on ice until use.
Each Elongation Reaction is performed by mixing 12 μl of Initiation Reaction, 11.5 μl of Phe-tRNAPhe Ternary Complex, and 2 μl of EF-G(GTP) and incubating for 5 min at 37 °C. Elongation Reactions are quenched by addition of 0.1× reaction volume 10 mM viomycin (Joseph and Noller, 1998), a ribosome-targeting antibiotic that strongly inhibits EF-G-promoted translocation. Primer Extension Reactions and all subsequent steps are performed as described in Section 4.1.1. Reactions in the absence and presence of Phe-tRNAPhe Ternary Complex and/or EF-G(GTP) are typically performed. In the absence of Phe-tRNAPhe Ternary Complex and EF-G(GTP), a strong +15 toeprint corresponding to the initiated ribosomal complex is observed. In the absence of EF-G(GTP), Phe-tRNAPhe binding at the A site of the initiated ribosomal complex shifts the strong +15 toeprint to +16. In the presence of EF-G(GTP), Phe-tRNAPhe binding at the A site of the initiated ribosomal complex followed by EF-G-catalyzed translocation further shifts the strong +16 toeprint to +18. Translocation efficiency is estimated by dividing the intensity of the +18 toeprint by the sum of the intensities of the +15, +16 and +18 toeprints; we typically achieve ~90% translocation efficiency in the first round of elongation.
Toeprinting assays to assess two rounds of elongation (generating a +21 toeprint) can be achieved by performing all reactions as outlined above, with the exception that a second ternary complex, Lys-tRNALys Ternary Complex (decoding the third codon, AAA, in the T4gp321-224 mRNA), is formed following the same procedure as that for Phe-tRNAPhe Ternary Complex formation above. Elongation Reactions are performed by mixing 12 μl of Initiation Reaction, 11.5 μl of Phe-tRNAPhe Ternary Complex, and 2.5 μl of EF-G(GTP), and incubating the reaction for 5 min at 37 °C. This is followed by addition of 11.5 ul of Lys-tRNALys Ternary Complex to the reaction and an additional incubation for 5 min at 37 °C. Under these conditions, we typically achieve ~90% and ~70% translocation efficiencies in the first and second rounds of elongation, respectively.
4.2.2. Polypeptide synthesis assay
In addition to the primer-extension inhibition assay, the activities of ribosomes, aa-tRNAs, and EFs in elongation can be independently assayed using a well-established polypeptide synthesis assay (Weinger et al., 2004). Each Elongation Reaction is performed in four steps:
An Initiation Reaction is prepared as described in Section 4.2.1, with the exception that the Primer Annealing Reaction is replaced with 9.2 pmol T4gp321-224 mRNA and the fMet-tRNAfMet is replaced with 0.3 pmol of f-[35S]Met-tRNAfMet (prepared by aminoacylating/formylating tRNAfMet as described in Section 3.4, with the exception that the 80 μM methionine is replaced with 16 μM methionine and 4 μM [35S]methionine (1,175 Ci mmol−1, Perkin Elmer)).
Ternary Complexes are formed as described in Section 4.2.1, with the exception that the 30 pmol of Phe-tRNAPhe and, if included, 30 pmol Lys-tRNALys are decreased to 4.5 pmol each.
EF-G(GTP) is prepared as described in Section 4.2.1.
Elongation Reactions are performed as described in Section 4.2.1.
Elongation Reactions are quenched by addition of 0.5 M KOH to a final concentration of 150 mM. Quenched reactions are spotted onto pre-coated, plastic-backed cellulose TLC plates (EMD Chemicals) and f-[35S]Met, f-[35S]Met-Phe, and f-[35S]Met-Phe-Lys products are separated using electrophoretic TLC (eTLC) as described in (Youngman et al., 2004) using a 0.5% pyridine/20% glacial acetic acid buffer. eTLCs are run for 30 min at 1200V, air-dried, phosphorimaged, and quantified in order to determine the percentage of f-[35S]Met that is converted to f-[35S]Met-Phe and the percentage of f-[35S]Met-Phe converted to f-[35S]Met-Phe-Lys. We typically achieve ~70% conversion of f-[35S]Met to f-[35S]Met-Phe and ~75% conversion of f-[35S]-Met-Phe to f-[35S]-Met-Phe-Lys using wild-type translation components.
4.3. Termination
Once translocated into the ribosomal A site, stop codons are decoded by the class I release factors, RF1 or RF2. In response to a stop codon, RF1/2 binds at the A site and catalyzes hydrolysis of the nascent polypeptide chain from the P-site peptidyl-tRNA. Subsequently, the GTPase class II release factor, RF3, binds to the post-hydrolysis, RF1/2-bound ribosomal complex in its GDP form, couples GDP-to-GTP exchange with the dissociation of RF1/2, and couples ribosome-stimulated GTP hydrolysis by RF3 with the dissociation of RF3 from the ribosomal complex. We typically use a standard polypeptide release assay, previously developed by (Freistroffer et al., 1997), to test the biochemical activities of termination components.
4.3.1. Polypeptide Release Assay
The activity of RF1/2 in polypeptide release is determined by performing a single-round fMet-[14C]Phe dipeptide release assay in the presence of excess RF3 without any guanine nucleotide (Zavialov et al., 2001). An Elongation Reaction is performed in four steps:
An Initiation Reaction is prepared as described in Section 4.2.1, with the exception that the Primer Annealing Reaction is replaced with 40 pmol of a variant T4gp321-224 mRNA containing AUG-UUU-UAA as the first three codons (i.e. encoding fMet-Phe-STOP).
Phe-tRNAPhe Ternary Complex is formed as described in Section 4.2.1, with the exception that the 30 pmol Phe-tRNAPhe is replaced with 15 pmol of [14C]Phe-tRNAPhe, prepared by aminoacylating tRNAPhe as described in Section 3.4, with the exception that the 55 μM phenylalanine is replaced with 55 μM [14C]phenylalanine (450 mCi mmol−1, Perkin Elmer).
EF-G(GTP) is prepared as described in Section 4.2.1.
An Elongation Reaction is performed by mixing 20 μl of Initiation Reaction, 20 μl of Phe-tRNAPhe Ternary Complex, and 4 μl EF-G(GTP) and incubating for 5 min at room temperature.
An Elongation Reaction prepared in this way is stalled such that the stop codon at the third codon position of the mRNA resides at the A site. Free GTP and GDP are removed from the Elongation Reaction by buffer exchanging into Tris-Polymix Buffer (5 mM Mg2+) using two successive Micro Bio-Spin 30 gel filtration spin columns. The stalled Elongation Reaction is then aliquoted, flash frozen in liquid nitrogen, and stored at −80 °C.
Release Reactions are performed in two steps:
An (RF1/2)/RF3 Mix is prepared by mixing 0.05 pmol RF1/2 and 2 pmol RF3 in a final volume of 5 μl, prepared in Tris-Polymix Buffer (5 mM Mg2+).
5 μl Elongation Reaction and 5 μl (RF1/2)/RF3 Mix are pre-incubated separately for 1 min at 37 °C, mixed together, and incubated for an additional 1 min at 37°C.
Release Reactions are quenched and ribosomal complexes are precipitated by addition of 1× reaction volume of ice-cold 25% formic acid, incubation for 15 min on ice, and centrifugation at 14,000×g. The amount of [14C]Phe in the resulting pellet (containing unreacted ribosomal complexes still carrying P-site fMet-[14C]Phe-tRNAPhe as well as any free [14C]Phe-tRNA) and in the supernatant (containing released fMet-[14C]Phe dipeptide) is measured by scintillation counting and a calibration curve is used to convert the resulting cpm into molar amount of dipeptide released.
