Abstract
Background
Late allergic reactions are common in the course of allergen-specific immunotherapy and even occur with allergy vaccines with reduced IgE reactivity.
Objective
We sought to study atopy patch test (APT) reactions and T-cell responses to the recombinant birch pollen allergen Bet v 1 and recombinant hypoallergenic T-cell epitope–containing Bet v 1 fragments in patients with birch pollen allergy with and without atopic dermatitis (AD).
Methods
A clinical study was conducted in 15 patients with birch pollen allergy with AD (group 1), 5 patients with birch pollen allergy without AD (group 2), 5 allergic patients without birch pollen allergy (group 3), and 5 nonallergic subjects (group 4) by performing skin prick tests and APTs with rBet v 1 and hypoallergenic rBet v 1 fragments. T-cell, cutaneous lymphocyte antigen (CLA)+ and CCR4+ T-cell and cytokine responses were studied by thymidine uptake, carboxyfluorescein diacetate succinimidyl ester staining, and Luminex technology, respectively.
Results
rBet v 1 and hypoallergenic rBet v 1 fragments induced APT reactions in not only most of the patients with birch pollen allergy with AD (11/15) but also in most of those without AD (4/5). Patients with birch pollen allergy with AD had higher Bet v 1–specific proliferation of CLA+ and CCR4+ T cells compared with patients with birch pollen allergy without AD. There were no differences in Bet v 1–specific CLA+ and CCR4+ proliferation and cytokine secretion in patients with and without APT reactions.
Conclusion
Hypoallergenic rBet v 1 fragments induce T cell–dependent late reactions not only in patients with birch pollen allergy with AD but also in those without AD, which can be determined based on APT results but not based on in vitro parameters.
Keywords: Allergy, allergen, recombinant hypoallergens, atopy patch testing, late-phase reaction, T-cell proliferation, cutaneous lymphocyte antigen, CCR4, birch pollen allergy, rBet v 1, rBet v 1 fragments, specific immunotherapy
Immediate-type inflammation caused by mast cell and basophil activation through IgE-allergen complexes is a hallmark of IgE-associated allergy, but there are also allergic manifestations, such as atopic dermatitis (AD), chronic asthma, and nasal polyposis, in which T-cell activation seems to play a major role.1-4 Results from clinical studies performed with drugs targeting IgE, such as omalizumab, an anti-IgE antibody,5-7 and in vitro data showing that IgE-facilitated allergen presentation8,9 is important for the activation of allergen-specific T cells suggest that IgE-mediated mechanisms also play a role in T cell–mediated allergic inflammation. However, studies performed with non–IgE-reactive allergen derivatives provided evidence that late-phase allergic inflammation can be elicited without involvement of IgE in patients with asthma, rhinoconjunctivitis, and AD.10-12
In an elegant study, Haselden et al10 have shown that late-phase asthmatic responses can be induced by injection of T-cell epitope–containing peptides without IgE reactivity in a T cell–dependent and MHC-restricted manner. Late-phase allergic side effects were also frequently observed in patients treated with chemically modified allergen extracts termed allergoids, which displayed reduced IgE reactivity,13 and in patients with birch pollen–induced rhinoconjunctivitis who were treated with allergen-specific immunotherapy (SIT) with hypoallergenic recombinant or synthetic fragments of the major birch pollen allergen Bet v 1.12,14 In a preliminary study we demonstrated that hypoallergenic recombinant fragments of the major birch pollen Bet v 1 induce atopy patch test (APT) reactions in patients with AD exacerbations induced by birch pollen.11 The latter studies provide evidence for the occurrence of an IgE-independent, T cell–mediated mechanism in late-phase allergic inflammation in allergic patients, but it has not been studied whether this mechanism is limited to certain allergic phenotypes/manifestations, such as AD, or occurs also in others (eg, rhinoconjunctivitis and asthma). Furthermore, it has not been investigated whether patients with IgE-independent T cell–mediated inflammation can be identified, and it is unclear what cell types, soluble factors, or both are precisely responsible for this type of allergic inflammation.15-18
The identification of patients with IgE-independent, T cell–mediated allergic inflammation would be important because these patients might benefit from T cell–targeting therapeutic strategies. Furthermore, knowledge regarding the mechanisms underlying non–IgE-mediated allergic inflammation is important for the design of SIT strategies that avoid T cell–mediated side effects, which in fact seem to be very common during SIT.19,20
We conducted a clinical trial with the IgE-reactive major birch pollen allergen rBet v 1 (amino acids 1-160) and 2 hypoallergenic rBet v 1 fragments containing the Bet v 1–specific T-cell epitopes (F1: amino acids 1-74 and F2: amino acids 75-160) for use in skin prick tests (SPTs) and APTs in patients with birch pollen allergy with and without AD to investigate whether hypoallergenic Bet v 1 fragments can induce APT reactions in patients with defined clinical phenotypes (ie, AD and rhinoconjunctivitis). Furthermore, we performed an extensive analysis of T-cell and cytokine responses and correlated these data with the presence or absence of positive APT reactions to study whether surrogate markers for the prediction of T cell–mediated allergic inflammation can be defined.
