Abstract
The n-3 polyunsaturated fatty acids act as substrates during the resolution phase of acute inflammation for the biosynthesis of specialized pro-resolving lipid mediators. One premier example is the C22-dihydroxy-polyunsaturated fatty acid protectin D1 (1). The human 15-lipoxygenase type I, via stereoselective processes and with docosahexaenoic acid as the substrate, enables the formation of this specialized pro-resolving lipid mediator. Herein, based on results from LC/MS-MS metabololipidomics, support is presented for the apprehended biosynthesis of 1 in human macrophages occurs via the intermediate 16S,17S-epoxy-protectin (5). Stereochemical pure 5 was obtained using the Katsuki-Sharpless epoxidation protocol, establishing the chirality at the C16 and C17 atoms, one Z-selective reduction as well as E- and Z-stereoselective Wittig reactions. In addition, information on the non-enzymatic aqueous hydrolysis products and the half-life of 16S,17S-epoxy-protectin (5) is presented.
Graphical Abstract
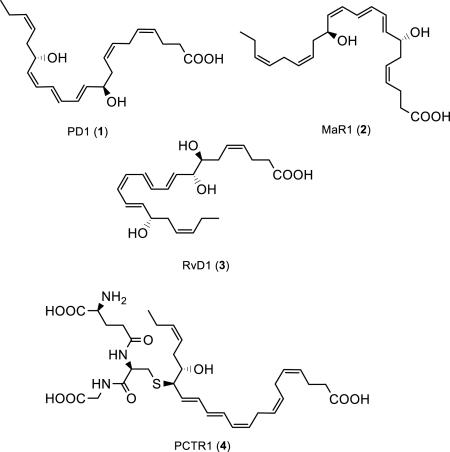
Several chemical mediators were recently identified that have the ability to initiate, modulate and dampen acute inflammation as well as stimulate resolution.1,2 Recent efforts have established that the return to homeostasis, i.e. catabasis,3 is due to the effective catalytic action by lipoxygenases and cyclooxygenases on the dietary n-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). During the resolution phase of acute inflammation these enzymatic processes stereoselectively form several distinct families of autacoids coined specialized pro-resolving mediators (SPMs),4-6 such as protectin D1 (PD1, 1), maresin 1 (2), resolvin D1 (3) and the protectin conjugate 16R-glutathionyl,17S-hydroxy-4Z,7Z,10Z,12E,14E,19Z-docosahexaenoic acid (PCTR1, 4).
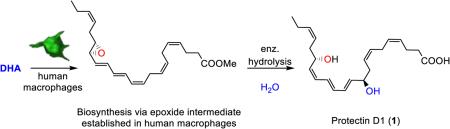
Protectin D1 (1),7-10 also known as neuroprotectin D1 (NPD1) when produced in neural systems,9 is a 15-lipoxygenase product of DHA formed in the presence of molecular oxygen.11 PD1 (1) is a significant example among the naturally occurring compounds belonging to the SPMs, exhibiting potent anti-inflammatory properties together with pro-resolving actions.11 PD1 (1) inhibits polymorphonuclear leukocytes infiltration and promotions of macrophage phagocytosis and efferocytosis.11,12 The exact biomolecular mechanisms underlying the resolution of many inflammatory diseases have been investigated with the use of SPMs.5,12 The lipid mediator PD1 (1) has been the subject of several pharmacological studies5 and some clinical trial development programs have been launched with PD1 (1).5
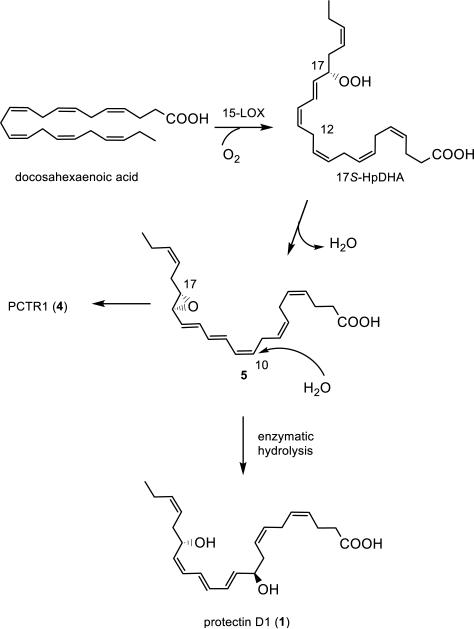
The exact structure of 1 was established in 2006 by direct comparison of isomers of 1 obtained by stereoselective synthesis.11,13 Protectin D1 (1) was disclosed to arise via an epoxide intermediate based on data from experiments using both isotopic oxygen incorporation and identification of acid alcohol trapping products via mass spectrometry (Figure 1).10,11
Figure 1.
Outline of the biosynthesis of protectin D1 (1) and the protectin conjugate PCTR1 (4).
The epoxide 5 is most likely also pivotal in the biosynthesis of novel protectin conjugates in tissue regeneration.14 Earlier studies of the biosynthesis of 1 yielded insufficient amounts to confirm the existence and the absolute configuration of this epoxide biosynthetic intermediate.
Due to the potent bioactions reported for protectin D1 (1)5 and the protectin glutathione conjugate PCTR1 (4),14 it is of considerable interest to investigate the involvement of the epoxide intermediate 5 in the biosynthesis of 1. Herein, support for the existence of such an intermediate, named 16S,17S-epoxy-protectin (5), is provided. In addition, data on the aqueous stability, the non-enzymatic aqueous hydrolysis products and the enzymatic product formed from 5, but also the half-life of 16S,17S-epoxy-protectin (5) under aqueous conditions, are reported. Together, these efforts renders support for the involvement of 5 in the biosynthesis of protectin D1 (1) in human macrophages.