Typically three reactions are performed to determine the activity of RF1/2 in polypeptide release. In the first reaction, the 5 μl (RF1/2)/RF3 Mix is replaced with 5 μl of 0.2 mM puromycin. Puromycin is a ribosome-targeting antibiotic that mimics the aminoacyl-end of an aa-tRNA and quantitatively deacylates the P-site peptidyl-tRNA via peptidyl transfer; thus, the puromycin reaction reports on the total amount of P-site fMet-[14C]-Phe-tRNAPhe that is competent for hydrolysis by RF1/2 (typically ~85%). The second and third reactions are performed in the absence and presence of (RF1/2)/RF3 Mix. The reaction in the absence of (RF1/2)/RF3 Mix reports the amount of uncatalyzed, background fMet-[14C]Phe dipeptide release, which is subtracted from the amount of fMet-[14C]Phe dipeptide released from the reaction in the presence of (RF1/2)/RF3. Dividing this corrected amount of fMet-[14C]Phe dipeptide released from the reaction in the presence of (RF1/2)/RF3 Mix by the amount of RF1/2 in the reaction yields the percent activity of RF1/2. Typically, wild-type RF1/2 exhibits a percent activity of 30–40%, in line with previous measurements (Zavialov et al., 2001). The stop-codon dependence of RF1/2-catalyzed peptide release is tested by replacing the mRNA in the Elongation Reactions such that ribosomes become stalled at a lysine sense codon (AAA) instead of at a stop codon (UAA); in this case, wild-type RF1/2 exhibits an undetected level of polypeptide release activity.
RF3 catalyzes the dissociation of RF1/2 from the ribosome following polypeptide release, and is itself dependent on GTP hydrolysis for recycling off the ribosome. We therefore test RF3 activity by following the extent of polypeptide release in cases where RF1 is limiting and RF3 is required to actively recycle RF1, thereby enabling multiple turnover (Zavialov et al., 2001). All reactions are performed identically as above, with two major exceptions: when present, 2 nmol guanine nucleotide is added to the (RF1/2)/RF3 Mix and, upon adding the (RF1/2)/RF3/Nucleotide Mix to the Elongation Reaction, reactions are incubated for 10 min instead of 1 min. Typically reactions are performed without any nucleotide, with GDP, and with GTP; the dependence of multiple turnover fMet-[14C]Phe dipeptide release on RF3 and GTP can be readily observed.
4.4. Ribosome Recycling
Following termination and the dissociation of both class I and II release factors, the resulting 70S posttermination complex, which contains just the mRNA and deacylated P-site tRNA, is dissociated into its respective 30S and 50S subunits through the joint action of RRF and EF-G in a GTP-dependent reaction (Hirokawa et al., 2005) (Fig. 1B). While the precise role of IF3 during recycling is still debated (Seshadri and Varshney, 2006), it has been suggested that IF3 is dispensable for actual subunit splitting, but plays a critical role by binding to the 30S subunit and both preventing dissociated subunits from re-associating and promoting the ejection of deacylated tRNA and mRNA (Peske et al., 2005; Zavialov et al., 2005) (Fig. 1B). Here we describe a general assay, previously developed by (Hirokawa et al., 2005), to monitor subunit dissociation by sucrose density gradient ultracentrifugation.
4.4.1. Subunit Dissociation Assay
Recycling Reactions are performed by mixing 8 pmol 70S ribosomes, 800 pmol RRF, 800 pmol EF-G, 200 pmol IF3, and 20 nmol GTP in a final reaction volume of 40 μl, prepared in Tris-Polymix Buffer (6 mM Mg2+), and incubating for 20 minutes at 37 °C. After a brief incubation on ice, Recycling Reactions are loaded onto a 10%–40% sucrose density gradient in the same Tris-Polymix Buffer, and 30S and 50S subunits are separated from 70S ribosomes by ultracentrifugation in an SW40 rotor (Beckman Coulter) at 25,000 rpm for 12 h at 4 °C. Gradients are analyzed by monitoring the absorbance at 254 nm with a density gradient fractionator (Brandel) and 70S ribosome dissociation is qualitatively assessed by comparing the area of absorbance peaks corresponding to the dissociated 30S and 50S subunits with that corresponding to intact 70S ribosomes. Reactions are typically performed in the absence of all factors (i.e., with only 70S ribosomes), in the absence of just RRF, in the absence of just IF3, and with all of the factors present. The experiment performed in the absence of all factors reports on the extent of intrinsic subunit dissociation and typically yields predominantly intact 70S ribosomes. Similarly, predominantly intact 70S ribosomes are obtained in the absence of just RRF (since RRF is required for optimal subunit dissociation) or in the absence of just IF3 (since IF3 is required to prevent dissociated subunits from re-associating). The reaction in the presence of all factors, however, yields a significant population of dissociated 30S and 50S subunits, thereby demonstrating RRF's subunit dissociation activity (Sternberg et al., 2009).
5. PREPARATION OF FLUORESCENTLY-LABELED TRANSLATION COMPONENTS
The spectroscopic properties of the Cy3 and Cy5 cyanine fluorophores make them an excellent donor (Cy3) and acceptor (Cy5) pair for smFRET studies of biomolecular systems. The efficiency of FRET between Cy3 and Cy5, characterized by a Förster distance (R0) of ~55 Å (Bastiaens and Jovin, 1996; Hohng et al., 2004), is most sensitive to the distance between Cy3 and Cy5 within a distance range of ~35–75 Å, a length scale that is ideal for probing conformational changes within the translational machinery (the E. coli ribosome has maximum dimensions of ~250 Å in each direction (Schuwirth et al., 2005) and tRNAs are expected to move through the ribosome in a series of steps that are 10s of Å each, along a total path of length >100 Å (Korostelev et al., 2008)). Thus, we have made extensive use of the Cy3/Cy5 FRET pair in our smFRET studies of protein synthesis. We routinely make use of amine-, thiol-, and aldehyde-/ketone-reactive derivatives of Cy3 and Cy5, which are commercially available from GE Healthcare. In our studies, we primarily use N-hydroxysuccimidyl (NHS) ester or maleimide derivatives of Cy3/5 to specifically label molecular constructs containing a single, unique amine or thiol group, respectively. In the sections below, we provide general protocols for designing labeling schemes and for specifically labeling ribosomes, tRNAs, and translation factors for smFRET studies of protein synthesis.
5.1. Phylogenetic analysis/structural modeling
Generally speaking, the choice of labeling positions is guided by two criteria: (1) labeling positions should not be located within active sites or other highly-conserved regions in order to minimize the risk of interfering with biological activity; (2) the distance between Cy3 and Cy5 should be close to R0, where the FRET efficiency will be most sensitive to changes in distance (Lakowicz, 1999). In order to achieve these criteria, phylogenetic analysis and structural modeling of the target molecules and/or complexes is usually necessary. For our smFRET studies of protein synthesis, we typically perform phylogenetic analysis using multiple sequence alignments of protein or RNA sequences from a variety of bacterial species (~20–50 species) using BLAST (www.ncbi.nlm.nih.gov/BLAST) (Altschul et al., 1990) and CLUSTAL-W (www.ebi.ac.uk/clustalw) (Thompson et al., 1994). Poorly-conserved amino acid residues or nucleotides that are distal from active sites can be identified based on the alignments and selected as candidate positions for labeling.
Structural modeling usually involves the comparison of coordinates derived from cryo-EM reconstructions and/or X-ray crystal structures of relevant ribosomal complexes using molecular visualization software such as PyMOL (pymol.sourceforge.net) (DeLano, 2008) or Swiss PDB Viewer (spdbv.vital-it.ch) (Guex and Peitsch, 1997). Typically, superpositions of various functionally-related complexes are performed in order to identify Cy3 and Cy5 labeling positions where the conformational rearrangement of interest is expected to result in a relative distance change between the two fluorophores that corresponds to a maximal change in FRET (bearing in mind that the FRET efficiency of the Cy3/Cy5 FRET pair is most sensitive to changes in distance for inter-fluorophore distances in the range of ~35–75 Å). Based on the results of phylogenetic analysis and structural modeling, we typically design a minimum of three candidate labeling constructs which are generated, fluorescently-labeled, biochemically assayed, and used for preliminary smFRET experiments. Based on the results of these experiments, the optimal construct is identified and chosen for detailed biochemical characterization and smFRET data collection.
5.2. Ribosome labeling
Ribosomes can be fluorescently labeled at either rRNA or ribosomal proteins (r-proteins), depending on the specific experiment. An approach for labeling the ribosome based on hybridization of fluorescently-labeled oligonucleotides to helical extensions engineered into surface-exposed rRNA hairpins has been described (Dorywalska et al., 2005) and one such construct has been recently used to conduct smFRET studies of ribosome dynamics (Marshall et al., 2009; Marshall et al., 2008b). Here we describe a method for labeling ribosomes that involves reconstitution of fluorescently-labeled r-proteins into mutant ribosomes lacking the target r-proteins. In the sections below we describe our general approach, developed using r-proteins L1 and L9 as targets (Fei et al., 2009; Fei et al., 2008; Sternberg et al., 2009).
