Abstract
Purpose
Sublethal damage repair (SLDR) is a type of repair that occurs in split dose irradiated cells, which was discovered more than 50 years ago. However, due to conflicting reported data, it remains unclear which DNA double strand break (DSB) repair pathway, non-homologous end-joining (NHEJ) repair, homologous recombination repair (HRR) or both, contributes to SLDR, particularly in human cells. We were interested in clarifying this question.
Methods and materials
Mammalian cell lines, including human, mouse and Chinese hamster ovary (CHO) cell lines, wild type, deficient in NHEJ or HRR were irradiated with either single dose or two split doses at 2 or 4 hour intervals. The clonogenic assay was used to evaluate these cell radiosensitivities.
Results
All wild type or HRR deficient cells (including human, mouse and CHO cells) showed a higher survival rate after exposure to split dose versus single dose radiation, however, all classical NHEJ deficient cells (including human, mouse and hamster cells) did not show any apparent sensitivity changes between single dose and split dose irradiation.
Conclusion
Classical NHEJ mainly contributes to SLDR in mammalian cells (including human cells) cells. These results have the potential to improve radiotherapy.
Keywords: DNA DSB, DNA repair, SLDR, NHEJ, HRR, Heavy ion
Introduction
Ionizing radiation (IR) kills cells primarily by generating DNA double strand breaks (DSB). To prevent death, mammalian cells have evolutionally developed two efficient pathways to repair DNA DSB: non-homologous end-joining (NHEJ) and homologous recombination repair (HRR). NHEJ includes Ku-dependent classical NHEJ that plays a major role in repair of DNA DSB for mammalian cells and alternative NHEJ that plays a backup role in in repair of DNA DSB for mammalian cells when the classical NHEJ is not available (Schipler et al. 2013). Sublethal damage (SLD) was first reported in x-ray-irradiated cells (chlamydomonas) in 1957 (Jacobson 1957). In this study, Jacobson showed that a radiation dose delivered in two fractions separated in time provided a higher survival rate than if the dose was given in one single fraction (Jacobson 1957). Two years later, Elkind and Sutton reported the sublethal damage repair (SLDR) in mammalian cells (V79 Chinese hamster and Chinese hamster ovary (CHO) cell lines), and showed that most SLDR occurred after a 2-hour incubation period prior to exposure to the second dose and gradually led to a plateau status (Elkind et al. 1959). Since IR kills cells mainly via generation of DNA DSB and the irradiated cell survival depends on the ability to repair DNA DSB, the SLDR phenotype reflects additional cell repair of DNA DSB prior to exposure to the second dose. SLDR is observed in exponential growth cells, which is different from other type of conditional DNA repair, potential lethal damage repair (PLDR). PLDR is defined as repair that occurs when plateau phase cells were allowed to remain in the density-inhibited state for 6 to 12 hours after irradiation (Hall et al. 2010). There are many reports that have studied different aspects of SLDR, such as cell cycle distribution (Zaider et al. 1996), later tissue response (Brenner et al. 1998), p53 (a tumor suppressor) involved effects (Pekkola-Heino et al. 1998); halftime for SLDR (Guerrero et al. 2006); intrinsic radiosensitivity and SLDR (Guerrero et al. 2006), implication (Liu et al. 2011) and inhibition of SLDR (Ben-Hur et al. 2012), etc. One earlier study reported that SLDR depended on HRR in chicken DT40 cells (Utsumi et al. 2001) and a recent study reported that SLDR mainly depended on Ku-dependent NHEJ in CHO cells (Somaiah et al. 2013). However, for mouse and human cells, the status remains unclear.
Unlike low linear energy transfer (LET) radiation (x-rays or gamma rays) that is generated by a traditional radiotherapy, radio-diagnosis machine or existed on Earth; high-LET radiation is generated by a heavy ion radiotherapy facility or exists in galactic space. High-LET IR kills more cells at the same dose than low-LET IR; however, the underlying mechanism is not completely understood. Previously, we and others reported that high-LET IR compared to low-LET IR interfered with only classical NHEJ in mice and human cells (Lind et al. 2003, Okayasu 2006, Wang et al. 2008). It was then demonstrated that high-LET IR does not affect HRR efficiency (Wang et al. 2008, Wang et al. 2010, Zafar et al. 2010). Interestingly, it was reported that after exposure to high-LET IR, the mammalian cells did not show SLDR as they did after exposure to low-LET IR (Hall et al. 1975). These results suggest that SLDR may depend mainly on classical NHEJ but not HRR in mice and human cells. The purpose of this study was to test this hypothesis. By examining the survival in mammalian (human, mouse and Chinese hamster ovary (CHO)) cell lines including wild type, deficient in NHEJ or in HRR, we irradiated the cells with either single dose or two split doses at 2 or 4 hour intervals, and demonstrated that Ku-dependent classical NHEJ is the main pathway for SLDR in mammalian cells (including human cells).