RESULTS AND DISCUSSION
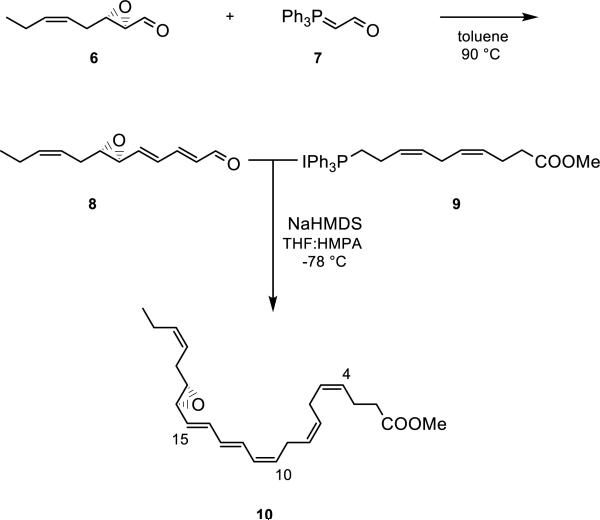
To establish evidence of the existence of an epoxide intermediate and its role as a biosynthetic precursor to bioactive protectins, stereochemically pure epoxide 5 was obtained by total synthesis. First, aldehyde 6 was prepared from commercially available cis-hex-3-en-1-ol in several steps, including a Katsuki-Sharpless asymmetric epoxidation reaction (Supporting Information).15 The absolute configuration set in the epoxidation process was assured via correlation with previously reported data.16 An E-selective Wittig reaction17 between 6 and the commercially available ylide (triphenylphosphoranylidene)acetaldehyde (7) furnished stereochemically pure aldehyde 8 in 61% yield after chromatographic purification. Next, Wittig salt 9 was prepared from (Z)-7-methoxy-7-oxohept-3-en-1-yl triphenylphosphonium iodide, the latter prepared as previously reported from 4-pentynoic acid.18 Lastly, aldehyde 6 was reacted in a Z-selective Wittig reaction18-22 with the ylide of 9, which was generated at at −78 °C after treatment with NaHMDS in THF and HMPA. This sequence yielded the epoxide ester 10 in 41% yield (Scheme 1). Thus, compound 10 was made in 10 steps, starting from commercially available 4-pentynoic acid, with an overall yield of 6% counting the longest linear sequence (Supporting Information). The synthesis of the chemically labile 16S,17S-epoxy-protectin (5) was achieved by a chemoselective basic aqueous hydrolysis18-22 of the methyl ester in 10 just prior to incubations (Supporting Information).
Scheme 1.
Synthesis of the methyl ester 10 of 16S,17S-epoxy-protectin (5).
The geometrical configurations of the olefins constituting the conjugated triene moiety of 10 were established using a COSY-45 NMR experiment. The results thus obtained are in accordance with an Z,E,E-triene present in 10. The following chemical shifts and coupling constants (J values) were recorded for the epoxy methyl ester 10: H12: 6.56 ppm, J = 14.9, 11.2 Hz; H14: 6.41 ppm, J = 15.2, 10.9 Hz; H13: 6.11 ppm, J = 14.9, 10.8 Hz; H11: 6.03 ppm, J = 11.2 Hz; H4, H5, H7, H8, H10, H15, H19, H20: 5.54-5.25 ppm. Notably, H11 has a 3J-coupling constant to H10 with a value of 11.0 Hz, which is consistent with the assigned Z- rather than an E-configured C10-C11 double bond.
Identification of Non-enzymatic Aqueous Hydrolysis Products from Synthetic 16S,17S-epoxy-Protectin (5)
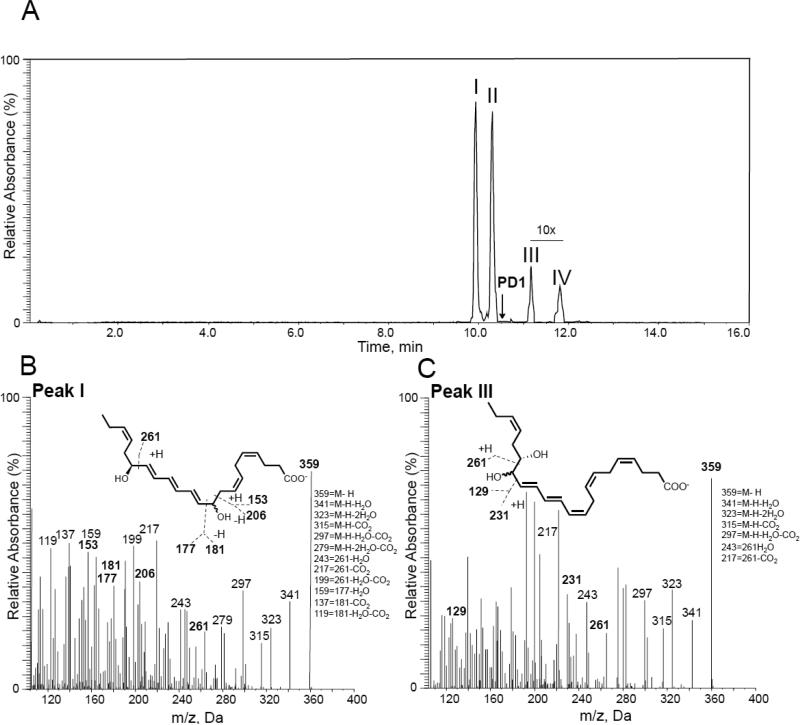
First 16S,17S-epoxy-protectin (5) was added to H2O (pH = 7.0) at room temperature. Incubations were quenched after 2 minutes and deuterated internal standards were added to facilitate identification. The products were extracted using solid phase extraction on C18 columns, and then the aqueous hydrolysis product profile of the synthetic material 5 was investigated using LC/MS-MS based lipid mediator metabololipidomics.23 Chromatographic analysis using multiple reaction monitoring gave four distinct peaks in incubations of synthetic 16S,17S-epoxy-protectin (5) with pH = 7.0 H2O (Figure 2A). Their chromatographic behavior and respective MS-MS spectra indicated the presence of two vicinal diol epoxide hydrolysis products (TR ~ 9.8 min and TR ~ 10.3 min) as well as two products (TR ~ 11.2 min and TR ~ 11.8 min) that arose from addition of H2O to the least sterically hindered end of the protectin carbocation intermediate.11,24 We next determined the identity of each of the products in these incubations assessing their respective MS-MS spectra and matching these with authentic and/or synthetic standards.24 These results demonstrated that the four products were isomers of protectin D1 (1) as authentic protectin D1 (1) eluted at a different retention time in this system (Figure 2A). The products beneath peaks I and II gave essentially identical MS-MS fragmentation spectra (Figure 2B) and were consistent with 10,17S-dihydroxydocosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid, with the double bonds at positions 4, 7 and 19 being uninvolved in the aqueous hydrolysis reaction and therefore their configurations are proposed to be retained in the cis configuration.25,26 Products under peaks III and IV also gave essentially identical MS-MS fragmentation spectra to each other (Figure 2C) and were consistent with the vicinal diol structure of 16,17S-dihydroxydocosa-4Z,7Z,10Z,12E,14E,19Z-hexaenoic acid. The double bonds at positions 4, 7 and 19 are also thought to retain their cis configuration given their non-involvement in the aqueous hydrolysis reaction. Because authentic PD1 (1) eluted at a different retention time to these four products, this indicated that the aqueous hydrolysis of this protectin intermediate 5 is analogous27 to that of leukotriene A4 (LTA4)28,29 and 13S,14S-epoxy maresin.30
Figure 2.