5.2.1. Preparation of mutant ribosomes lacking target r-proteins
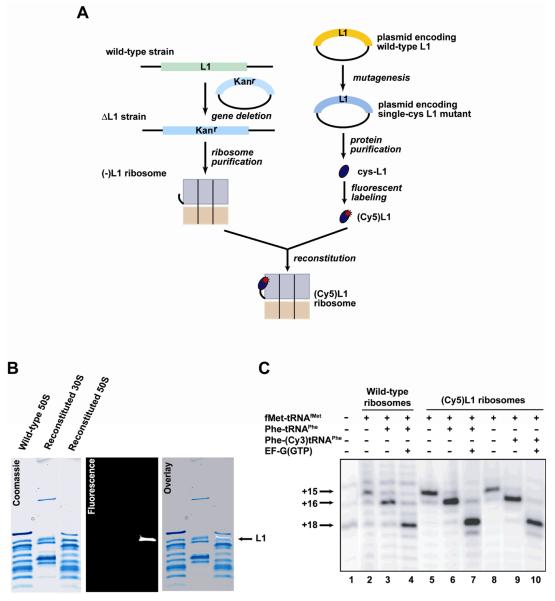
Ribosomes lacking a single r-protein (Fei et al., 2008) are obtained from single-deletion E. coli strains generated using a one-step gene deletion technique originally developed by (Baba et al., 2006; Datsenko and Wanner, 2000) (Fig. 2A). Briefly, strain BW25113, a recombination-proficient derivative of E. coli K12, is transformed with the Red helper plasmid pKD46 encoding the λ Red recombination system under the control of the arabinose-inducible, ParaB promoter. A linear DNA fragment targeting the r-protein gene of interest is constructed by PCR amplification using plasmid pKD13 (carrying a kanamycin resistance cassette) or pKD3 (carrying a chloramphenicol resistance cassette) as a template. The 3'-ends of the PCR primers used to generate the linear DNA fragment contain ~20 nucleotides complementary to the sequences flanking the antibiotic resistance genes in pKD13 or pKD3 while the 5'-ends contain ~50 nucleotide extensions homologous to E. coli chromosomal sequences immediately upstream and downstream of the gene encoding the target r-protein. 500–800 ng of linear DNA fragment is electroporated into electrocompetent BW25113(pKD46) cells. Cells are grown in antibiotic-free SOC media supplemented with 1 mM of L-arabinose for 2 h at 37 °C, spread onto agarose plates supplemented with the appropriate antibiotic (30 μg ml−1), and incubated at 37 °C. Antibiotic resistant colonies are selected and grown in Luria-Bertani (LB) media (Difco) and gene deletion is verified by PCR amplification of the targeted region of the chromosome and DNA sequencing.
Figure 2.
Preparation and characterization of (Cy5)L1 ribosomes. (A) Generation of (Cy5)L1 ribosomes. Ribosomes lacking r-protein L1 ((−)L1 ribosomes) are purified from an E. coli strain in which the gene encoding r-protein L1 has been deleted by an in-frame knock out (ΔL1). In parallel, r-protein L1 is cloned and mutagenized to generate a single-cysteine variant. The single-cysteine mutant L1 is purified and labeled with Cy5-maleimide. (Cy5)L1 is then in vitro reconstituted with (−)L1 ribosomes in order to generate (Cy5)L1-labeled ribosomes. (B) Incorporation of (Cy5)L1 into (−)L1 ribosomes. Coomassie staining (left), fluorescence scanning (middle), and overlay (right) of an SDS-PAGE gel containing ribosomal proteins extracted from wild-type and reconstituted ribosomal subunits. (C) Elongation toeprinting assay. The activities of unlabeled and (Cy3/5)-labeled translation elongation components are tested by a standard toeprinting assay. cDNA bands corresponding to mRNA positions +15, +16 and +18, relative to the A of the AUG start codon, report on the formation of a 70S initiation complex (+15), the incorporation of the first A-site aa-tRNA (Phe-tRNAPhe) (+16), and a single translocation step (+18). Lane 1 is a control primer extension of the mRNA in the absence of any translation components that is used to detect sites of reverse transcriptase inhibition caused by local secondary structures within the mRNA. The intensities of the bands corresponding to the +15, +16, and +18 toeprints in this lane are used to correct the raw intensities of the +15, +16, and +18 toeprints in Lanes 2–10. The activities of unlabeled ribosomes with unlabeled Phe-tRNAPhe (compare Lane 2 with Lanes 3 and 4), (Cy5)L1 ribosomes with unlabeled Phe-tRNAPhe (compare Lane 5 with Lanes 6 and 7), and (Cy5)L1 ribosomes with Phe-(Cy3)tRNAPhe (compare Lane 8 with Lanes 9 and 10) are indistinguishable. Comparison of the corrected intensities of the +15 and +18 toeprints in Lanes 4, 7, and 10 suggests that for all combinations of unlabeled and labeled components, 70S initiation complexes are ~90% active in the first round of elongation.
Ribosomes lacking two r-proteins (Fei et al., 2009) are obtained from double-deletion E. coli strains generated by P1 vir phage transduction of a donor single-deletion strain into a recipient single-deletion strain (Goldberg et al., 1974; Moore, 2009; Wall and Harriman, 1974). In our case, we construct a chloramphenicol resistant donor single-deletion strain using pKD3 and a kanamycin resistant recipient single-deletion strain using pKD13. Using these donor and recipient single-deletion strains, we then follow a variation of the phage P1 vir transduction protocols developed by Sauer and co-workers (http://openwetware.org/wiki/Sauer:P1vir_phage_transduction). Briefly, a 2.5 ml culture of the donor single-deletion strain is infected with P1 vir phage and grown for 1–3 h until the culture becomes clear, indicating that the cells have been completely lysed. The resulting lysate contains transducing particles which carry random fragments of the donor single-deletion strain genome, including the kanamycin resistance gene; this lysate is used to infect a liquid culture of the recipient single-deletion strain. Double-deletion mutants are selected on agarose plates supplemented with both kanamycin and chloramphenicol. Antibiotic resistant colonies are selected and grown in LB media, and gene deletion is verified by PCR amplification and DNA sequencing.
Single- and double-deletion strains may exhibit a slow-growth phenotype whose severity will depend on the specific r-protein(s). In our case, the growth rate of the L9 single-deletion strain was comparable to that of the wild-type BW25113 strain, while the doubling times of the L1 single-deletion strain and L1/L9 double-deletion strain were ~2-fold and ~6-fold slower than the wild-type strain, respectively. Tightly-coupled 70S ribosomes lacking one or two r-proteins are purified from single- or double-deletion BW25113 strains, respectively, using the protocol described in Section 3.2.
5.2.2. Preparation of fluorescently-labeled r-proteins
Fluorescently-labeled r-proteins are prepared in four steps:
The target r-protein genes are PCR-amplified from C600 genomic DNA and cloned into the pProEX-HTb plasmid system (Section 3.5).
Cloned r-protein genes are mutagenized using the QuickChange Mutagenesis Kit (Stratagene) to mutate wild-type cysteine residues to non-reactive amino acids (serine is a typical structurally- and chemically-conservative choice) and to introduce a unique cysteine residue at a position selected through phylogenetic analysis and structural modeling (Section 5.1).
Single-cysteine r-protein mutants are overexpressed and purified under denaturing conditions (described below).
Single-cysteine r-protein mutants are labeled with maleimide derivatives of Cy3/5 (described below).