METHODS
Subjects and study design
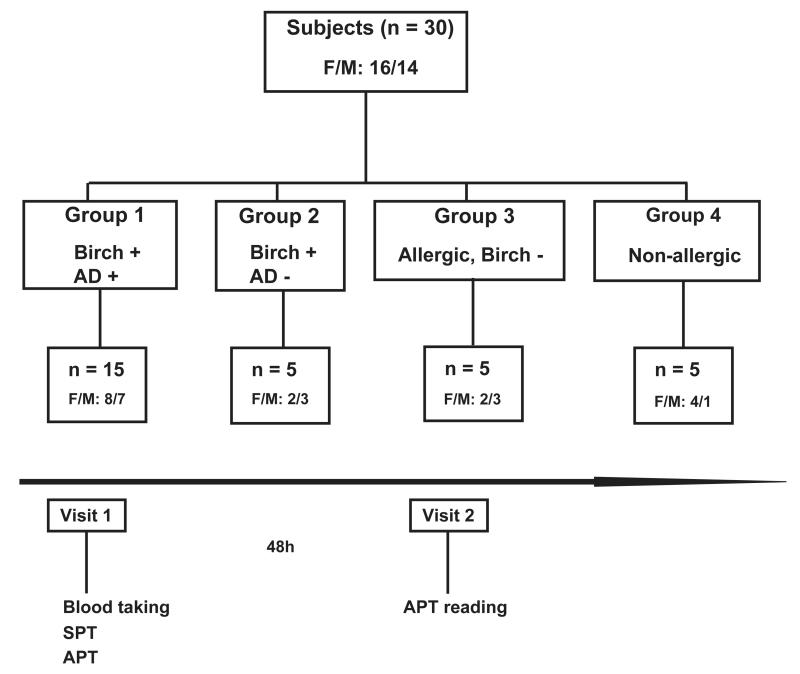
The present study was conducted at the Department of Dermatology of the Medical University of Vienna as a National Institutes of Health (NIH)–registered clinical trial (2009-011859-51) with the approval of the Ethics Committee of the Medical University of Vienna (EK147/2009) and the Austrian health authorities in accordance with the guidelines of the Declaration of Helsinki. Fig 1 provides a summary of the study. A total of 30 subjects (18-65 years of age) were enrolled and allocated to 4 groups.Table I summarizes the demographic and clinical characteristics of the study population. Group 1 comprised patients who, according to clinical history, had exacerbation of AD during the birch pollen season (n = 15); group 2 comprised patients with birch pollen–related rhinoconjunctivitis who had never experienced any AD symptoms (n = 5); group 3 comprised allergic patients without birch pollen allergy (n = 5); and group 4 comprised nonallergic subjects (n = 5, Fig 1 and Table I). The group sizes were influenced by results from a pilot study in which we found that non–IgE-reactive rBet v 1 fragments induced positive APT results in 4 of 6 patients with birch pollen allergy.11 In our current study the sample size per group was enlarged 5-fold for groups 1 to 3 and 2.5-fold for control group 4. Exclusion criteria are described in the Methods section in this article’s Online Repository at www.jacionline.org.
FIG 1.
Study design. Four groups of subjects, group 1 (patients with birch pollen allergy with AD, n = 15), group 2 (patients with birch pollen allergy with rhinoconjunctivitis without AD, n = 5), group 3 (allergic patients without birch pollen allergy and without AD, n = 5), and group 4 (nonallergic subjects, n = 5), were subjected to SPTs and APTs.
TABLE I.
Demographic and clinical characteristics of subjects
| Characteristics of subjects | Group 1 (n = 15) | Group 2 (n = 5) | Group 3 (n = 5) | Group 4 (n = 5) |
|---|---|---|---|---|
| Sex | ||||
| Male, no. (%) | 8 (53.3) | 3 (60) | 3 (60) | 1 (20) |
| Female, no. (%) | 7 (46.7) | 2 (40) | 2 (40) | 4 (80) |
| Age (y) | ||||
| Mean ± SD | 28.7 ± 6.4 | 34.4 ± 9.3 | 30.8 ± 1.9 | 29.8 ± 5 |
| Range | 19-44 | 25-47 | 28-33 | 23-36 |
| Allergies, no. (%) | ||||
| Birch | 15 (100) | 5 (100) | – | – |
| Animals | 14 (93.3) | 5 (100) | – | – |
| Grass | 12 (80) | 5 (100) | 3 (60) | – |
| Plant food | 9 (60) | 4 (80) | 1 (20) | – |
| Non–plant-derived food | 6 (40) | 1 (20) | – | – |
| Molds | 8 (53.3) | 2 (40) | 1 (20) | – |
| Mites | 14 (93.3) | 4 (80) | 3 (60) | – |
| Weeds | 4 (26.7) | 1 (20) | 1 (20) | – |
| Symptoms, no. (%) | ||||
| Atopic dermatitis | 15 (100) | – | – | – |
| Rhinoconjunctivitis | 15 (100) | 5 (100) | 5 (100) | – |
| Asthma | 4 (26.7) | – | – | – |
| Oral allergy syndrome | 9 (60) | 5 (100) | – | – |
The trial comprised 2 visits. At visit 1 (day 1), the participants were subjected to anamnesis and clinical documentation, a blood sample was drawn, and thereafter SPTs and APTs were performed with an equimolar mix of F1+F2 with rBet v 1. The blood samples were used for determination of total IgE and specific IgE reactivities, for basophil activation testing, and to study T-cell proliferation, proliferation of cutaneous lymphocyte antigen (CLA)+, and CCR4+ CD3 T cells and cytokine responses. The second visit (day 3) took place 48 hours after skin testing and included the reading and photodocumentation of APT reactions (Fig 1).
To investigate whether positive APT reactions to rBet v 1 or to the F1+F2 mix are associated with specific cellular responses, the 30 study subjects were regrouped according to their APT reactions and the presence or absence of birch pollen allergy into group A (patients with positive APT reactions and birch pollen allergy), group B (patients with lack of APT reactions and presence of birch pollen allergy), and group C (subjects with lack of APT reactions and absence of birch pollen allergy).
Study materials, SPTs, and APTs
Purified folded and IgE-reactive rBet v 1, as well as hypoallergenic rBet v 1 fragment 1 (F1: amino acids 1-74) and rBet v 1 fragment 2 (F2: amino acids 75-160), were produced according to good manufacturing practice guidelines by Biomay AG (Vienna, Austria) as sterile stock solutions. Dilutions used as test solutions were freshly prepared by the investigator shortly before testing and used for SPTs and APTs, as described in the Methods section in this article’s Online Repository.
Total and specific IgE levels
Serum samples were obtained from each participant at visit 1 before SPTs and APTs were performed and stored at −20°C until use. Total IgE levels and specific IgE levels to natural birch pollen extract and rBet v 1 were measured by using the ImmunoCAP system (Phadia, Uppsala, Sweden). IgE reactivity to rBet v 1 and hypoallergenic rBet v 1 fragments was determined in a RAST-based nondenaturing dot blot assay, as described in the Methods section in this article’s Online Repository.
Lymphocyte proliferation assays and detection of secreted cytokines
Lymphocyte proliferation assays and detection of secreted cytokines were performed as described in the Methods section in this article’s Online Repository. Flow cytometric analysis of CLA+ and CCR4+ T cells were performed as described in the Methods section in this article’s Online Repository.