Methods
Cell lines and culture
The cell lines used in this study included human immortalized fibroblast cell lines MRC5SV (wild type), 180BRM, ligase IV (Lig4) mutant, NHEJ deficient (Riballo et al. 1999)) and AT5BISV (Ataxia telangiectasia mutated (ATM)−/−, HRR deficient (Golding et al. 2004)); CHO cell lines: AA8 (wild type), irs1-SF (without XRCC3 and HRR deficient (Fuller et al. 1988, Liu et al. 1998)), V3 (DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) deficient) and V3WT (V3 transfected with DNA-PKcs) were obtained from Dr. Benjamin PC Chen’s lab (Chen et al. 2005); and mouse immortalized embryo fibroblast (MEF) cell lines: Ku80+/+ (wild type) and Ku80−/− (without Xrcc5 and NHEJ deficient) cells. MRC5SV and 180BRM were obtained from Dr. Iliakis’s lab with Dr. Arlett’s permission (Wang et al. 2001). AT5BISV, CHO and mouse cell lines. The cell culture condition was as described previously (Wang et al. 2003, Yu et al. 2011).
Vector construction and transfection
The mouse wild type Ku80 coding sequence was amplified from cDNA that was prepared using C57BL/6 mouse small intestine samples with primers (Forward: CCGGGAATTCAGGCGTGGTCCGGTAATAAG (EcoRI) and Reverse: GCAAGCGGCCGCTATAT CATGTCCAGTAAATCATC (NotI)). After the sequence was verified, the cDNA was inserted into a pCMV-HA expression vector (Clontech). The NHEJ deficient MEF (Ku80−/−) cells were transfected with the vector (1 μg) using Lipofectamine3000 (Invitrogen, Carlsbad, CA, USA). At 48h after transfection the cells were collected for further experiments.
Immunoblotting
The whole cell lyses were prepared for immunoblotting as described previously (Wang et al. 2009). The antibodies against Actin were purchased from Santa Cruz Biotechnology Inc (Dallas, TX, USA). The antibody against HA was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA).
Radiation
Radiation was performed in our laboratory using an x-ray machine (X-RAD 320, North Brandford, CT, USA) at 320 kV, 10 mA with a 2-mm aluminum filter and the dose rate was 2 Gy/min for cells. The x-ray dose was verified using a monitor control system that was periodically calibrated by the company engineer.
Cell radio-sensitivity assay
Cell radiation sensitivity was evaluated by loss of colony-forming ability. After exposure to radiation with different doses, the cells were collected immediately or incubated at 37°C for different time periods and then collected, and plated to obtain a density of 20-100 colonies per dish. Duplicate dishes were prepared for each radiation dose. The cells were incubated for 7-10 days and the colonies were stained with crystal violet in 100% methanol solution.
Statistical analysis
The data were statistically analyzed using a Student's t test. Differences with p < 0.05 are considered significant.