Overexpression and purification of r-proteins follows the protocol for translation factor purification presented in Section 3.5, with the following modifications. Cells from a 500 ml culture are lysed in r-Protein Buffer A (50 mM Tris-HCl (pH4°C=8), 5 mM MgCl2, 0.1 mM PMSF, and 5 mM BME) and the resulting lysate is cleared by centrifugation at 10,000×g for 45 min at 4 °C. An SDS-PAGE gel is used to determine whether the majority of the overexpressed r-protein partitions into the supernatant or into insoluble inclusion bodies that co-sediment with the cell pellet. For r-proteins that primarily partition into inclusion bodies, such as L1 and L9, the pellet is resuspended in r-Protein Buffer B (10 mM Tris-HCl (pH4°C=8), 100 mM NaH2PO4 (pH=8), 6 M urea, 0.1 mM PMSF, and 5 mM BME) by gently stirring overnight at 4 °C. For r-proteins that primarily partition into the supernatant, the supernatant is dialyzed against r-Protein Buffer B overnight at 4 °C. The resulting r-protein mixture is cleared again by centrifugation at 12,000×g for 30 min at 4 °C. 6xHis-tagged r-proteins are purified as described in Section 3.5 with the exception that the Ni2+-NTA column is washed with 8 column volumes of r-Protein Buffer C (r-Protein Buffer B adjusted to pH4°C=6.7) and r-proteins are eluted with r-Protein Buffer D (r-Protein Buffer B adjusted to pH4°C=5.5). r-Protein-containing fractions are combined, diluted to an r-protein concentration of 0.1–0.2 mg ml−1 (as measured by the Bradford assay), and dialyzed extensively against r-Protein Buffer E (50 mM Na2HPO4 (pH=7.0), 100 mM NaCl, and 2 mM BME) to remove urea and renature the r-protein. Renatured r-protein is concentrated to 0.5–1 mg ml−1, 6xHis-tagged TEV protease is added, and dialysis against r-Protein Buffer E is continued. Cleaved r-protein is separated from uncleaved r-protein, 6xHis-tag fragments, and 6xHis-tagged TEV protease using a second Ni2+-NTA column as described in Section 3.5 with the exception that the Ni2+-NTA resin is pre-equilibrated against r-Protein Buffer E. The cleaved, purified r-protein is dialyzed or gel filtered into 2× r-Protein Buffer F (50 mM Na2HPO4 (pH=7.0), 200 mM NaCl, and 2 mM BME), concentrated using a centrifugal filtration device, diluted to 1× r-Protein Buffer F by addition of 100% glycerol, and stored at −20 °C. Final yields of ~10–20 mg of r-protein per L culture are typically obtained.
Fluorescent labeling of r-proteins is generally performed in a Tris- or phosphate-based Labeling Buffer at pH=7.0–7.5, with the exact composition varying depending on the specific r-protein. As examples, L1 Labeling Buffer is composed of 100 mM Na2HPO4 (pH = 7.2), 100 mM NaCl, and a 100-fold molar excess of tris(2-carboxyethyl)phosphine hydrochloride (TCEP, a non-thiol-containing reducing agent which selectively reduces disulfides) over L1, while L9 Labeling Buffer is composed of 50 mM Tris-HCl (pHRT=7.2), 200 mM KCl, 4 M urea, and a 100-fold excess of TCEP over L9. r-Protein is buffer exchanged into Labeling Buffer and concentrated to ~40 μM using a centrifugal filtration device, and the resulting solution is incubated for 30 min at room temperature in order to fully reduce r-protein disulfide bonds. A 20-fold molar excess of Cy3/5-maleimide, pre-dissolved in a minimum volume (typically less than 5% of the total reaction volume) of anhydrous dimethyl sulfoxide (DMSO), is added to the r-protein solution and the labeling reaction is incubated for 2 h at room temperature followed by a minimum of 5 h at 4 °C. The reaction is quenched by adding BME to a final concentration of 6 mM. Labeled proteins are separated from unreacted, free Cy3/Cy5 using a HiLoad 16/60 Superdex 75 prep grade gel filtration column (GE Healthcare) pre-equilibrated against Gel Filtration Buffer. Again, the exact composition of the Gel Filtration Buffer will vary depending on the r-protein; L1 Gel Filtration Buffer is 20 mM Tris-HCl (pHRT=7.8), 200 mM NaCl, 2 mM MgCl2, and 6 mM BME) and L9 Gel Filtration Buffer is 20 mM Tris-HCl (pHRT=7.8), 400 mM NH4Cl, 4 mM MgCl2, and 6 mM BME. The labeling efficiencies are typically 65–100% for L1 and ~50% for L9.
5.2.3. Reconstitution of fluorescently-labeled r-proteins into mutant ribosomes lacking target r-proteins
Reconstitution generally involves incubation of mutant ribosomes lacking the target r-protein(s) with a molar excess of the purified r-protein(s). The specific concentrations of ribosomes and r-protein(s), as well as the buffer conditions, incubation time, and temperature, will generally need to be optimized for specific r-protein(s). As a starting point, here we provide references and protocols for reconstituting (Cy3/5)L1 and (Cy3/5)L9 into 50S subunits lacking L1 ((−)L1), L9 ((−)L9), or both L1 and L9 ((−)L1/L9). (Cy3/5)L1 is reconstituted into (−)L1 50S subunits by incubating 1.8 nmol (Cy3/5)L1 and 1.2 nmol (−)L1 50S subunits in 300 μl of L1 Reconstitution Buffer (10 mM Tris-HCl (pH37°C=7.5), 8 mM Mg(OAc)2, 150 mM NH4Cl, and 5 mM BME) for 10 min at 35 °C (Odom et al., 1990) (Fig. 2A). (Cy3/5)L9 is reconstituted into (−)L9 50S subunits by incubating 1.8 nmol (Cy3/5)L9 and 1.2 nmol (−)L9 50S subunits in 300 μl of L9 Reconstitution Buffer (50 mM HEPES(KOH) (pH37°C=7.5), 4 mM MgCl2, 400 mM NH4Cl, 6 mM BME, and 0.1% Nikkol) for 15 min at 37 °C (Ermolenko et al., 2007). (Cy3/5)L1 and (Cy3/5)L9 are reconstituted into (−)L1/L9 50S subunits by incubating 1.8 nmol (Cy3/5)L1 and 1.2 nmol (−)L1/L9 50S subunits in 300 μl of L1/L9 Reconstitution Buffer (20 mM Tris-HCl (pHRT=7.85), 4 mM MgCl2, 400 mM NH4Cl, and 6 mM BME) for 15 min at 37 °C followed by addition of 1.8 nmol (Cy3/5)L9 and an additional 10 min incubation at 37 °C. Reconstituted, fluorescently-labeled 50S subunits are purified from unincorporated (Cy3/5)L1 and/or (Cy3/5)L9 using sucrose density gradient ultracentrifugation (Section 3.2). Under these conditions we achieve reconstitution efficiencies of ~100% for (Cy3/5)L1 (Fig. 2B) and ~60% for (Cy3/5)L9 (Fei et al., 2009; Fei et al., 2008). Reconstituted, fluorescently-labeled 50S subunits are fully active in the elongation toeprinting assay described in Section 4.2.1 (Fig. 2C) (Fei et al., 2009; Fei et al., 2008).
5.3. tRNA labeling
5.3.1. tRNAfMet labeling
Fluorescent labeling of initiator tRNAfMet at the 4-thiouridine at nucleotide position 8 (s4U8) is achieved via reaction with Cy3/5-maleimide using slight modifications of a previously published protocol (Carbon and David, 1968). Labeling is achieved by incubating 13 nmol tRNAfMet and 650 nmol Cy3/5-maleimide in 150 μl tRNAfMet Labeling Buffer (50 mM Tris-HCl (pH37°C=7.8)) for 5 h at 37 °C. The labeling reaction is quenched with 0.1× reaction volume of 3 M NaOAc (pH=5.5). Multiple extractions with 1× reaction volume phenol are performed until unreacted Cy3/5 is no longer visibly extracted (this typically requires approximately 6 phenol extractions). Phenol phases are saved and back-extracted with 0.25× volume of 0.4 M NaOAc (pH=5.5) and the back-extracted aqueous phase is combined with the original aqueous phase. The pooled sample is extracted twice with 1× reaction volume chloroform, and ethanol precipitated by addition of 3× reaction volume of −20°C ethanol and overnight incubation at −20°C, and finally centrifuged at 18,000×g for 20 min at 4 °C.