Statistical analysis
The nonparametric Kruskal-Wallis test was used for comparison between the APT-positive and APT-negative groups. In case of statistical significance, retesting with the Mann-Whitney U test was used to assess statistical differences of pairwise comparisons between the APT-positive and APT-negative groups. P values of less than .05 were considered statistically significant.
Basophil activation determined by means of flow cytometry: CD203c and CD63 assay
Basophil activation was detected by means of flow cytometry with CD203c and CD63 assays, as described in the Methods section in this article’s Online Repository.
RESULTS
Characteristics of study subjects and study design
Thirty subjects (16 female and 14 male subjects) were recruited and allocated to 4 groups (Fig 1 and Table I). Group 1 included 15 patients with exacerbation of AD induced by birch pollen (age, 19-44 years; mean age, 28.7 ± 6.4 years). Each of these patients also had birch pollen–related rhinoconjunctivitis, 4 had asthma, and 9 had birch pollen-related oral allergy syndrome (Table I). Patients from group 1 were allergic to several other allergen sources as well (eg, house dust mites, animals, grass pollen, and molds).
Group 2 consisted of 5 patients with birch pollen–related rhinoconjunctivitis who, like patients in group 1, were allergic to several other allergen sources as well (eg, house dust mites, animals, grass pollen, and molds; age, 25-47 years; mean age, 34.4 ± 9.3 years; Table I). However, unlike patients from group 1, group 2 patients never had AD.
Two control groups containing subjects without birch pollen allergy were also studied. Group 3 contained 5 patients with only respiratory allergy to allergen sources other than birch pollen (age, 28-33 years; mean age, 30.8 ± 1.9 years). Group 4 included 5 nonallergic subjects (age, 23-36 years; mean age, 29.8 ± 5 years; Fig 1 and Table I). Table I provides a summary of the demographic and clinical characteristics of the subjects, whereas Table E1 in this article’s Online Repository at www.jacionline.org shows additional serologic and immunologic parameters of the study subjects.
Each of the patients with birch pollen allergy in groups 1 and 2 showed specific IgE reactivity to rBet v 1 (group 1: median, 12.8 kUA/L; group 2: median, 13.8 kUA/L) and birch pollen extract (group 1: median, 13.2 kUA/L; group 2: median, 12 kUA/L), whereas subjects from groups 3 and 4 did not (see Table E1).
Subjects from the 4 groups were also characterized regarding IgE reactivity to rBet v 1, F1, F2, and F1+F2 by using RAST-based dot blotting. Each of the patients with birch pollen allergy from groups 1 and 2 displayed IgE binding to rBet v 1, whereas none showed IgE binding to F1 (see Fig E1 in this article’s Online Repository at www.jacionline.org). Only 1 patient from group 2 (ie, patient 10) had a slightly positive IgE reactivity to F2 (see Fig E1 and Table E1). Eight of the 20 patients with birch pollen allergy from groups 1 and 2 displayed weak IgE binding (according to densitometry, <20% of IgE reactivity to Bet v 1) to the F1+F2 mix (group 1: subjects 18, 22, 25, and 26; group 2: subjects 6, 9, 10 and 13; see Fig E1). Sera from subjects of group 3 and 4 did not show any detectable IgE reactivity to dot-blotted rBet v 1 or to hypoallergenic rBet v 1 fragments (see Fig E1). During visit 1, blood samples were taken from each of the 30 subjects, SPTs were performed, and APTs were mounted, the results of which were then read 48 hours later during visit 2.
rBet v 1, but not rBet v 1 fragments, induce immediate-type skin reactions in patients with birch pollen allergy
SPTs demonstrated that all patients with birch pollen allergy (groups 1 and 2) had immediate-type skin reactions to birch pollen extract and to each of the 2 concentrations of rBet v 1 (ie, 20 and 40 μg/mL; Table II). By contrast, none of the 20 patients with birch pollen allergy exhibited immediate-type skin reactions to any of the 2 concentrations (ie, 20 and 40 μg/mL) of the hypoallergenic rBet v 1 fragment mix (F1+F2, Table II) or to the hypoallergenic rBet v 1 fragments F1 or F2 (data not shown). Interestingly, none of the 8 subjects with residual IgE reactivity to rBet v 1 fragments (see Table E1) showed any immediate skin response to the fragments or fragment mix (Table II and see Fig E2 in this article’s Online Repository at www.jacionline.org). None of the control subjects (group 3: allergic without birch pollen allergy; group 4: nonallergic) had positive skin reactions to birch pollen extract, rBet v 1, the F1+F2 mix, or the individual fragments (Table II, data not shown). Each of the subjects had immediate SPT responses to histamine.
TABLE II.