LC/MS-MS profile of synthetic 16S,17S-epoxy-protectin (5) non-enzymatic aqueous hydrolysis products. Synthetic 16S,17S-epoxy-protectin (5, 200 ng/200 μL) was placed in double distilled H2O (pH = 7.0), products were extracted and profiled using lipid mediator metabololipidomics. (A) MRM chromatogram of products m/z 359 > 217. Arrow denotes retention time of synthetic and authentic protectin D1 (1). (B, C) MS-MS spectra employed for identification of products under peaks (B) I and (C) III. Results are representative of n = 4 incubations.
Matching of Products from Synthetic 16S,17S-Epoxy-Protectin (5) and Products from Macrophage Epoxide Intermediate
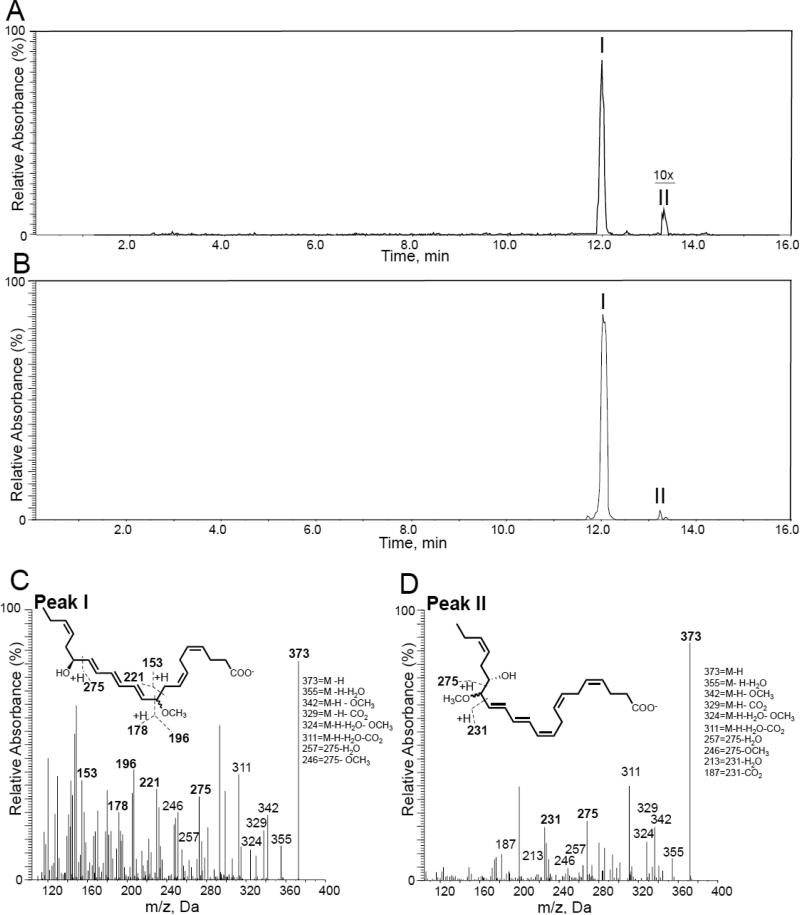
To assess the production of this protectin epoxide intermediate by human macrophages we sought evidence for the formation of methoxy-trapping products following acidic MeOH trapping. This approach was taken because the amounts of this intermediate produced by human cells are less than what is required for direct NMR analysis. Protectin D1 (1) is produced during self-resolving Escherichia coli (E. coli) infections,31 and given the fact that the lipid mediator 1 accelerates the resolution of E.coli infections as well as stimulates the phagocytosis of E.coli by macrophages, we tested if these human macrophages would produce 16S,17S-epoxy-protectin (5). E. coli does not form protectin D1 (1).31 Using multiple reaction monitoring of products obtained with human macrophage incubations gave two distinct peaks (Figure 3A); the major peak I gave a retention time (TR) of 12.0 min and peak II gave TR 13.4 min. Incubation of synthetic 16S,17S-epoxy-protectin (5) with acidified methanol also gave two peaks with TR 12.0 and 13.4 min, respectively (Figure 3B). Assessment of the MS-MS fragmentation spectra for the product beneath peak I gave characteristic fragmentation of 10-methoxy,17S-hydroxydocosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid (likely a pair of diasteromers that co-elute) and display m/z 373 = M-H, m/z 355 = M-H-H2O, m/z 342 = M-H-OCH3, m/z 329 = M-H-CO2, m/z 324 = M-H-H2O-OCH3, m/z 311 = M-H-H2O-CO2 and m/z 246 = 275-OCH3 (Figure 3C). Assessment of the MS-MS fragmentation spectra for the product under peak II gave characteristic fragmentation of 16-methoxy,17S-hydroxydocosa-4Z,7Z,10Z,12E,14E,19Z-containing products, (also likely a pair of diasteromers that co-elute) (Figure 3D). The configurations of the double bonds at carbon positions 4, 7 and 19 are likely to be retained in the cis configuration since they are uninvolved in the reaction with MeOH. These results provide evidence that the acid alcohol trapping products of synthetic 16S,17S-epoxy-protectin (5) match those derived from human macrophage incubations and are in accord with previous findings.8 In both experiments using acidified MeOH, the formation of the two 10-methoxy-17S-hydroxy isomeric products dominated.