The tRNAfMet pellet is resuspended in tRNA HIC Buffer A and (Cy3/5)tRNAfMet is separated from unlabeled tRNAfMet using HIC as described in Section 3.4. (Cy3)tRNAfMet elutes from the Phenyl-5PW column at ~34.5% tRNA HIC Buffer B whereas (Cy5)tRNAfMet typically elutes as two peaks at ~36.5% and ~45% tRNA HIC Buffer B. While it is currently not known why (Cy5)tRNAfMet elutes as two peaks, it is possible that the two peaks arise from the distinct hydrophobicities of two interconverting isomers of (Cy5)tRNAfMet; in support of this possibility, when the peak eluting at 45% tRNA HIC Buffer B is collected, incubated at 37 °C for 10 min, and re-injected onto the Phenyl-5PW column, two peaks are again eluted with the same retention times as before. Using this protocol, a labeling efficiency of ~2–5% is typically achieved. HIC fractions containing the ~95–98% of unlabeled tRNAfMet can be re-labeled as described above, yielding a similar, ~2–5% labeling efficiency; this observation suggests that the degree of s4U8 modification within the tRNAfMet sample is not limiting the reaction. Instead, it is likely that hydrolysis of the Cy3/5-maleimide to Cy3/5-maleamic acid effectively outcompetes reaction of Cy3/5-maleimide with the thione group of s4U8 (Carbon and David, 1968). Attempts to further optimize reaction conditions in order to obtain labeling efficiencies above 5% have not been successful. The eluted (Cy3/5)tRNAfMet is buffer exchanged and concentrated into Barnstead NANOpure water using a centrifugal filter device (MWCO=10,000). We routinely achieve >90% aminoacylation/formylation efficiency of (Cy3/5)tRNAfMet using the procedures described in Section 3.4, with the exception that the concentrations of methionyl tRNA synthetase and formylmethionyl-tRNA formyltransferase are increased to 0.2 μM and 2 μM, respectively. fMet-(Cy3)tRNAfMet elutes from the Phenyl-5PW column at ~41.5% tRNA HIC Buffer B and the two fMet-(Cy5)tRNAfMet peaks elute at ~43% and ~51% tRNA HIC Buffer B. fMet-(Cy3/5)tRNAfMet are fully active in the IF2γ toeprinting assay described in Section 4.1.1 (J.W. and R.L.G., unpublished data) as well as the elongation toeprinting assay described in Section 4.2.1 (Blanchard et al., 2004b).
5.3.2. tRNAPhe labeling
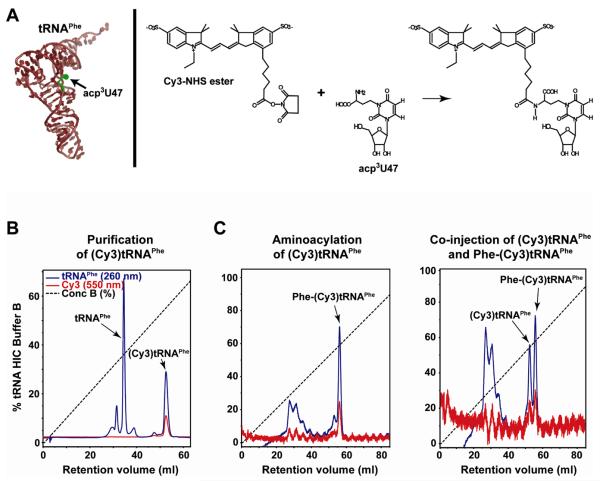
Fluorescent labeling of E. coli tRNAPhe (Sigma) at the primary aliphatic amino group of the 3-(3-amino-3-carboxypropyl)-uridine at position 47 (acp3U47) is achieved by reaction with Cy3/5-NHS esters (Fig. 3A) using slight modifications of a previously published protocol (Plumbridge et al., 1980). Labeling is achieved by incubating 10 nmol of tRNAPhe and ~200 nmol Cy3/5-NHS ester in 75 μl tRNAPhe Labeling Buffer (50 mM HEPES (pH=8.0), 0.9 M NaCl) for 8 h at 30 °C, followed by overnight incubation at 4 °C. The reaction is quenched, phenol extracted, chloroform extracted, and ethanol precipitated as described above for (Cy3/5)tRNAfMet. (Cy3/5)tRNAPhe is separated from unlabeled tRNAPhe using HIC (Fig. 3B) as described in Section 3.4. (Cy3)tRNAPhe and (Cy5)tRNAPhe elute from the Phenyl-5PW column at ~55% and ~61% tRNA HIC Buffer B, respectively. Using this protocol, a labeling efficiency of ~30% is routinely achieved. (Cy3/5)tRNAPhe can be aminoacylated with >90% efficiency (Fig. 3C) using the method described in Section 3.4 and is fully active in the elongation toeprinting assay described in Section 4.2.1 (Fig. 2C) (Blanchard et al., 2004b; Fei et al., 2008).
Figure 3.
Preparation of Phe-(Cy3/5)tRNAPhe. (A) Left panel: Structure of tRNAPhe, indicating the 3-(3-amino-3-carboxypropyl)-uridine residue at position 47 (acp3U47) (indicated in green) whose primary aliphatic amino group is reacted with Cy3-NHS ester. Right panel: Reaction chemistry involved in labeling acp3U47 with Cy3-NHS ester. (B) HIC chromatogram demonstrating the separation of (Cy3)tRNAPhe from unlabeled tRNAPhe. Peaks corresponding to (Cy3)tRNAPhe and tRNAPhe are labeled. (C) Left panel: HIC chromatogram demonstrating that (Cy3)tRNAPhe can be aminoacylated with phenylalanine with an efficiency of >90%. Right panel: HIC chromatogram of a co-injection of equimolar amounts of (Cy3)tRNAPhe and Phe-(Cy3)tRNAPhe, demonstrating the shift in elution position of Phe-(Cy3)tRNAPhe relative to (Cy3)tRNAPhe, thereby confirming the assignment of the peak in the left panel as Phe-(Cy3)tRNAPhe and confirming the >90% aminoacylation efficiency.
5.4. Translation factor labeling
Single-cysteine translation factor mutants are designed and constructed as described above for single-cysteine r-proteins, and are overexpressed and purified as described in Section 3.5. The general labeling procedure described in the following paragraph was developed using a single-cysteine RF1 mutant (Sternberg et al., 2009), but can be easily extended to any translation factor containing a unique cysteine.
Fluorescent labeling of translation factors generally follows the procedures described above for fluorescent labeling of r-proteins (Section 5.2.2). Briefly, labeling is performed in TF Labeling Buffer (100 mM Tris-HOAc (pH25°C=7.0), 50 mM KCl, and a 10-20-fold molar excess of TCEP over translation factor). The translation factor is buffer exchanged into TF Labeling Buffer, concentrated to 50–100 μM, and incubated for 15–30 min at room temperature in order to fully reduce translation factor disulfide bonds. A 10-20-fold molar excess of Cy3/5-maleimide, pre-dissolved in a minimum amount of anhydrous DMSO, is added to the translation factor solution and the labeling reaction is incubated for 1–2 h at room temperature with occasional mixing followed by an overnight incubation at 4 °C. The reaction is quenched with BME and the translation factor is separated from free Cy3/5 on an appropriately sized gel filtration column (a HiLoad 16/60 Superdex 75 prep grade column is appropriate for all translation factors except IF2γ, EF-G, and RF3, where a HiLoad 16/60 Superdex 200 prep grade should be used instead) using TF Buffer E (Section 3.5) as a column pre-equilibration and running buffer. Translation factor-containing fractions are pooled, buffer exchanged and concentrated into 2× TF Buffer E using a centrifugal filtration device, diluted to 1× TF Buffer E by addition of 100% glycerol, and stored at −20 °C. The labeling efficiency can be estimated from the gel filtration chromatogram using the translation factor and Cy3/5 extinction coefficients and the integrated absorbance at 280 nm (translation factor) and 550 nm (Cy3) or 650 nm (Cy5) of the protein peak. Using these protocols, the labeling efficiency of a single-cysteine RF1 mutant is 40–60% (Sternberg et al., 2009).
Depending on the labeling efficiency, it may be necessary or desirable to further separate (Cy3/5)translation factor from unlabeled translation factor. Experiments that involve the binding of (Cy3/5)translation factor to (Cy3/5)ribosomes will suffer from a low detection of smFRET events when the ratio of unlabeled translation factor to (Cy3/5)translation factor is high. Additionally, the interpretation of biochemical activity assays may be complicated when unlabeled translation factor may compete with (Cy3/5)translation factor. Taking advantage of the added hydrophobicity that Cy3/5 imparts onto the translation factor, (Cy3/5)translation factor can be efficiently separated from unlabeled translation factor using HIC. Translation factor-containing fractions from the gel filtration purification are buffer exchanged into TF HIC Buffer A (100 mM Na2HPO4 (pH25°C=7.0), 1 M (NH4)2SO4), concentrated using a centrifugal filtration device and subsequently injected onto the Phenyl-5PW column (Section 3.4) pre-equilibrated against TF HIC Buffer A. (Cy3/5)translation factor is then separated from unlabeled translation factor by elution with a linear gradient of 0–100% TF HIC Buffer B (100 mM Na2HPO4, pH25°C = 7.0) over 16 column volumes. This purification procedure yields 100% homogenously-labeled (Cy3/5)translation factor, which is buffer exchanged into 2× TF Buffer E (Section 3.5) and concentrated using a centrifugal filtration device, diluted to 1× TF Buffer E with 100% glycerol, and stored at −20°C. (Cy5)RF1, prepared and purified as described here, is fully active in the polypeptide release assay described in Section 4.3.1 (Sternberg et al., 2009).