SPT and APT responses
| SPT (mm2) |
APT |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject | Group | Histamine | Birch | rBet v 1 (20 μg/mL) |
rBet v 1 (40 μg/mL) |
F1+F2 (20 μg/mL) |
F1+F2 (40 μg/mL) |
rBet v 1 | F1+F2 |
| 1 | 1 | 82.29 | 124.80 | 134.32 | 152.70 | − | − | ++++ | ++++ |
| 5 | 1 | 76.26 | 91.27 | 56.64 | 76.57 | − | − | +++ | ++ |
| 7 | 1 | 157.06 | 173.38 | 190.06 | 187.13 | − | − | +++ | ++ |
| 8 | 1 | 184.98 | 137.88 | 201.50 | 220.83 | − | − | ++++ | ++++ |
| 15 | 1 | 115.17 | 152.10 | 104.68 | 236.41 | − | − | +++ | ++ |
| 18 | 1 | 37.20 | 62.84 | 67.17 | 84.41 | − | − | +++++ | +++++ |
| 20 | 1 | 102.79 | 139.73 | 95.90 | 111.55 | − | − | ++ | ++ |
| 21 | 1 | 40.38 | 46.49 | 39.09 | 42.69 | − | − | + | +++ |
| 22 | 1 | 148.30 | 87.79 | 122.01 | 170.25 | − | − | +++++ | +++++ |
| 26 | 1 | 59.59 | 64.79 | 49.70 | 94.92 | − | − | +++++ | +++++ |
| 19 | 1 | 144.71 | 96.06 | 141.87 | 223.65 | − | − | ++ | − |
| 24 | 1 | 50.68 | 50.52 | 61.33 | 78.70 | − | − | − | − |
| 25 | 1 | 236.38 | 235.59 | 227.69 | 394.59 | − | − | − | − |
| 27 | 1 | 115.47 | 120.12 | 242.12 | 243.92 | − | − | − | − |
| 28 | 1 | 128.67 | 137.17 | 191.41 | 267.77 | − | − | − | − |
| 9 | 2 | 121.10 | 217.00 | 201.28 | 410.51 | − | − | +++++ | ++ |
| 10 | 2 | 54.56 | 56.20 | 51.12 | 61.84 | − | − | +++ | +++ |
| 13 | 2 | 116.13 | 104.07 | 132.37 | 324.44 | − | − | +++++ | +++ |
| 11 | 2 | 133.51 | 119.15 | 190.26 | 285.30 | − | − | ++ | − |
| 6 | 2 | 120.78 | 108.69 | 73.00 | 89.83 | − | − | − | − |
| 2 | 3 | 122.58 | − | − | − | − | − | − | − |
| 3 | 3 | 192.57 | − | − | − | − | − | − | − |
| 4 | 3 | 89.89 | − | − | − | − | − | − | − |
| 12 | 3 | 76.05 | − | − | − | − | − | − | − |
| 23 | 3 | 66.26 | − | − | − | − | − | − | − |
| 14 | 4 | 51.2 | − | − | − | − | − | − | − |
| 16 | 4 | 79.89 | − | − | − | − | − | − | − |
| 17 | 4 | 43.62 | − | − | − | − | − | − | − |
| 29 | 4 | 197.43 | − | − | − | − | − | − | − |
| 30 | 4 | 142.45 | − | − | − | − | − | − | − |
APT reactions to rBet v 1 and rBet v 1 fragments occur only in patients with birch pollen allergy
In parallel to SPTs, APTs were performed on the backs of the subjects with 160 μg of rBet v 1 or with an equimolar mix containing 80 μg of each hypoallergenic rBet v 1 fragment (F1+F2, Fig 1). Fifteen of the 30 subjects had positive APT responses to 1 or both of the antigens. Each of the subjects with positive responses belonged to group 1 or 2 and was birch pollen allergic, whereas none of the subjects from groups 3 or 4 had a positive APT result (Table II). Interestingly, APT reactions were induced by IgE-reactive antigens, as well as by hypoallergenic rBet v 1 fragments and occurred in patients with exacerbation of AD induced by birch pollen, as well as in patients with birch pollen–induced respiratory allergy without AD (see Fig E3 in this article’s Online Repository at www.jacionline.org for some examples). APT reactions with single fragments and control APTs performed with pure Vaseline (Unilever, London, United Kingdom) were negative (data not shown).
Frequent occurrence of APT reactions to hypoallergenic rBet v 1 fragments in patients with AD
We found that APTs with rBet v 1 induced an eczematous reaction in 11 (73.3%) of 15 patients with exacerbation of AD induced by birch pollen (group 1; Table II and Fig E3 in this article’s Online Repository at www.jacionline.org shows subjects 19 and 26 as examples). APTs with a mix of hypoallergenic F1+F2 induced a positive eczematous reaction in 10 (66.7%) of 15 patients with exacerbation of AD induced by birch pollen (group 1; Table II and Fig E3 shows an example given for subject 26). Only 3 of the 10 subjects with positive APT reactions to the hypoallergenic F1+F2 mix had residual IgE reactivity to the F1+F2 mix, whereas the other 7 subjects had no detectable IgE specific for F1+F2. Therefore, an involvement of IgE (eg, contribution of mast cell or basophil activation or IgE-facilitated allergen presentation) in the APT reactions can be excluded for the latter subjects.
Frequent occurrence of APT reactions to rBet v 1 and rBet v 1 fragments in patients with only respiratory birch pollen allergy
Interestingly, we observed that APTs with rBet v 1 also induced eczematous skin reactions in 4 (80%) of 5 patients who had only birch pollen–related rhinoconjunctivitis but who never experienced exacerbation of AD induced by birch pollen (group 2; Table II and see Fig E3 for results for subjects 11 and 13 as example). Again, we found that the mix of hypoallergenic F1+F2 induced eczematous skin reactions in 3 of the 5 patients with birch pollen allergy who had only respiratory forms of birch pollen allergy without AD (group 2; Table II and see Fig E3 for subject 13 shown as an example).
Neither rBet v 1– nor rBet v 1 fragment–specific T-cell proliferation were significantly associated with the occurrence of APT reactions
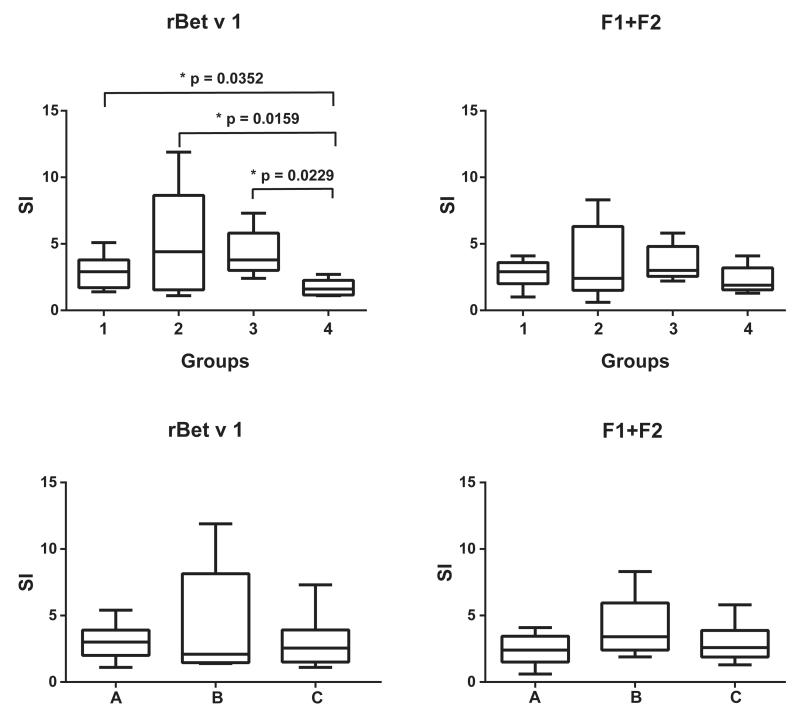
In a first set of experiments, we analyzed lymphocyte proliferation toward rBet v 1 and hypoallergenic F1+F2 in subjects of groups 1 to 4 (Fig 2, upper panel). We found that rBet v 1–induced T-cell proliferation was significantly higher in patients with exacerbation of AD induced by birch pollen (group 1) compared with that in nonallergic subjects (group 4; Fig 2, upper panel). It was also significantly higher in patients with respiratory allergy to birch without AD and allergic patients without birch pollen allergy compared with that in nonallergic subjects (Fig 2, upper panel). However, no significant differences were observed when groups 1, 2, and 3 were compared with each other. Furthermore, no statistically significant differences regarding T-cell proliferations specific for hypoallergenic F1+F2 among groups 1 to 4 were found (Fig 2, upper panel).