Figure 3.
Evidence for the production of 16S,17S-epoxy-protectin by human macrophages. Human macrophages (5 × 106 cells/mL) were incubated (PBS, pH = 7.45, 37 °C) and quenched after 2 min with acidified (apparent pH = ~3) MeOH and products were extracted and profiled using lipid mediator metabololipidomics. (A) Synthetic 16S,17S-epoxy-protectin (5, 200 ng/200 μL) was placed in acidified methanol (apparent pH = ~3), products were profiled using lipid mediator metabololipidomics. MRM chromatogram for transition m/z 373 > 246. (B) MRM chromatogram of acid alcohol trapping products of the protectin intermediate from macrophages, transition m/z 373 - 246. Synthetic 16S,17S-epoxy-protectin (5, 200 ng/200 μL) was employed. (C, D) MS-MS spectra employed for the identification of products under peaks C) I and D) II. Results are representative of n = 3 separate healthy donors for A, C and D. For B results are representative of n = 3 incubations.
Conversion of 16S,17S-Epoxy-Protectin (5) to Protectin D1 and Isomers (1) by Human Macrophages
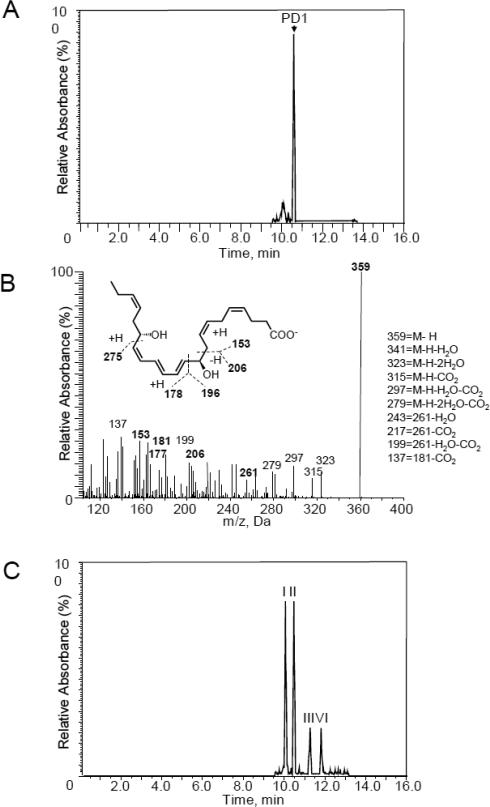
We next investigated the conversion of 16S,17S-epoxy-protectin (5) to protectin D1 (1) and isomers of 1. To this end, we incubated human macrophages with synthetic 5 and profiled the products using LC-MS-MS based lipid mediator profiling.23 Liquid chromatographic analysis of the products obtained using MRM for the ion pair m/z 359>153 gave a sharp peak with retention time (TR) = 10.5 min (Figure 4A). Moreover, an MS-MS fragmentation pattern with m/z 359 = M-H, m/z 341 = M-H-H2O-CO2, m/z 323 = M-H-2H2O, m/z 315 = M-H-CO2, m/z 297 = M-H-H2O-CO2, m/z 279 = M-H-2H2O-CO2, m/z 243 = 261-H2O, m/z 217 = 261-CO2, m/z 199 = 261-H2O-CO2 and m/z 137 = 181-CO2, characteristic of protecin D1 (1), was observed (Figure 4B).7,11,18 Hence, enzymatic conversion of 5 to protectin D1 (1) was observed. Moreover, when the 16S,17S-epoxy-protectin (5) was incubated with macrophages that were incubated for 1h at 100°C prior to addition of 5, four isomeric products were observed (Figure 4C). The peaks I and II gave characteristic retention times and MS-MS spectra corresponding with 10, 17S-dihydroxydocosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid isomers. In addition, peaks III and IV gave retention times and MS-MS products consistent with 16,17S-dihydroxydocosa-4Z,7Z,10Z,12E,14E,19Z-hexaenoic acid isomers. The same MS-MS fragmentation patterns were observed for these four products using denatured macrophages prior to addition of 5, as reported in Figure 2B and Figure 2C, consistent with isomers of 10,17S-dihydroxydocosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid13,32 and two vicinal diol isomers of 16,17S-dihydroxydocosa-4Z,7Z,10Z,12E,14E,19Z-hexaenoic acid, respectively. Altogether, data from these experiments reveal that stereochemically pure epoxide 5 was converted to protectin D1 (1). In the absence of enzymatic activity, epoxide 5 was also converted to the same isomers of protectin D1 (1) shown in Figure 2.
Figure 4.
Sterochemical pure 16S,17S-epoxy-protectin (5) is converted to protectin D1 (1) and isomers of protectin D1 (1) by human macrophages. Human macrophages (5×107 cells/ml) obtained from peripheral blood mononuclear cells were incubated with 16S,17S-epoxy-protectin (5) (1.0 μM) and E. coli (2.5×108 cells/ml; DPBS containing 10% BSA; pH = 7.45; 37°C). Incubations were quenched after 30 min using ice-cold methanol and products were assessed using metabololipidomics. A) Selected ion chromatogram (m/z 359>153) depicting protectin D1 (1). B) MS-MS spectrum employed for identification of protectin D1 (1). C) Human macrophages were incubated at 100°C for 1 h, then with 16S,17S-epoxy-protectin (5) (1.0 μM) and E. coli (2.5×108 cells/ml; DPBS containing 10% BSA; pH = 7.45; 37°C), incubations were quenched after 60 min and products assessed using metabololipidomics. Results are representative of n = 4 separate cell preparations.