6. CONCLUSIONS AND FUTURE PERSPECTIVES
Over the past few years, the highly-purified, fluorescently-labeled in vitro translation system described here, and similar systems in our colleagues' laboratories, have been successfully used in numerous smFRET studies of protein synthesis (reviewed in (Blanchard, 2009; Frank and Gonzalez Jr., 2010; Marshall et al., 2008a)). Going forward it is imperative that the basic experimental system described here be expanded in several important directions. For example, smFRET studies of functionally-disrupted mutant ribosomes, tRNAs, and translation factors will be crucial for determining the molecular basis through which ribosome, tRNA, and translation factor dynamics are coupled to each other and to the mechanisms underlying protein synthesis; to date only one such smFRET study of mutant ribosomes has been performed (Munro et al., 2007).
In addition to mutant translation components, relatively straightforward expansions of the experimental system described here should allow smFRET studies to test the hypothesis that regulatory factors might exert their control over protein synthesis by specifically altering the dynamics of the translating ribosome. To name a few candidate factors: the reverse translocase LepA (EF-4), which promotes reverse translocation of the ribosome along its mRNA template by precisely one codon (Qin et al., 2006); elongation factor P (EF-P), which stimulates formation of the first peptide bond at the interface between the initiation and elongation stages of protein synthesis (Aoki et al., 1997); the ribosomal protection protein Tet(O), which catalyzes the dissociation of the ribosome-targeting antibiotic tetracycline from ribosomes, thereby protecting ribosomes from inhibition by tetracycline (Spahn et al., 2001); and the specialized, EF-Tu-like elongation factor SelB, which, in response to a cis-acting mRNA structural element, recodes a stop codon with a selenocysteine tRNA in order to incorporate selenocysteine into selenoproteins (Forchhammer et al., 1989).
Perhaps the most important and challenging extension of our experimental platform is the establishment of a eukaryotic-based, highly-purified, fluorescently-labeled in vitro translation system. Development of such a system would open the door to detailed mechanistic studies of eukaryotic translation initiation and its regulation. This is a critical area of ongoing mechanistic research that is driven by the increased complexity of the eukaryotic translation initiation machinery relative to its bacterial counterpart (Kapp and Lorsch, 2004), the prominent role of translation initiation in the translational control of eukaryotic gene expression (Sonenberg and Hinnebusch, 2009), and the increasingly apparent correlation between the deregulation of translation initiation and human diseases such as cancer (Clemens, 2004) and viral infections (Schneider and Mohr, 2003).
ACKNOWLEDGMENTS
R.L.G. would like to acknowledge scientific and financial support from Profs. Joseph D. Puglisi (Stanford University School of Medicine) and Steven Chu (formerly at Stanford University, currently at the U.S. Department of Energy), in whose laboratories early work on the development of the highly-purified, fluorescently-labeled in vitro translation system described here was performed. R.L.G. would also like to acknowledge Scott C. Blanchard (formerly at the Stanford University School of Medicine, currently at Weill Cornell Medical School) and Harold D. Kim (formerly at Stanford University, currently at the Georgia Institute of Technology), with whom the early work in the Puglisi and Chu laboratories was collaboratively performed. R.L.G.'s postdoctoral work in the Puglisi and Chu laboratories was funded by postdoctoral fellowships from the American Cancer Society (PF-01-119-01-GMC) and the Burroughs Wellcome Fund (CABS 1004856).
Work in the Gonzalez laboratory is supported by a Burroughs Wellcome Fund CABS Award (CABS 1004856), an NSF CAREER Award (MCB 0644262), an NIH-NIGMS R01 grant (R01 GM084288), an American Cancer Society Research Scholar Grant (RSG GMC-117152), and a Columbia University Research Initiatives in Science and Engineering (RISE) Award to R.L.G. S.H.S. was supported by the Columbia University Langmuir Scholars Program. M.M.E. and M.T.E. are supported by Columbia University's NIH Training Program in Molecular Biophysics (T32-GM008281).
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aoki H, Adams SL, Turner MA, Ganoza MC. Molecular characterization of the prokaryotic efp gene product involved in a peptidyltransferase reaction. Biochimie. 1997;79:7–11. doi: 10.1016/s0300-9084(97)87619-5. [DOI] [PubMed] [Google Scholar]
- Antoun Ayman, Pavlov Michael Y., Tenson Tanel, Ehrenberg M. Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering. Biol. Proced. Online. 2004;6:35–54. doi: 10.1251/bpo71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2 doi: 10.1038/msb4100050. 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens PI, Jovin TM. Microspectroscopic imaging tracks the intracellular processing of a signal transduction protein: fluorescent-labeled protein kinase C beta I. Proc. Natl. Acad. Sci. U. S. A. 1996;93:8407–12. doi: 10.1073/pnas.93.16.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC. Single-molecule observations of ribosome function. Curr. Opin. Struct. Biol. 2009;19:103–9. doi: 10.1016/j.sbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. tRNA selection and kinetic proofreading in translation. Nat. Struct. Mol. Biol. 2004a;11:1008–14. doi: 10.1038/nsmb831. [DOI] [PubMed] [Google Scholar]
- Blanchard SC, Kim HD, Gonzalez RL, Jr., Puglisi JD, Chu S. tRNA dynamics on the ribosome during translation. Proc. Natl. Acad. Sci. U. S. A. 2004b;101:12893–12898. doi: 10.1073/pnas.0403884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BR, Ames BN. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 1982;257:9759–69. [PubMed] [Google Scholar]
- Brandi L, Marzi S, Fabbretti A, Fleischer C, Hill WE, Gualerzi CO, Lodmell JS. The translation initiation functions of IF2: Targets for thiostrepton inhibition. J. Mol. Biol. 2004;335:881–894. doi: 10.1016/j.jmb.2003.10.067. [DOI] [PubMed] [Google Scholar]
- Cammack KA, Wade HE. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem. J. 1965;96:671–80. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon J, David H. Studies on the thionucleotides in transfer ribonucleic acid. Addition of N-ethylmaleimide and formation of mixed disulfides with thiol compounds. Biochemistry. 1968;7:3851–8. doi: 10.1021/bi00851a010. [DOI] [PubMed] [Google Scholar]
- Chambliss GH, Henkin TM, Leventhal JM. Bacterial in vitro protein-synthesizing systems. Methods Enzymol. 1983;101:598–605. doi: 10.1016/0076-6879(83)01040-x. [DOI] [PubMed] [Google Scholar]
- Clemens MJ. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. 2004;23:3180–8. doi: 10.1038/sj.onc.1207544. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviter T, Gromadski KB, Rodnina MV. The ribosome's response to codon-anticodon mismatches. Biochimie. 2006;88:1001–11. doi: 10.1016/j.biochi.2006.04.013. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL molecular graphics system. DeLano Scientific LLC; Palo Alto, CA, USA: 2008. http://www.pymol.org. [Google Scholar]
- Dincbas-Renqvist V, Engstrom A, Mora L, Heurgue-Hamard V, Buckingham R, M., E. A post-translational modification in the GGQ motif of RF2 from Escherichia coli stimulates termination of translation. EMBO J. 2000;19:6900–6907. doi: 10.1093/emboj/19.24.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorywalska M, Blanchard SC, Gonzalez RL, Kim HD, Chu S, Puglisi JD. Site-specific labeling of the ribosome for single-molecule spectroscopy. Nucleic Acids Res. 2005;33:182–9. doi: 10.1093/nar/gki151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnoff JS, Maitra U, Kivie Moldave, Lawrence G. Isolation and properties of protein factors involved in polypeptide chain initiation in Escherichia coli. Methods Enzymol. 20:248–261. [Google Scholar]