FIG 2.
T-cell proliferation toward rBet v 1 or F1+F2. Shown are PBMC proliferations (y-axes: stimulation indices as box-and-whisker plots showing minimum, quartiles, median, and maximum values) in response to rBet v 1 or the rBet v 1 fragments (F1+F2) for study groups 1 to 4 (upper panel) and (lower panel) for patients with birch pollen allergy with positive APT reactions (group A), patients with birch pollen allergy with negative APT reactions (group B), and subjects without birch pollen allergy with negative APT reactions (group C). Significant differences between the groups are indicated.
We did not find any statistically significant differences regarding T-cell proliferation to rBet v 1 and the mix of hypoallergenic F1+F2 when the 30 study subjects were regrouped according to the presence of APT reactions and birch pollen allergy (ie, group A), lack of APT reactions and presence of birch pollen allergy (ie, group B), and lack of APT reactions and absence of birch pollen allergy (ie, group C; Fig 2, lower panel).
Proliferation of Bet v 1–specific skin-homing T cells is highest in patients with birch pollen allergy with AD but is not significantly correlated with positive APT reactions
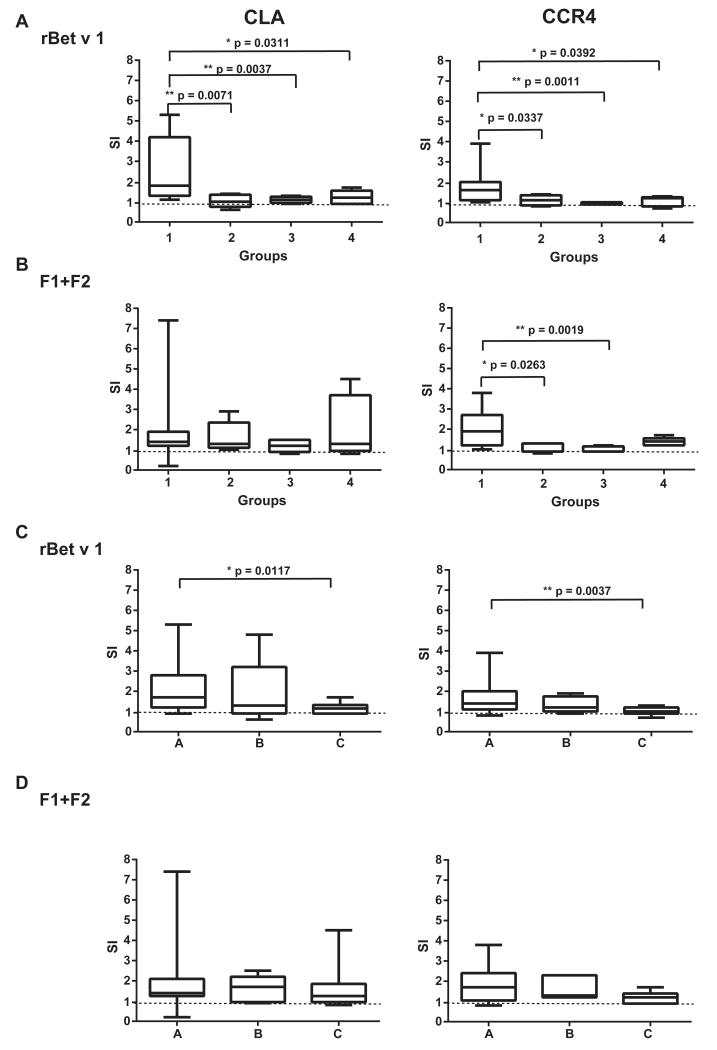
It is well established that patients with AD have increased levels of skin-homing CLA+ and CCR4+ T cells.21 However, thus far, it has not been studied whether there is an association between allergen-induced proliferation of these cells and allergen-induced eczematous skin reactions. We found that stimulation with rBet v 1 induced a specific CD3+CD4+ cell proliferation in the CLA+ and CCR4+ cells that was significantly higher in the group of patients with exacerbations of AD induced by birch pollen compared with groups 2, 3, and 4 (ie, subjects without birch pollen–induced AD; Fig 3, A). A similar trend was observed when cells were stimulated with the mix of hypoallergenic F1+F2 for CCR4+ cells (Fig 3, B). The proliferation was significantly higher in group 1 when compared with that in groups 2 and 3 but was not significant when compared with that in group 4. For CLA+ cells, differences were not significant.
FIG 3.
Activation of CLA+ or CCR4+CD3+ cells by rBet v 1 or F1+F2 with carboxyfluorescein diacetate succinimidyl ester staining. A and B, Shown are the stimulation indices as box-and-whisker plots showing minimum, quartiles, median, and maximum values in response to rBet v 1 (Fig 3, A) or to the rBet v 1 fragment mix (F1+F2; Fig 3, B) for study groups 1 to 4. C and D, Results for patients with birch pollen allergy with positive APT reactions (Fig 3, A), patients with birch pollen allergy with negative APT reactions (Fig 3, B), and subjects without birch pollen allergy with negative APT reactions (Fig 3, C) in response to rBet v 1 and F1+F2, respectively. Significant differences between the groups are indicated. The horizontal dashed line represents the cutoff for positive proliferation (stimulation index > 1).