Results and discussion
SLDR exists in HRR deficient cells
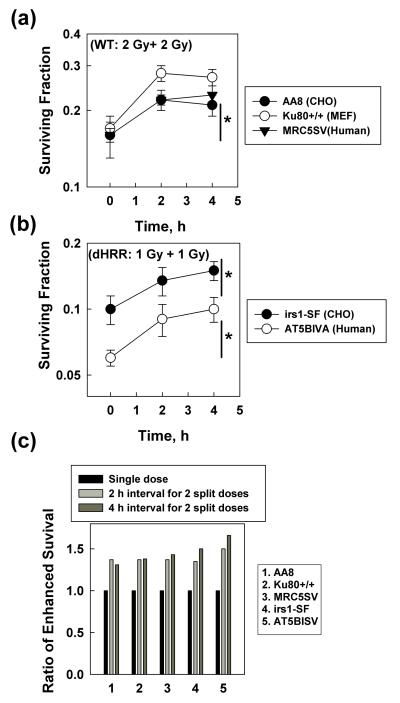
The wild type cell lines, including CHO (AA8), MEF (Ku80+/+) and human (MRC5SV), showed greater cell survival when the single dose (4 Gy) was split into two doses (2 Gy + 2 Gy) at ≥ 2 h intervals (Figure 1a), indicating that the increased survival was via the SLDR process. We then performed a similar experiment with the HRR deficient CHO (irs1-SF) and human (AT5BISV, ATM−/−) cells (Golding et al. 2004), but reduced the dose to 2 Gy that was split into two doses (1 Gy + 1 Gy) in order to get a similar survival range to their wild type counterparts for comparison. Similar to the results from wild type cells, the HRR deficient cells also showed greater cell survival when the single dose was split into two doses at ≥ 2 h intervals (Fig. 2B), indicating that SLDR occurs in these cells. Although these HRR deficient cells are more sensitive than their wild counterparts to radiation-induced killings, they showed a similar level of increased cell survival to their wild counterparts (Fig. 1C). These results indicate that these HRR deficient cells have a functional SLDR and, therefore, exclude the possibility that HRR contributes to SLDR. Our conclusion is different from one previous study (Utsumi et al. 2001) that indicates HRR is required for SLDR. The varying conclusions may be due to the different species. The other study used DT40 (chicken cell lines) that mainly depend on HRR to repair DNA DSB and we used mammalian cells that depend on both NHEJ and HRR to repair DNA DSB. Our data are supported by the data obtained from another group using CHO cells (Somaiah et al. 2013).
Figure 1.
SLDR is present in HRR deficient cells. (a) Wild type (WT) cells including CHO (AA8), MEF (Ku80+/+) and human (MRC5SV) were either exposed to single dose (4 Gy, 0 h point) or split doses (2 Gy + 2 Gy) using different time intervals. A clonogenic assay was used to detect the cell sensitivity to radiation-induced killings. The plating efficiency is 71% for AA8 cells, 40% for Ku80+/+ cells and 25% for MRC5SV cells. Data shown are the mean and SD from five independent experiments, *, P< 0.05 between the survival fraction data obtained from 2 or 4 h time points (interval time between the split doses) compared to the 0 h time point (single dose). (b) HRR deficient cell (dHRR) including CHO (irs1-SF) and human (AT5BIVA, ATM−/−) were exposed to either single dose (2 Gy, 0 h point) or split doses (1 Gy + 1 Gy) with different time intervals. A clonogenic assay was used to detect the cell sensitivity to radiation-induced killings. The plating efficiency is 15% for AT5BIVA cells and 28% for irs1-SF cells. Data shown are the mean and SD from five independent experiments, *, P< 0.05 between the survival fraction data obtained from 2 or 4 h time points (interval time between the split doses) compared to the 0 h time point (single dose). (c) The ratio of enhanced cell survival was calculated from the data shown in panel (a) and (b).
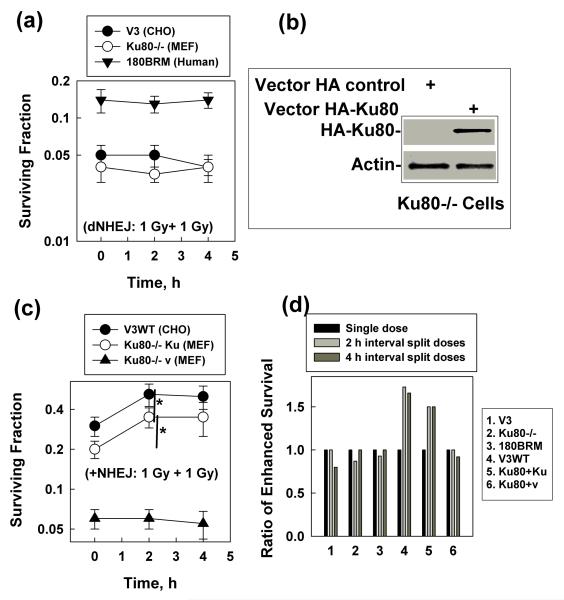
Figure 2.