Investigations on Aqueous Stability and Determination of Half-life
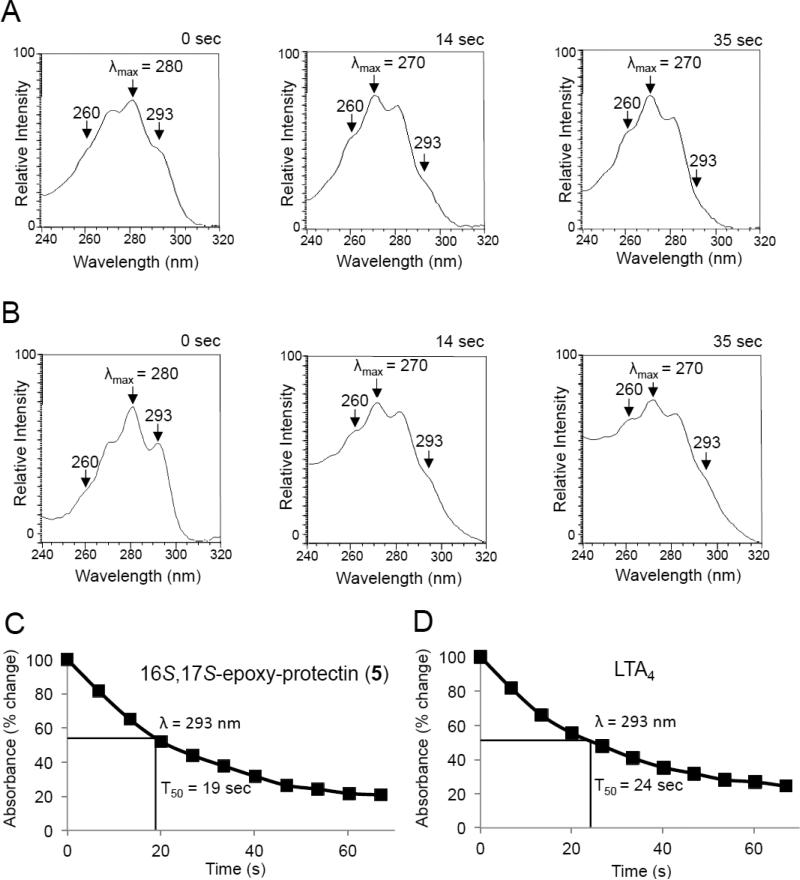
We next tested the aqueous stability of synthetic 16S,17S-epoxy-protectin (5), see Supporting Information for details. Assessment of UV absorbance for this synthetic product in phosphate buffered saline (pH = 7.45) demonstrated a rapid shift in the λmax from 280 nm, characteristic of a conjugated triene allylic to an auxochrome, to 270 nm (Figure 5). For comparison, LTA4 also gave a shift in λmax from 280 nm to 270 nm (Figure 5). In these incubations, we also found a reduction in absorbance at 293 nm giving an estimated half-life of ~19 seconds for 16S,17S-epoxy-protectin (5) and ~24 seconds for LTA4 (Figure 5). Together these results establish the identity of the non-enzymatic hydrolysis products for the protectin epoxide biosynthetic intermediate and the configuration of the conjugated triene in this human macrophage intermediate 5. The time course of 16S,17S-epoxy-protectin (5) stability was similar to that of LTA4 and this suggests also that the protectin epoxide 5, like that of LTA4 in the biosynthesis of for example LTB4, can be a key intermediate in transcellular biosynthesis during cell-cell interactions in complex tissues.27-29
Figure 5.
Aqueous stability of 16S,17S-epoxy-protectin (5). UV chromophores of A) LTA4 and B) 16S,17S-epoxy-protectin (5) and their non-enzymatic hydrolysis products (PBS, pH = 7.45, room temperature) at indicated intervals. UV absorbance at 293 nm for C) Synthetic 16S,17S-epoxy-protectin (5, 200 ng/200 μL, PBS, pH = 7.45, room temperature) and D) LTA4 (200 ng/200 μL, PBS, pH = 7.45, room temperature). A and B are representative of n = 3 experiments. C and D are mean of n = 3 experiments. The methyl ester of LTA4 was purchased from Cayman Chemical Company and hydrolyzed just prior to use, see Supporting Information.