- Elbing K, Brent R. Escherichia coli, plasmids, and bacteriophages. Curr. Protoc. Mol. Biol. 2002;59:1.1.1–1.1.7. [Google Scholar]
- Ermolenko DN, Majumdar ZK, Hickerson RP, Spiegel PC, Clegg RM, Noller HF. Observation of intersubunit movement of the ribosome in solution using FRET. J. Mol. Biol. 2007;370:530–40. doi: 10.1016/j.jmb.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Fei J, Bronson JE, Hofman JM, Srinivas RL, Wiggins CH, Gonzalez RLJ. Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15702–15707. doi: 10.1073/pnas.0908077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei J, Kosuri P, MacDougall DD, Gonzalez RL., Jr. Coupling of ribosomal L1 stalk and tRNA dynamics during translation elongation. Mol. Cell. 2008;30:348–59. doi: 10.1016/j.molcel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Forchhammer K, Leinfelder W, Bock A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989;342:453–6. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- Fourmy D, Mechulam Y, Brunie S, Blanquet S, Fayat G. Identification of residues involved in the binding of methionine by Escherichia coli methionyl-tRNA synthetase. FEBS Lett. 1991;292:259–63. doi: 10.1016/0014-5793(91)80879-8. [DOI] [PubMed] [Google Scholar]
- Frank J, Gonzalez RL., Jr. Annu. Rev. Biochem. 2010. Structure and dynamics of a processive Brownian motor: the translating ribosome. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrick K, Noller H. Catalysis of ribosomal translocation by sparsomycin. Science. 2003;300:1159–1162. doi: 10.1126/science.1084571. [DOI] [PubMed] [Google Scholar]
- Freistroffer DV, Pavlov MY, MacDougall J, Buckingham RH, Ehrenberg M. Release factor RF3 in E.coli accelerates the dissociation of release factors RF1 and RF2 from the ribosome in a GTP-dependent manner. EMBO J. 1997;16:4126–33. doi: 10.1093/emboj/16.13.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher SR. One-dimensional SDS gel electrophoresis of proteins. Curr. Protoc. Mol. Biol. 2006;75:10.2A.1–10.2A.37. doi: 10.1002/0471142727.mb1002as75. [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Bender RA, Streicher SL. Direct selection for P1-sensitive mutants of enteric bacteria. J. Bacteriol. 1974;118:810–4. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RL, Jr., Chu S, Puglisi JD. Thiostrepton inhibition of tRNA delivery to the ribosome. RNA. 2007;13:2091–7. doi: 10.1261/rna.499407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Ha T. Single-Molecule Fluorescence Resonance Energy Transfer. Methods. 2001;25:78–86. doi: 10.1006/meth.2001.1217. [DOI] [PubMed] [Google Scholar]
- Hapke B, Noll H. Structural dynamics of bacterial ribosomes. IV. Classification of ribosomes by subunit interaction. J. Mol. Biol. 1976;105:97–109. doi: 10.1016/0022-2836(76)90196-0. [DOI] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Gold L. Selection of the initiator tRNA by Escherichia coli initiation factors. Genes Dev. 1989;3:1899–1912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- Hartz D, McPheeters DS, Traut R, Gold L. Extension Inhibition Analysis of Translation Initiation Complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Heurgue-Hamard V, Champ S, Engstrom A, Ehrenberg M, Buckingham RH. The hemK gene in Escherichia coli encodes the N(5)-glutamine methyltransferase that modifies peptide release factors. EMBO J. 2002;21:769–78. doi: 10.1093/emboj/21.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa G, Nijman RM, Raj VS, Kaji H, Igarashi K, Kaji A. The role of ribosome recycling factor in dissociation of 70S ribosomes into subunits. RNA. 2005;11:1317–28. doi: 10.1261/rna.2520405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Roeder RG. Purification of his-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 1991;19:6337–8. doi: 10.1093/nar/19.22.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohng S, Joo C, Ha T. Single-molecule three-color FRET. Biophys. J. 2004;87:1328–37. doi: 10.1529/biophysj.104.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc PC, Kurland CG. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc. Natl. Acad. Sci. U. S. A. 1979;76:3174–8. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S, Noller HF. EF-G-catalyzed translocation of anticodon stem-loop analogs of transfer RNA in the ribosome. EMBO J. 1998;17:3478–83. doi: 10.1093/emboj/17.12.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp LD, Lorsch JR. The molecular mechanics of eukaryotic translation. Annu. Rev. Biochem. 2004;73:657–704. doi: 10.1146/annurev.biochem.73.030403.080419. [DOI] [PubMed] [Google Scholar]
- Korostelev A, Ermolenko DN, Noller HF. Structural dynamics of the ribosome. Curr. Opin. Chem. Biol. 2008;12:674–83. doi: 10.1016/j.cbpa.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR. Principles of Fluorescence Spectroscopy. Springer; New York: 1999. [Google Scholar]
- Lee TH, Blanchard SC, Kim HD, Puglisi JD, Chu S. The role of fluctuations in tRNA selection by the ribosome. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13661–5. doi: 10.1073/pnas.0705988104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Agirrezabala X, Lei J, Bouakaz L, Brunelle JL, Ortiz-Meoz RF, Green R, Sanyal S, Ehrenberg M, Frank J. Recognition of aminoacyl-tRNA: a common molecular mechanism revealed by cryo-EM. EMBO J. 2008;27:3322–31. doi: 10.1038/emboj.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljas A. Structural aspects of protein synthesis. World Scientific Publishing Co. Pte. Ltd.; Singapore: 2004. [Google Scholar]
- Liu C, Wang R, Varlamova O, Leyh TS. Regulating energy transfer in the ATP sulfurylase-GTPase system. Biochemistry. 1998;37:3886–92. doi: 10.1021/bi971989d. [DOI] [PubMed] [Google Scholar]
- Maar D, Liveris D, Sussman JK, Ringquist S, Moll I, Heredia N, Kil A, Blasi U, Schwartz I, Simons RW. A single mutation in the IF3 N-terminal domain perturbs the fidelity of translation initiation at three levels. J. Mol. Biol. 2008;383:937–44. doi: 10.1016/j.jmb.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Marshall RA, Aitken CE, Dorywalska M, Puglisi JD. Translation at the single-molecule level. Annu. Rev. Biochem. 2008a;77:177–203. doi: 10.1146/annurev.biochem.77.070606.101431. [DOI] [PubMed] [Google Scholar]
- Marshall RA, Aitken CE, Puglisi JD. GTP hydrolysis by IF2 guides progression of the ribosome into elongation. Mol. Cell. 2009;35:37–47. doi: 10.1016/j.molcel.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RA, Dorywalska M, Puglisi JD. Irreversible chemical steps control intersubunit dynamics during translation. Proc. Natl. Acad. Sci. U. S. A. 2008b;105:15364–9. doi: 10.1073/pnas.0805299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna SA, Kim I, Puglisi EV, Lindhout DA, Aitken CE, Marshall RA, Puglisi JD. Purification and characterization of transcribed RNAs using gel filtration chromatography. Nat. Protoc. 2007;2:3270–7. doi: 10.1038/nprot.2007.480. [DOI] [PubMed] [Google Scholar]
- Milligan JF, Groebe DR, Witherell GW, Uhlenbeck OC. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987;15:8783–98. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D, Wintermeyer W, Rodnina MV. GTPase activation of elongation factors Tu and G on the ribosome. Biochemistry. 2002;41:12520–8. doi: 10.1021/bi026301y. [DOI] [PubMed] [Google Scholar]
- Moore DD. Commonly used reagents and equipment. Curr. Protoc. Mol. Biol. 2000;35:A.2.5. doi: 10.1002/0471142727.mba02s35. [DOI] [PubMed] [Google Scholar]
- Moore S, Sauer RT. P1vir phage transduction. 2009 http://openwetware.org/wiki/Sauer:P1vir_phage_transduction.