SLDR is not present in NHEJ deficient cells. (a) NHEJ deficient (dNHEJ) cells including CHO (V3), MEF (Ku80−/−) and human (180BRM, Lig4 mutant) were exposed to either single dose (2 Gy, 0 h point) or split doses (1 Gy + 1 Gy) with different time intervals. A clonogennic assay was used to detect the cell sensitivity to radiation-induced killings. The plating efficiency is 48% for V3 cells, 30% for Ku80−/− cells and 10% for 180BRM cells. Data shown are the mean and SD from five independent experiments, *, P< 0.05 between the survival fraction data obtained from 2 or 4 h time points (interval time between the split doses) compared to the 0 h time point (single dose). (b) Ku80−/− cells were transiently transfected with either the HA- vector (vector HA control) or the vector encoding Ku80 (vector HA-Ku80). The transfection efficiency is 60-70%. At 24 h after transfection, a portion of the cells was collected for Western blot. HA antibody was used for detecting the HA-Ku80 expression level, and Actin was used as the internal loading control. (c) V3WT, Ku80−/− re-expressed with Ku80 (Ku80−/− Ku) (+NHEJ), or Ku80−/− cells transfected with the vector control (Ku80−/− v) that were a portion of cells from the transfected experiments as described in (b), were exposed to either single dose (2 Gy, 0 h point) or split doses (1 Gy + 1 Gy) at different time intervals. A clonogennic assay was used to detect the cell sensitivity to radiation-induced killings. The plating efficiency is 88% for V3WT, 38% for (Ku80−/− Ku) and 30% for Ku80−/− v. Data shown are the mean and SD from five independent experiments, *, P< 0.05 between the survival fraction data obtained from 2 h time point (interval between the split doses) compared to the 0 h time point (single dose). (d) The ratio of enhanced cell survival was calculated from the data shown in panel (a) and (c).
The interval time between split doses (~ 2 h) has efficiently increased cell survival, suggesting that most SLDR had already finished. These results are also consistent with the results where the halftime for SLDR in human cells after exposure to 2-4 Gy was 0.2-0.4 h (Guerrero et al. 2006). Considering that HRR needs a homologous DNA template for a sister chromatin conversion–related repair and mainly occurs in the S and G2 phases of cell cycle, it is also reasonable to exclude HRR as the major contributor to SLDR.
SLDR is not present in Ku-dependent classical NHEJ deficient cells
Next we examined whether SLDR occurred through the Ku-dependent classical NHEJ pathway in mammalian (including human) cells by detecting the cell sensitivity to single dose and split doses at the same intervals (as described in figure 1). The NHEJ deficient cell lines include CHO (V3), mouse (Ku80−/−) and human (180BRM) cells. All of the NHEJ deficient cells showed no significant changes in sensitivity to single dose (2 Gy) or split doses (1 Gy + 1 Gy) at ≥ 2 h intervals (Fig. 2A). These results indicate that these cells lack SLDR and suggest that Ku-dependent classical NHEJ contributes mainly to SLDR. To confirm this hypothesis, we performed rescue experiments on V3 and Ku80−/− cells. V3WT cells are V3 cells (deficient in DNA-PKcs) re-expressed with DNA-PKcs as described previously (Chen et al. 2005). We used the HA-coding vector (as a control) to generate HA-Ku80 expression plasmid. We transiently transfected the vectors into Ku80−/− cells and showed an HA-Ku80 expression well in the cells at 24 h after transfection (Fig. 2B). We then examined the sensitivity of these cells to either single dose (2 Gy) or split doses (1 Gy + 1 Gy) at ≥ 2 h intervals. After the DNA-PKcs or Ku80 gene was re-expressed in the NHEJ deficient cells, the cell resistance to radiation-induced killing increased dramatically (Fig. 2C). More importantly, cell survival increased after exposure to two doses (1 Gy + 1 Gy) at ≥ 2 h intervals versus exposure to single dose (2 Gy) (Fig. 2C), indicating that the cells re-obtained SLDR ability. The levels of increased cell survival in the rescue cells are similar to that of their wild counterparts (Fig. 1C, Fig. 2D). These results confirm that Ku-dependent classical NHEJ is the major player in SLDR in mammalian cells.