The first step in the biosynthesis of protectin D1 (1) depicted in Scheme 1 is a stereoselective abstraction of the pro-S hydrogen atom at C-17 of DHA to form 17S-HpDHA mediated by 15-lipoxygenase type I. This hydroperoxide is converted to the 16S,17S-configurated epoxide 5 with a concurrent second hydrogen abstraction at C-12 mediated by the same lipoxygenase.5,24 These processes result in the formation of the 10Z,12E,14E-configured triene in 5. Then 5, under enzymatically hydrolysis conditions, is regio- and stereoselectively attacked by water at the C-10 position, via a transient allylic carbocation specie, that results in the formation of the 10R-configured allylic alcohol and the 11E,13E,15Z-configured triene in protectin D1 (1). During these enzymatically controlled steps the S-configuration at C-17 is not altered.5 This is consistent with the accepted biosynthesis of other known pro-resolving mediators25,26 and the leukotrienes, such as LTB4, the latter class of mediators via the LTA4 epoxide.28,29 Based on these observations, and by comparison of data obtained from LC-MS/MS experiments using synthetic isomers of 1,13,32,33 it is unlikely that the enantiomeric 16R,17R-configurated epoxide of 5 is formed in human macrophages. Moreover, the formation of the 17R-HpDHA stereoisomer has been observed in the presence of aspirin and recombinant isolated COX-2 enzyme,7 that results in the formation of the 17R-epimer of protectin D1 (1), coined AT-protectin D1, most likely via a 16R,17R-configurated epoxide intermediate. The absolute configuration of AT-protectin D1 was determined based on results from LC/MS-MS metabololipidomics experiments32 using synthetic standards.33
To summarize, we have now provided additional support for the biosynthesis of protectin D1 (1) in human macrophages occurs as depicted in Scheme 1 via the intermediate 16S, 17S-epoxy-protectin (5). Recently Rodriguez and Spur reported a steroselective total synthesis of PCTR1 (4) that established the absolute configuration of 4.35,36 Notably, the SPM 4 contains the 10Z,12E,14E-triene and an anti-disubstituted thioether alcohol moiety with R- and S-configurations at the C16 and C17 carbon atoms, respectively. These structural features also render support for the existence and involvement of 16S,17S-epoxy-protectin (5) as an intermediate in the biosynthesis of the protectins.14 The biosynthesis of the protectin members of the SPMs involved in human physiological processes differs from other oxygenated natural products, such as the oxylipins.37 For the latter class of natural products, lipoxygenase and allene oxide synthase are the enzymes involved in the formation of epoxy allylic cation derived intermediates.38 In human physiological systems the biosynthesis of the protectin class of SPMs results in natural products, with a E,E,Z-triene moiety, exhibiting potent pro-resolving actions. Altogether, these features distinguish the SPMs from other oxygenated natural products of non-human origin. Further investigations are on-going towards elucidating the role of the intermediate 5 in the biosynthesis of novel SPMs and protectin conjugates in tissue regeneration.14,34 Since the SPMs are of interest as agonists in resolution physiology, these natural products will continue to attract interest from the research community.4
EXPERIMENTAL SECTION
General Experimental Procedures
Optical rotations were measured using a 1 mL cell with a 1.0 dm path length on a Perkin Elmer 341 polarimeter. The UV/Vis spectra from 190-900 nm were recorded using a Biochrom Libra S32PC spectrometer using quartz cuvettes. NMR spectra were recorded on a Bruker AVII400 spectrometer at 400 MHz or a Bruker AVII600 spectrometer at 600 MHz. for 1H NMR, and at 101 MHz or 150 MHz for 13C NMR. Spectra are referenced relative to the central residual protium solvent resonance in 1H NMR (CDCl3 δ = 7.27, C6D6 δ = 7.16, and MeOH-d4 δ = 3.31) and the central carbon solvent resonance in 13C NMR (C6D6 δ = 128.06 ppm, CDCl3 δ = 77.00 ppm, and MeOH-d4 = δ 49.00). Mass spectra were recorded at 70 eV on a Waters Prospec Q spectrometer using EI, ES or CI as the method of ionization. High-resolution mass spectra were recorded on a Waters Prospec Q spectrometer using EI or ES as the methods of ionization. Thin layer chromatography was performed on silica gel 60 F254 aluminum-backed plates fabricated by Merck. Flash column chromatography was performed on silica gel 60 (40-63 μm) produced by Merck. HPLC analyses for chemical purities were performed on an Agilent Technologies 1200 Series instrument with diode array detector set at 254 nm and equipped with a C18 stationary phase (Eclipse XDB-C18 5 μm 4.6 × 150 mm), applying the conditions stated. Unless stated otherwise, all commercially available reagents and solvents were used in the form they were supplied without any further purification. The stated yields are based on isolated material. Diastereomeric ratios reported in this paper have not been validated by calibration, see Wernerova and Hudlicky, for discussions and guidelines.39 Liquid chromatography (LC)-grade solvents were purchased from Fisher Scientific. The Eclipse Plus C18 column (100 × 4.6 mm × 1.8 μm) was obtained from Agilent and C18 SPE columns were from Waters. The synthetic standards for LC-tandem mass spectrometry (MS-MS) quantitation and deuterated (d) internal standards (d4-PGE2 and d4-LTB4) were purchased from Cayman Chemicals. Zymosan A was purchased from Sigma Aldrich, anti-mouse CD11b and anti-mouse Ly6Gwere purchased from Biolegend.
(2E,4E)-5-((2S,3S)-3-((Z)-Pent-2-en-1-yl)oxiran-2-yl)penta-2,4-dienal (8)
Aldehyde 6 (100 mg, 0.71 mmol, 1.0 equiv) was dissolved in anhydrous toluene (4 mL) and solid (triphenylphosphoranylidene)acetaldehyde (7) (446 mg, 1.50 mmol, 2.1 equiv.) was added in one portion. The reaction setup was thoroughly evacuated and re-filled with argon (3×) and then heated to 90 °C for 4.5 h. The resulting deep red solution was allowed to cool to room temperature and concentrated in vacuo. The residue was loaded onto a silica gel column and eluted with 26% Et2O in heptane to yield product 8 (84 mg, 0.44 mmol, 61%) as a colorless oil. - 57 (c 0.10, CHCl3); 1H NMR (600 MHz, CDCl3) δH 9.57 (d, J = 7.9 Hz, 1H), 7.07 (dd, J = 15.3, 11.0 Hz, 1H), 6.62 (dd, J = 15.3, 11.0 Hz, 1H), 6.16 (dd, J = 15.3, 7.9 Hz, 1H), 5.98 (dd, J = 15.3, 7.4 Hz, 1H), 5.61 – 5.49 (m, 1H), 5.40 – 5.30 (m, 1H), 3.25 (dd, J = 7.4, 2.0 Hz, 1H), 2.95 (td, J = 5.3, 2.0 Hz, 1H), 2.49 – 2.41 (m, 1H), 2.41 – 2.32 (m, 1H), 2.06 (p, J = 6.8, 6.2 Hz, 2H), 0.98 (t, J = 7.5 Hz, 3H), 13C NMR (151 MHz, CDCl3) δC 193.7, 150.1, 141.4, 135.5, 132.3, 131.0, 121.9, 61.0, 56.9, 29.7, 20.9, 14.3. HRESITOFMS m/z 215.1050 [M+Na]+ (calcd for C12H16O2Na, 215.1047); TLC (heptane/Et2O 74:26, KMnO4 stain): Rf = 0.12. Note: A significant amount of the α,β-unsaturated aldehyde resulting from a single Wittig reaction between 6 and 7 pre-elutes product 8 under these purification conditions.