- Mora L, Heurgue-Hamard V, de Zamaroczy M, Kervestin S, Buckingham RH. Methylation of bacterial release factors RF1 and RF2 is required for normal translation termination in vivo. J. Biol. Chem. 2007;282:35638–45. doi: 10.1074/jbc.M706076200. [DOI] [PubMed] [Google Scholar]
- Munro JB, Altman RB, O'Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol. Cell. 2007;25:505–17. doi: 10.1016/j.molcel.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom OW, Picking WD, Hardesty B. Movement of tRNA but not the nascent peptide during peptide bond formation on ribosomes. Biochemistry. 1990;29:10734–44. doi: 10.1021/bi00500a004. [DOI] [PubMed] [Google Scholar]
- Ogle JM, Ramakrishnan V. Structural insights into translational fidelity. Annu. Rev. Biochem. 2005;74:129–177. doi: 10.1146/annurev.biochem.74.061903.155440. [DOI] [PubMed] [Google Scholar]
- Pavlov MY, Ehrenberg M. Rate of translation of natural mRNAs in an optimized in vitro system. Arch. Biochem. Biophys. 1996;328:9–16. doi: 10.1006/abbi.1996.0136. [DOI] [PubMed] [Google Scholar]
- Peske F, Rodnina MV, Wintermeyer W. Sequence of steps in ribosome recycling as defined by kinetic analysis. Mol. Cell. 2005;18:403–12. doi: 10.1016/j.molcel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Plumbridge JA, Baumert HG, Ehrenberg M, Rigler R. Characterisation of a new, fully active fluorescent derivative of E. coli tRNA Phe. Nucleic Acids Res. 1980;8:827–43. [PMC free article] [PubMed] [Google Scholar]
- Powers T, Noller HF. A functional pseudoknot in 16S ribosomal RNA. EMBO J. 1991;10:2203–2214. doi: 10.1002/j.1460-2075.1991.tb07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Polacek N, Vesper O, Staub E, Einfeldt E, Wilson DN, Nierhaus KH. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell. 2006;127:721–33. doi: 10.1016/j.cell.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Raleigh EA, Elbing K, Brent R. Escherichia coli, plasmids, and bacteriophages. Curr. Protoc. Mol. Biol. 2002;59:1.4.1–1.4.14. [Google Scholar]
- Recht MI, Douthwaite S, Dahlquist KD, Puglisi JD. Effect of mutations in the A site of 16S rRNA on aminoglycoside antibiotic-ribosome interaction. J. Mol. Biol. 1999;286:33–43. doi: 10.1006/jmbi.1998.2446. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Wintermeyer W. Effect of translocation on topology and conformation of anticodon and D loops of tRNAPhe. J. Mol. Biol. 1981;151:57–79. doi: 10.1016/0022-2836(81)90221-7. [DOI] [PubMed] [Google Scholar]
- Roy R, Hohng S, Ha T. A practical guide to single-molecule FRET. Nat. Methods. 2008;5:507–16. doi: 10.1038/nmeth.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse J, Gallagher SR. Staining proteins in gels. Curr. Protoc. Mol. Biol. 2009;85:10.6.1–10.6.27. doi: 10.1002/0471142727.mb1006s85. [DOI] [PubMed] [Google Scholar]
- Schmitt E, Blanquet S, Mechulam Y. Crystallization and preliminary X-ray analysis of Escherichia coli methionyl-tRNAMet(f) formyltransferase complexed with formyl-methionyl-tRNAMet(f) Acta Crystallogr., Sect. D: Biol. Crystallogr. 1999;55(Pt 1):332–4. doi: 10.1107/S0907444998011780. [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem. Sci. 2003;28:130–6. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- Schuette JC, Murphy F. V. t., Kelley AC, Weir JR, Giesebrecht J, Connell SR, Loerke J, Mielke T, Zhang W, Penczek PA, Ramakrishnan V, Spahn CM. GTPase activation of elongation factor EF-Tu by the ribosome during decoding. EMBO J. 2009;28:755–65. doi: 10.1038/emboj.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JHD. Structures of the bacterial ribosome at 3.5 A resolution. Science. 2005;310:827–834. doi: 10.1126/science.1117230. [DOI] [PubMed] [Google Scholar]
- Selmer M, Dunham CM, Murphy F. V. t., Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–42. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- Seshadri A, Varshney U. Mechanism of recycling of post-termination ribosomal complexes in eubacteria: a new role of initiation factor 3. J. Biosci. 2006;31:281–9. doi: 10.1007/BF02703921. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, Ueda T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001;19:751–5. doi: 10.1038/90802. [DOI] [PubMed] [Google Scholar]
- Simonian MH, Smith JA. Spectrophotometric and colorimetric determination of protein concentration. Curr. Protoc. Mol. Biol. 2006;76:10.1A.2–10.1A.9. doi: 10.1002/0471142727.mb1001as76. [DOI] [PubMed] [Google Scholar]
- Slatko BE, Albright LM. Denaturing gel electrophoresis for sequencing. Curr. Protoc. Mol. Biol. 1992;16:7.6.1–7.6.13. doi: 10.1002/0471142727.mb0706s16. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn CM, Blaha G, Agrawal RK, Penczek P, Grassucci RA, Trieber CA, Connell SR, Taylor DE, Nierhaus KH, Frank J. Localization of the ribosomal protection protein tet(o) on the ribosome and the mechanism of tetracycline resistance. Mol. Cell. 2001;7:1037–45. doi: 10.1016/s1097-2765(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Stark H, Rodnina MV, Wieden HJ, Zemlin F, Wintermeyer W, van Heel M. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codon-recognition complex. Nat. Struct. Biol. 2002;9:849–54. doi: 10.1038/nsb859. [DOI] [PubMed] [Google Scholar]
- Sternberg SH, Fei J, Prywes N, McGrath KA, Gonzalez RL., Jr. Translation factors direct intrinsic ribosome dynamics during translation termination and ribosome recycling. Nat. Struct. Mol. Biol. 2009;16:861–868. doi: 10.1038/nsmb.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Li W, Stagg SM, Sengupta J, Nielsen RC, Nissen P, Harvey SC, Ehrenberg M, Frank J. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Mol. Biol. 2003;10:899–906. doi: 10.1038/nsb1003. [DOI] [PubMed] [Google Scholar]
- Villa E, Sengupta J, Trabuco LG, LeBarron J, Baxter WT, Shaikh TR, Grassucci RA, Nissen P, Ehrenberg M, Schulten K, Frank J. Ribosome-induced changes in elongation factor Tu conformation control GTP hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1063–8. doi: 10.1073/pnas.0811370106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EG, Jelenc PC, Ehrenberg M, Kurland CG. Rate of elongation of polyphenylalanine in vitro. Eur. J. Biochem. 1982;122:193–7. doi: 10.1111/j.1432-1033.1982.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Wall JD, Harriman PD. Phage P1 mutants with altered transducing abilities for Escherichia coli. Virology. 1974;59:532–44. doi: 10.1016/0042-6822(74)90463-2. [DOI] [PubMed] [Google Scholar]
- Weinger JS, Parnell KM, Dorner S, Green R, Strobel SA. Substrate-assisted catalysis of peptide bond formation by the ribosome. Nat. Struct. Mol. Biol. 2004;11:1101–6. doi: 10.1038/nsmb841. [DOI] [PubMed] [Google Scholar]
- Wilson DN, Blaha G, Connell SR, Ivanov PV, Jenke H, Stelzl U, Teraoka Y, Nierhaus KH. Protein synthesis at atomic resolution: mechanistics of translation in the light of highly resolved structures for the ribosome. Curr. Protein Pept. Sci. 2002;3:1–53. doi: 10.2174/1389203023380846. [DOI] [PubMed] [Google Scholar]
- Wilson K. Curr. Protoc. Mol. Biol. 1987. Preparation of genomic DNA from bacteria; pp. 2.4.1–2.4.2. [DOI] [PubMed] [Google Scholar]
- Wyatt JR, Chastain M, Puglisi JD. Synthesis and purification of large amounts of RNA oligonucleotides. BioTechniques. 1991;11:764–69. [PubMed] [Google Scholar]
- Youngman EM, Brunelle JL, Kochaniak AB, Green R. The active site of the ribosome is composed of two layers of conserved nucleotides with distinct roles in peptide bond formation and peptide release. Cell. 2004;117:589–99. doi: 10.1016/s0092-8674(04)00411-8. [DOI] [PubMed] [Google Scholar]
- Zavialov AV, Buckingham RH, Ehrenberg M. A posttermination ribosomal complex is the guanine nucleotide exchange factor for peptide release factor RF3. Cell. 2001;107:115–124. doi: 10.1016/s0092-8674(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Zavialov AV, Hauryliuk VV, Ehrenberg M. Splitting of the posttermination ribosome into subunits by the concerted action of RRF and EF-G. Mol. Cell. 2005;18:675–86. doi: 10.1016/j.molcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Bartley LE, Babcock HP, Russell R, Ha T, Herschlag D, Chu S. A single-molecule study of RNA catalysis and folding. Science. 2000;288:2048–51. doi: 10.1126/science.288.5473.2048. [DOI] [PubMed] [Google Scholar]