The main reason that Ku-dependent classical NHEJ but not HRR plays a major role in SLDR is not due to the inefficiency of the induction/activation of the HRR genes after IR, but is due to the short time frame for SLDR. The maximum SLDR is completed within 2 hours in most mammalian cell lines, and the 2 h window only allows Ku-dependent classical NHEJ but not HRR to efficiently work since HRR needs a much longer time frame for cells to provide homologue templates while entering the S/G2 phases. Unlike HRR, Ku-dependent classical NHEJ does not need a homologue template at the DNA DSB ends and is independent of the cell cycle (Rothkamm et al. 2003), so the 2 h interval between the split doses is enough for NHEJ to occur. These results also explain why high-LET irradiated cells did not show efficient SLDR: since high-LET radiation already interferes with Ku-dependent classical NHEJ, therefore high-LET radiation inhibits SLDR as well. The mechanism underlying why SLDR has only a short period of time (~2 h) needs more studies to elucidate in the future.
Since radiotherapy is completed through multiple fractionations, SLDR plays an important role in protecting the survival of the irradiated human tumor cells that are proficient in Ku-dependent classical NHEJ. Our results indicate that inhibiting Ku-dependent classical NHEJ with inhibitors or high-LET radiation can efficiently inhibit SLDR, and subsequently promote more cancer cell killings, which will ultimately improve radiotherapy.
Acknowledgements
We thank Dr. Iliakis and Dr. Chen for providing the cell lines, Ms. Doreen Theune for editing the manuscript. This work is supported by grants from the National Aeronautics and Space Administration (NNX11AC30G to Y.W.) and the National Cancer Institute (P30CA138292 to the Institute).
Abbreviation
- SLDR
Sublethal damage repair
- IR
Ionizing radiation
- DSB
double strand breaks
- NHEJ
non-homologous end-joining
- HRR
homologous recombination repair
- PLDR
potential lethal damage repair
- LET
Linear energy transfer
- CHO
Chinese hamster ovary
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ben-Hur E, Elkind MM, Bronk BV. Thermally Enhanced Radioresponse of Cultured Chinese Hamster Cells: Inhibition of Repair of Sublethal Damage and Enhancement of Lethal Damage. Radiat Res. 2012;178:AV139–AV145. doi: 10.1667/rrav11.1. [DOI] [PubMed] [Google Scholar]
- Brenner DDS, Armour EPD, Corry PPD, Hall EDS. Sublethal Damage Repair Times for a Late-Responding Tissue Relevant to Brachytherapy (and External-Beam Radiotherapy): Implications for New Brachytherapy Protocols. Int J Rad Onc Bio Phys. 1998;41:135–138. doi: 10.1016/s0360-3016(98)00029-7. [DOI] [PubMed] [Google Scholar]
- Chen BPC, Chan DW, Kobayashi J, Burma S, Asaithamby A, Morotomi-Yano K, Botvinick E, Qin J, Chen DJ. Cell Cycle Dependence of DNA-dependent Protein Kinase Phosphorylation in Response to DNA Double Strand Breaks. J Biol Chem. 2005;280:14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- Elkind M, Sutton H. X-ray damage and recovery in mammalian cells in culture. Nature. 1959;184:1293–1295. doi: 10.1038/1841293a0. [DOI] [PubMed] [Google Scholar]
- Fuller LF, Painter RB. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mut Res. 1988;193:109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- Golding SE, Rosenberg E, Khalil A, McEwen A, Holmes M, Neill S, Povirk LF, Valerie K. Double strand break repair by homologous recombination is regulated by cell cycle-independent signaling via ATM in human glioma cells. J Biol Chem. 2004;279:15402–15410. doi: 10.1074/jbc.M314191200. [DOI] [PubMed] [Google Scholar]
- Guerrero M, Li X. Halftime for repair of sublethal damage in normal bladder and rectum: an analysis of clinical data from cervix brachytherapy. Phys Med Biol. 2006;51:4063–4071. doi: 10.1088/0031-9155/51/16/012. [DOI] [PubMed] [Google Scholar]
- Hall E, Giaccia A. Radiobiology for the Radiologist. Lippincott Williams &Wilkins; 2010. [Google Scholar]
- Hall EJ, Roizin-Towle L, Theus RB, August LS. Radiobiological Properties of High—Energy Cyclotron-Produced Neutrons Used for Radiotherapy. Radiology. 1975;117:173–178. doi: 10.1148/117.1.173. [DOI] [PubMed] [Google Scholar]