Interestingly, when patients with birch pollen allergy were regrouped according to the occurrence (ie, group A) or absence (ie, group B) of a positive APT reaction, there was no significant difference between the groups regarding proliferation induced with Bet v 1 in CLA+ and CCR4+ cells and also proliferation induced with the mix of hypoallergenic F1+F2 in CLA+ and CCR4+ cells (Fig 3, C and D).
Patients with birch pollen allergy with and without Bet v 1–specific APT reactions have similar cytokine responses to Bet v 1 and Bet v 1 fragments
No statistically significant differences were found regarding levels of any of the cytokines (ie, IL-1, IL-2, IL-4; IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, IL-22, IFN-γ, TNF-α, granulocyte colony-stimulating factor, GM-CSF, monocyte chemoattractant protein 1, and macrophage inflammatory protein 1) induced in PBMCs with rBet v 1 (see Fig E4 in this article’s Online Repository at www.jacionline.org) or with the hypoallergenic F1+F2 mix (see Fig E5 in this article’s Online Repository at www.jacionline.org) from patients with positive APT reactions (ie, group A) compared with those from patients with negative APT reactions (ie, group B: birch pollen allergy; group C: absence of birch pollen allergy). Yet some other differences were noted that were not related to the presence or absence of ATP reactions. For example, hypoallergenic F1+F2 induced significantly higher levels of the TH1 cytokine IFN-γ in PBMCs from patients without birch pollen allergy (see Fig E5). A similar trend without significance was observed for Bet v 1 (see Fig E4). An inverse situation was found for IL-4 levels, which were significantly higher in PBMCs from patients with birch pollen allergy stimulated with the mix of hypoallergenic F1+F2 (see Fig E5). A similar trend without significance was found for Bet v 1 (see Fig E4). In accordance with the results for IFN-γ and IL-4, we found that levels of IL-12 induced with Bet v 1 and the mix of hypoallergenic F1+F2 were highest in PBMC cultures from subjects without birch pollen allergy. The latter findings thus seem to reflect the TH1/TH2 dysbalance of the Bet v 1–specific response in subjects with and without birch pollen allergy.
Interestingly, IFN-γ levels were significantly higher in PBMCs of the patients with birch pollen allergy with positive APT reactions (ie, group A) when compared with those in patients with birch pollen allergy without positive APT reactions (ie, group B) when the mix of hypoallergenic F1+F2 was used for stimulation. However, IFN-γ levels were also significantly higher in PBMCs of subjects without birch pollen allergy and negative APT reactions (ie, group C) when compared with those in patients with birch pollen allergy without positive APT reactions (ie, group B).
Furthermore, we noted that IL-7 levels in PBMC cultures from patients with birch pollen allergy stimulated with Bet v 1 and the mix of hypoallergenic F1+F2 were significantly higher compared with levels found in cultures of PBMCs from subjects without birch pollen allergy (see Figs E4 and E5).
We also studied whether supernatants from stimulated PBMCs of the study subjects would be able to activate basophils by studying upregulation of basophil activation markers (ie, CD63 and CD203c). However, we could not detect any increase in upregulation of these markers when basophils were exposed to supernatants from PBMCs stimulated with Bet v 1 or the fragment mix compared with medium (data not shown).
DISCUSSION
In this clinical study we used hypoallergenic T-cell epitope–containing fragments of the major birch pollen allergen Bet v 1 to investigate the occurrence of IgE-independent, T cell–mediated allergic skin inflammation in patients with birch pollen allergy with different disease manifestations, allergic patients without birch pollen allergy, and nonallergic subjects by using APTs. We found that 11 (73.3%) of 15 patients with birch pollen allergy with AD had T cell–mediated APT reactions to rBet v 1, and 10 of these patients (10/15 [66.6%]) had late eczematous reactions to the mix of hypoallergenic rBet v 1 fragments. These results demonstrate that non–IgE-mediated mechanisms are very frequent in chronic allergen-induced skin inflammation. More important, and quite unexpected, was the finding that T cell–mediated APT reactions to rBet v 1 (4/5 [80%]) were also very common in patients with birch pollen allergy who had only allergic rhinoconjunctivitis but never experienced an exacerbation of AD induced by birch pollen. Here again, the eczematous reactions were often IgE-independent, appearing in 3 (60%) of 5 patients. In fact, there are not many studies in which APTs were performed with allergen extracts in allergic patients without AD, and there is no systematic study in which hypoallergenic allergen derivatives were used for APTs. In studies comparing allergic patients with and without AD, positive APT reactions were much more frequent in patients with AD or confined to this group when compared with patients with only respiratory allergy without a history of eczema.22 In our study, the appearance of Bet v 1–specific APT reactions was strictly confined to patients with birch pollen allergy and was not observed in allergic patients without birch pollen allergy or nonallergic subjects. In fact, others have reported that positive APT reactions to aeroallergens are very common in patients with AD,23 but our study is the first to use hypoallergenic allergen derivatives to demonstrate that the reactions are often non–IgE mediated. Even for those patients who showed some residual IgE reactivity to the mix of rBet v 1 fragments (ie, <20% of the Bet v 1–specific IgE reactivity), it is very unlikely that the APT reactions were IgE mediated because the magnitudes of the APT reactions were similar between those induced by fully IgE-reactive Bet v 1 and those induced by the hypoallergenic Bet v 1 fragments, despite extremely low IgE reactivity of the rBet v 1 fragments and their lack of allergenic activity, as shown by SPTs.