Methyl (4Z,7Z,10Z,12E,14E)-15-((2S,3S)-3-((Z)-pent-2-en-1-yl)oxiran-2-yl)pentadeca-4,7,10,12,14-pentaenoate (10)
Wittig salt 9 (50 mg, 0.088 mmol, 1.3 equiv.) was dissolved in THF (1 mL) and HMPA (0.15 mL) and cooled to −78 °C. NaHMDS (0.6 M in toluene, 0.15 mL, 0.088 mmol, 1.3 equiv.) was added dropwise and the reaction mixture was stirred for 30 min, giving an orange, completely homogenous solution. Aldehyde 8 (13 mg, 0.067 mmol, 1.0 equiv.) was azeotroped twice with 2-methyltetrahydrofuran, dissolved in THF (0.5 mL), cooled to −78 °C and added dropwise to the reaction flask via cannula. After 1 h, the flask was rapidly warmed to −20 °C for a few minutes and then quenched by the addition of phosphate buffer (pH = 7, 2 mL). The phases were separated and the aqueous phase was extracted with Et2O (3 × 1 mL). The combined organic layers were dried (Na2SO4), filtrated and concentrated in vacuo. The material thus obtained was purified twice by microscale flash column chromatography employing a Pasteur pipette; the silica gel to which was added as a slurry prepared using 3% Et3N, 20% Et2O in heptane, and the column was eluted with 25% Et2O in heptane to provide the epoxy methyl ester 10 in 42% yield (10 mg) as a colourless oil. - 44 (c = 0.30, CHCl3); UV (hexane) λmax 272 (log ε 3.61), 280 (log ε 4.05), 291 (log ε 2.79) nm. 1H NMR (400 MHz, C6D6) δH 6.56 (dd, J = 14.9, 11.3 Hz, 1H), 6.41 (dd, J = 15.2, 10.9 Hz, 1H), 6.11 (dd, J = 14.9, 10.8 Hz, 1H), 6.09 – 5.98 (m, 1H), 5.54 – 5.25 (m, 8H), 3.34 (s, 3H), 3.06 (dd, J = 7.8, 2.0 Hz, 1H), 2.96 – 2.86 (m, 2H), 2.85 – 2.76 (m, 2H), 2.72 (td, J = 5.2, 2.0 Hz, 1H), 2.31 (q, J = 7.6 Hz, 2H), 2.26 – 2.16 (m, 2H), 2.18 – 2.07 (m, 2H), 1.92 (p, J = 7.3 Hz, 2H), 0.88 (t, J = 7.5 Hz, 3H), 13C NMR (101 MHz, C6D6) δC 172.8, 134.7, 134.2, 132.4, 131.4, 131.1, 129.3, 129.2, 129.0, 128.8, 128.5, 128.5, 123.2, 60.3, 57.8, 51.1, 34.0, 30.1, 26.6, 26.0, 23.2, 21.0, 14.4. HRESITOFMS m/z 379.2252 [M+Na]+ (calcd for C23H32O3Na, 379.2249); TLC (heptanes/Et2O 74:26, CAM stain): Rf = 0.23. The spectral data were in accord by those reported by Rodriguez and Spur.35
Lipid Mediator Metabololipidomics.23
The LC-MS-MS system included a Shimadzu LC-20AD HPLC and a Shimadzu SIL-20AC autoinjector (Shimadzu) paired with a QTrap 5500 (ABSciex) coupled to a Poroshell 120 EC column (100 mm × 4.6 mm × 2.7 μm; Agilent) maintained at 50 °C using a column oven (ThermaSphere TS-130; Phenomenex) employed for identification and quantification. For product profiling the following mobile phase was employed: MeO/H2O/acetic acid of 55:45:0.1 (vol:vol:vol) with a gradient ramped to 80:20:0.1 (vol:vol:vol) for 8 min, that was held for 3 min and then ramped to 98:2:0.1 (vol:vol:vol) for the next 0.1 min and maintained at 98:2:0.1 (vol:vol:vol) for 4 min, with a flow rate maintained at 0.6 mL/min. The QTrap 5500 was operated in negative ionization mode using scheduled multiple reaction monitoring coupled with information-dependent acquisition (IDA) and an enhanced product ion scan (EPI).
Incubations with Human Macrophages
Human peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient Ficoll-Histopaque isolation as described earlier.40 Isolated PBMCs were washed three times to remove platelets, and monocytes purified by adhesion to tissue culture plates for 30 min (37 °C, DPBS+/+, pH = 7.45). These were then cultured for 7 days in RPMI-1640 medium supplemented with 10% FCS and 20 ng/mL GM-CSF. Macrophages (~1 × 106 cells/mL) were suspended in PBS+/+ containing DHA (1 μM) and E. coli (5 × 107 CFU) were added. After 2 min (37 °C, pH = 7.45) the incubation was stopped with 2 volumes acidified ice-cold MeOH (apparent pH = ~3), deuterated internal standards were added to facilitate identification, products extracted using C18 solid phase extraction, and profiled using LC/MS-MS based lipid mediator metabololipidomics.23 In separate experiments human macrophages (~1 × 106 cells/mL) were suspended in PBS+/+ then incubated with 5 (200 ng/200μl) and E. coli (5 × 107 CFU). After 5 min the incubations were quenched and products profiled as above. The solution of 5 was kept on solid dry ice in a closed container prior to use.
Human peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient Ficoll-Histopaque isolation.30 Isolated PBMCs were washed 3 times to remove platelets, and monocytes were purified by adhesion to tissue culture plates for 30 min (37°C, DPBS+/+, pH = 7.45). Cells were then cultured for 7 days in RPMI 1640 medium supplemented with 10% FCS and 20 ng/ml GM-CSF. Macrophages (5×107 cells) were suspended in PBS+/+ containing 10% BSA and incubated for 10 min (37°C, pH 7.45) prior to the addition of 16S,17S-epoxy-protectin (5) (~1.0 μM) and E. coli (2.5×108 cells/ml). The solution of 5 was kept on solid dry ice in a closed container prior to use. After 30 min, incubations were stopped with 2 volumes ice-cold methanol, d4-LTB4 was added to facilitate quantification and identification, and products were extracted using C18 columns and quantified by LC-MS-MS metabololipidomics.23 In select experiments macrophages were incubated at 100°C for 1h prior to addition of 16S,17S-epoxy-protectin (5) and E. coli and incubations conducted as detailed above.