- Jacobson B. Evidence for recovery from x-ray damage in Chlamydomonas. Radiat Res. 1957;7:394–406. [PubMed] [Google Scholar]
- Lind B, Persson L, Edgren M, Hedlöf I, Brahme A. Repairable–Conditionally Repairable Damage Model Based on Dual Poisson Processes. Radiat Res. 2003;160:366–375. doi: 10.1667/0033-7587(2003)160[0366:rrdmbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Liu N, Lamerdin JE, Tebbs RS, Schild D, Tucker JD, Shen MR, Brookman KW, Siciliano MJ, Walter CA, Fan W, et al. XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- Liu Q, Schneider F, Ma L, Wenz F, Herskind C. Sublethal Damage (SLD) Repair: Relation to DNA Repair and Implications for Protracted Irradiation with Low-energy X-rays. Int J Rad Onc Bio Phys. 2011;81:S715–S716. [Google Scholar]
- Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA Damage Induced by Accelerated Heavy Ions in Mammalian Cells Proficient and Deficient in the Non-homologous End-Joining Pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- Pekkola-Heino K, Servomaa K, Kiuru A, Grenman R. Sublethal damage repair capacity in carcinoma cell lines with p53 mutations. Head & Neck. 1998;20:298–303. doi: 10.1002/(sici)1097-0347(199807)20:4<298::aid-hed3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Riballo E, Critchlow SE, Teo S-H, Doherty AJ, Priestley A, Broughton B, Kysela B, Beamish H, Plowman N, Arlett CF, et al. Identification of a defect in DNA ligase IV in a radiosensitive leukaemia patient. Current Biol. 1999;9:699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipler A, Iliakis G. DNA double-strand–break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic Acids Res. 2013;41:7589–7605. doi: 10.1093/nar/gkt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaiah N, Yarnold J, Lagerqvist A, Rothkamm K, Helleday T. Homologous recombination mediates cellular resistance and fraction size sensitivity to radiation therapy. Radiother Oncol. 2013;108:155–161. doi: 10.1016/j.radonc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Utsumi H, Tano K, Takata M, Takeda S, Elkind MM. Requirement for repair of DNA double-strand breaks by homologous recombination in split-dose recovery. Radiat Res. 2001;155:680–686. doi: 10.1667/0033-7587(2001)155[0680:rfrodd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang X, Zhang P-Y, Wang Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair. 2008;7:725–733. doi: 10.1016/j.dnarep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Zeng Z, Perrault A, Cheng X, Qin W, Iliakis G. Genetic evidence for the involvement of DNA ligase IV in the DNA-PK-dependnet pathway of non-homologous end joining in mammalian cells. Nucleic Acids Res. 2001;29:1653–1660. doi: 10.1093/nar/29.8.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang X, Wang P, Yu X, Essers J, Chen D, Kanaar R, Takeda S, Wang Y. Characteristics of DNA-binding proteins determine the biological sensitivity to high-linear energy transfer radiation. Nucleic Acids Res. 2010;38:3245–3251. doi: 10.1093/nar/gkq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Liu S, Zhang P, Zhang S, Naidu M, Wang HC, Wang Y. S-phase cells are more sensitive to high linear energy transfer radiation. Int J Rad Onc Bio Phys. 2009;74:1236–1241. doi: 10.1016/j.ijrobp.2008.12.089. [DOI] [PubMed] [Google Scholar]
- Wang X, Khadpe J, Hu B, Iliakis G, Wang Y. An over-activated ATR/CHK1 pathway is responsible for the prolonged G2 accumulation in irradiated AT cells. J Biol Chem. 2003;278:30869–30874. doi: 10.1074/jbc.M301876200. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang H, Wang P, Guida P, Chen B, Wang Y. The Ku dependent non-homologous end-joining pathway contributes to low dose radiation-stimulated cell survival. J Cell Physiol. 2011;226:369–374. doi: 10.1002/jcp.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous Recombination Contributes to the Repair of DNA Double-Strand Breaks Induced by High-Energy Iron Ions. Radiat Res. 2010;173:27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]
- Zaider M, Wuu CS, Minerbo GN. The Combined Effects of Sublethal Damage Repair, Cellular Repopulation and Redistribution in the Mitotic Cycle. I. Survival Probabilities after Exposure to Radiation. Radiat Res. 1996;145:457–466. [PubMed] [Google Scholar]