According to the type of reaction (ie, hypersensitivity type IV like) and the time of appearance (ie, 48 hours after application, delayed type), the APT reactions were most likely T cell mediated. Therefore we searched for surrogate markers of Bet v 1–specific T-cell activation and Bet v 1–specific induction of T cell–derived cytokines, which might be associated with the APT reactions. Much to our surprise, we did not find any significant correlation between positive Bet v 1–specific APT reactions and Bet v 1–specific T-cell proliferation in cultured PBMCs. Because in the present study we could analyze only T-cell and cytokine responses in peripheral blood, we next sought to focus on T cells that home to the skin and express CLA, CCR4, or both.24,25 In fact, it has been reported that CLA+CCR4+ T cells are upregulated in lesional skin of patients with AD, but it has not yet been investigated whether allergen-induced activation of such cells is associated with allergen-induced APT reactions. We found an increased expression of skin-homing (CLA+) and CCR4+CD4+ T cells in the rBet v 1–stimulated PBMCs from patients with AD compared with those from patients with only birch pollen–related rhinoconjunctivitis and nonallergic subjects. These results are comparable with those of Nakatani et al,21 indicating a predominant expression of skin-homing cells in the peripheral blood of patients with AD. However, again, analysis of allergen-specific activation and proliferation of in vitro–cultured skin-homing cells in patients with positive allergen-specific APT reactions were not significantly correlated. In our analysis we investigated only CD3+CD4+ T cells. Therefore it is possible that the development of positive APT reactions might be due to other types of T cells, such as CD8+ T cells, which were reported to occur in increased numbers in patients with positive APT reactions when compared with numbers in normal skin,25 and there is evidence that allergen-primed CD8+ T cells are required for the development of AD-like lesions.24
We also performed an extensive analysis of inflammatory and tolerogenic cytokines, including IL-10 and IL-22, which has been suggested to be secreted specifically by a subset of human skin-homing T cells26 in supernatants of PBMCs stimulated with rBet v 1 and recombinant hypoallergenic Bet v 1 fragments. However, we did not find significant correlations between any of the tested allergen-induced cytokines and the presence or absence of allergen-specific APT reactions.
Together with studies performed in patients with AD using Bet v 1–homologous food allergens, which are not stable when cooked or digested in the gastrointestinal tract,27,28 our study provides evidence that non–IgE-mediated, T cell–mediated mechanisms are very common in patients with AD. It has been also shown for patients with allergen-induced asthma that non–IgE-mediated, T cell–dependent late-phase reactions can occur when patients are exposed to T-cell epitope–containing non–IgE-reactive allergen derivatives.10 Interestingly, our study also shows that hypoallergenic Bet v 1 derivative–induced APT reactions occur frequently in patients with birch pollen–induced rhinoconjunctivitis. Since it has been reported that patients who were treated with recombinant and synthetic hypoallergenic Bet v 1 fragments frequently experience late-phase side effects,12,14 it is very likely that these side effects are non–IgE mediated and T cell dependent. Thus our study shows that APTs with hypoallergenic allergen derivatives but no other of the tested in vitro surrogate markers are useful to identify patients who show T cell–mediated allergic inflammation. Therefore one might consider using APTs with hypoallergens to identify patients who are at risk of having non–IgE-mediated, T cell–mediated allergic inflammation because they might benefit from T cell–targeted therapeutic strategies. Furthermore, patients with T cell–mediated side effects during SIT might benefit from new forms of SIT, which reduce the presence of allergen-specific T-cell epitopes in the vaccine, such as recombinant peptide carrier vaccines that have a strongly reduced ability to induce allergen-specific T-cell activation and APT reactions.29,30 Therefore it will be interesting to perform follow-up studies in which APTs are performed before SIT with hypoallergenic Bet v 1 derivatives to determine whether positive APT reactions elicited by hypoallergenic Bet v 1 derivatives might predict late-phase side effects during birch pollen SIT.
Supplementary Material
FIG E1. IgE reactivity to rBet v 1 and rBet v 1 fragments. Dot-blotted purified recombinant antigens (rBet v 1, rBet v 1 fragments F1 and F2, an rBet v 1 fragment mix [F1+F2], and BSA) were incubated with sera from the study subjects from groups 1 to 4 (1-30) or with buffer alone as a negative control (NC). Bound IgE antibodies were detected with iodine 125–labeled anti-human IgE antibodies and visualized by means of autoradiography.
FIG E2. Immediate-type skin reactions to rBet v 1 and rBet v 1 fragments in subjects with residual IgE reactivity to the F1+F2 mix in RAST-based dot blotting assay. SPTs were performed with birch pollen extract (1), rBet v 1 (2 and 3), and the mix of rBet v 1 fragments F1+F2 (4 and 5). SPTs were performed with antigen concentrations of 20 μg/mL (2 and 4) or 40 μg/mL (3 and 5).
FIG E3. Delayed-type skin reactions to rBet v 1 and rBet v 1 fragments in selected subjects. Shown are APT reactions to rBet v 1 and the rBet v 1 fragment mix (F1+F2) in patients from study groups 1 to 4 (group 1, subjects 26 and 19; group 2, subjects 13 and 11; group 3, subject 4; and group 4, subject 16). APTs were performed with 160 μg of rBet v 1 and a mix containing 80 μg of each rBet v 1 fragment (F1+F2).
FIG E4. Cytokine levels measured in PBMC cultures on stimulation with rBet v 1 shortly before SPTs and APTs. Shown are cytokine levels (in picograms per milliliter) determined for triplicate cultures as box-and-whisker plots showing minimum, quartiles, median, and maximum values (y-axes) for APT-positive patients with birch pollen allergy with positive APT reactions (A), patients with birch pollen allergy with negative APT reactions (B), and subjects without birch pollen allergy with negative APT reactions (C; x-axes). Statistically significant differences (P < .05) are indicated. G-CSF, Granulocyte colony-stimulating factor; MCP-1, monocyte chemoattractant protein 1; MIP-1b, macrophage inflammatory protein 1.
FIG E5. Cytokine levels measured in PBMC cultures on stimulation with the F1+F2 mix shortly before SPTs and APTs. Shown are cytokine levels (in picograms per milliliter) determined for triplicate cultures as box-and-whisker plots showing minimum, quartiles, median, and maximum values (y-axes) for APT-positive patients with birch pollen allergy with positive APT reactions (A), patients with birch pollen allergy with negative APT reactions (B), and subjects without birch pollen allergy with negative APT reactions (C; x-axes). Statistically significant differences (P < .05) are indicated. G-CSF, Granulocyte colony-stimulating factor; MCP-1, monocyte chemoattractant protein 1; MIP-1b, macrophage inflammatory protein 1.
TABLE E1. Clinical and serologic characterization of subjects
Clinical implications.
Late-phase reactions to hypoallergenic T-cell epitope–containing allergen derivatives occur frequently and can be determined based on APT reactions.