Supplementary Material
ACKNOWLEDGMENT
The Research Council of Norway (KOSK-II 197704/V30) and The School of Pharmacy, University of Oslo, are gratefully acknowledged for Ph.D.-scholarships to M.A. and J.E.T., respectively. I.V., R.A.C. and J.D. are supported by the National Institutes of Health GM Grant PO1GM095467 (C.N.S.).
Footnotes
Supporting Information. Experimental procedures for the synthesis and characterization data, i.e. 1H-, 13C- spectra data, HRMS and UV/VIS spectra, as well as chromatograms from GLC and HPLC analyses of intermediates 6, 8, and 9 and the epoxy methyl ester 10, as well as for the intermediates employed in the preparation of 6, are available in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
C.N.S. has filed patents on PD1 (1) and related compounds. C.N.S.'s interests are reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict of interest policies.
REFERENCES
- 1.Tabas I, Glass CK. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown HA, Marnett LJ. Chem. Rev. 2011;111:5817–5820. doi: 10.1021/cr200363s. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Nat. J. Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Petasis NA. Chem. Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Proc. Natl. Acad. Sci. USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariel A, Pin-Lan L, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN. J. Biol. Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 11.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. J. Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 12.Schwab JM, Chiang N, Arita M, Serhan CN. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The following stereoisomers were employed: 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (protectin D1 (1)); 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid; 10S,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid and 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid.
- 14.Dalli J, Ramon S, Norris PC, Colas RA, Serhan CN. FASEB J. 2015;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar DA, Meshram HM. Synth. Commun. 2013;43:1145–1154. [Google Scholar]
- 16.Kang S, Kim Y, Lim J, Kim K, Kim S. Tetrahedron Lett. 1991;32:363–366. [Google Scholar]
- 17.Maryanoff BE, Reitz AB. Chem. Rev. 1989;89:863–927. [Google Scholar]
- 18.Aursnes M, Tungen JT, Vik A, Dalli J, Hansen TV. Org. Biomol. Chem. 2014;12:432–437. doi: 10.1039/c3ob41902a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aursnes M, Tungen JT, Vik A, Collas R, Cheng C-Y, Dalli J, Serhan CN, Hansen TV. J. Nat. Prod. 2014;77:910–916. doi: 10.1021/np4009865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tungen JE, Aursnes M, Dalli J, Arnardottir H, Serhan CN, Hansen TV. Chem. Eur. J. 2014;20:14575–14578. doi: 10.1002/chem.201404721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tungen JE, Aursnes M, Hansen TV. Tetrahedron Lett. 2015;56:1843–1846. [Google Scholar]
- 22.Tungen JE, Aursnes M, Vik A, Ramon S, Colas R, Dalli J, Serhan CN, Hansen TV. J. Nat. Prod. 2014;77:2241–2247. doi: 10.1021/np500498j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Am. J. Physiol. Cell. Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serhan CN, Dalli J, Colas RA, Chiang N. Biochim. Biophys. Acta. 2015;1851:397–413. doi: 10.1016/j.bbalip.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corey EJ, Mehrota MM. Tetrahedron Lett. 1986;27:5173–5176. [Google Scholar]
- 26.Serhan CN. Biochim. Biophys. Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 27.Wong PYK, Serhan CN. Cell-cell interactions in the release of inflammatory mediators: eicosanoids, cytokines, and adhesion. Plenum Press; New York; London: 1991. [PubMed] [Google Scholar]
- 28.Rådmark O, Malmsten C, Samuelsson B, Clark DA, Goto DA, Marfat A, Corey EJ. Biochem. Biophys. Res. Commun. 1980;92:954–961. doi: 10.1016/0006-291x(80)90795-0. [DOI] [PubMed] [Google Scholar]
- 29.Haegström JZ. J. Biol. Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 30.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan, Charles N. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Nature. 2012;484:524–529. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Friedman G, Yang R, Karamanov S, Belayev LS, Bazan NG, Zhu M, Winkler JW, Petasis NA. Chem. Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. In the 2011 study, the following stereoisomers obtained by stereoselective synthesis were employed: 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (protectin D1 (1)); 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (AT-PD1); 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid; 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexaenoic acid; 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13Z,15E,19Z-hexaenoic acid; 10R,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid and 10S,17R-dihydroxy-docosa-4Z,7Z,11E,13E,15E,19Z-hexaenoic acid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petasis NA, Yang R, Winkler JW, Zhu M, Uddin J, Bazan NG, Serhan CN. Tet. Lett. 2012;53:1695–1698. doi: 10.1016/j.tetlet.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalli J, Chiang N, Serhan CN. Proc. Natl. Acad. Sci. USA. 2014;111:E4753–61. doi: 10.1073/pnas.1415006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez AR, Spur BW. Tet. Lett. 2015;56:5811–5815. [Google Scholar]
- 36.The synthetic material of PCTR1 (4) was matched with endogenously formed 4 that displayed essentially near identical chromatographic properties and MS-MS fragmentation spectra with synthetic 4. Rodriguez; A. R.; Spur, B. W.; Serhan, C. N. unpublished results. See reference 23 for details on LC/MS-MS metabololipidomics.
- 37.Gerwick WH. Chem. Soc. Rev. 1993;93:1807–1823. [Google Scholar]
- 38.Gerwick WH. Lipids. 1996;31:1215–1231. doi: 10.1007/BF02587906. [DOI] [PubMed] [Google Scholar]
- 39.Wernerova M, Hudlicky T. Synlett. 2010;18:2701–2707. [Google Scholar]
- 40.Dalli J, Serhan CN. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.