Acknowledgments
Supported by grants F4605 and F4611 of the Austrian Science Fund (FWF), by grant 323183 from the European Research Council, and by a research grant from Biomay AG, Vienna, Austria.
A. Neubauer is employed by Biomay AG. H. Huber has received research support from FFG and is a board member for, is employed by, and has stock/stock options in Biomay AG. R. Henning has received consultancy fees from, is employed by, has a patent with, has received royalties from, and has stock/stock options in Biomay AG. P. Valent has received research support from FWF (SFB 4610), Ariad, Celgene, Novartis, and Capella; has received consultancy fees from Novartis; and has received lecture fees from Novartis, Celgene, Ariad, and Pfizer. F. Sallusto has received research support from ERC, SNSF. S. Wöhrl has received consultancy fees from Novartis and Thermo Fisher, has provided expert testimony for AFFiRiS, and has received lecture fees from ALK-Abelló, Bencard, MEDA, Stallergenes, and Thermo Fisher. R. Valenta has received research support from the Austrian Science Fund (FWF), Biomay AG (Vienna, Austria), and Thermo Fisher (Uppsala, Sweden) and has received consultancy fees from Biomay AG, Thermo Fisher, Fresenius Medical Care (Bad Homburg, Germany), and Boehringer Ingelheim (Biberach, Germany).
Abbreviations used
- AD
Atopic dermatitis
- APT
Atopy patch test
- CLA
Cutaneous lymphocyte antigen
- FACS
Fluorescence-activated cell sorting
- MFI
Mean fluorescence intensity
- SIT
Allergen-specific immunotherapy
- SPT
Skin prick test
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–91. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DM, O’Byrne PM. Recent advances in pathophysiology of asthma. Chest. 2010;137:1417–26. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 3.Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 4.Gevaert P, Holtappels G, Johansson SGO, Cuvelier C, van Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 5.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–6. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 6.Holgate ST, Djukanovic R, Casale T, Bousquet J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin Exp Allergy. 2005;35:408–16. doi: 10.1111/j.1365-2222.2005.02191.x. [DOI] [PubMed] [Google Scholar]
- 7.Klunder S, Saggar LR, Seyfert-Margolis V, Asare AL, Casale TB, Durham SR, et al. Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120:688–95. doi: 10.1016/j.jaci.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Novak N, Bieber T, Kraft S. Immunoglobulin E-bearing antigen-presenting cells in atopic dermatitis. Curr Allergy Asthma Rep. 2004;4:263–9. doi: 10.1007/s11882-004-0069-2. [DOI] [PubMed] [Google Scholar]
- 9.van Neerven RJ, Knol EF, Ejrnaes A, Würtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141:119–29. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 10.Haselden BM, Kay AB, Larchè M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campana R, Mothes N, Rauter I, Vrtala S, Reininger R, Focke-Tejkl M, et al. Non-IgE-mediated chronic allergic skin inflammation revealed with rBet v 1 fragments. J Allergy Clin Immunol. 2008;121:528–30. doi: 10.1016/j.jaci.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin Exp Allergy. 2008;38:1514–25. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 13.Bousquet J, Maasch HJ, Skassa-Brociek W, Wahl R, Dhivert H, Michel FB, et al. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. III. Efficacy and safety of unfractionated and high-molecular-weight preparations in rhinoconjunctivitis and asthma. J Allergy Clin Immunol. 1989;84:546–56. doi: 10.1016/0091-6749(89)90369-2. [DOI] [PubMed] [Google Scholar]
- 14.Spertini F, Perrin Y, Audran R, Pellaton C, Boudousquié C, Barbier N, et al. Safety and immunogenicity of immunotherapy with Bet v 1-derived contiguous overlapping peptides. J Allergy Clin Immunol. 2014;134:239–40.e13. doi: 10.1016/j.jaci.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, et al. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. 1996;107:871–6. doi: 10.1111/1523-1747.ep12331164. [DOI] [PubMed] [Google Scholar]
- 16.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–98. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 17.Hennino A, Vocanson M, Toussaint Y, Roder K, Benetiere J, Schmitt AM, et al. Skin-infiltrating CD8+ T cells initiate atopic dermatitis lesions. J Immunol. 2007;178:5571–7. doi: 10.4049/jimmunol.178.9.5571. [DOI] [PubMed] [Google Scholar]
- 18.Hennino A, Jean-Decoster C, Giordano-Labadie F, Debeer S, Vanbervliet B, Rozieres A, et al. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J Allergy Clin Immunol. 2011;127:1064–7. doi: 10.1016/j.jaci.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Winther L, Malling HJ, Mosbech H. Allergen-specific immunotherapy in birch- and grass-pollen-allergic rhinitis. II. Side-effects. Allergy. 2000;55:827–35. doi: 10.1034/j.1398-9995.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 20.Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy. A prospective multi-centre study. Clin Exp Allergy. 2006;36:254–60. doi: 10.1111/j.1365-2222.2006.02340.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakatani T, Kabugari Y, Shimada Y, Inaoki M, Takehara K, Mukaida N, et al. CCR4+ memory CD4+ T lymphocytes are increased in peripheral blood and lesional skin from patients with atopic dermatitis. J Allergy Clin Immunol. 2001;107:353–8. doi: 10.1067/mai.2001.112601. [DOI] [PubMed] [Google Scholar]
- 22.Fuiano N, Fusilli S, Incorvaia C. House dust mite-related allergic diseases: role of skin prick test, atopy patch test, and RAST in the diagnosis of different manifestations of allergy. Eur J Pediatr. 2010;169:819–24. doi: 10.1007/s00431-009-1118-6. [DOI] [PubMed] [Google Scholar]
- 23.Darsow U, Laifaoui J, Kerschenlohr K, Wollenberg A, Przybilla B, Wüthrich B, et al. The prevalence of positive reactions in the atopy patch test with aeroallergens and food allergens in subjects with atopic eczema: a European multicenter study. Allergy. 2004;59:1318–25. doi: 10.1111/j.1398-9995.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- 24.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Am J Pathol. 1990;136:1053–68. [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–80. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 26.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Protection of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 27.Reekers R, Busche M, Wittmann M, Kapp A, Werfel T. Birch pollen-related foods trigger atopic dermatitis in patients with specific cutaneous T-cell responses to birch pollen antigens. J Allergy Clin Immunol. 1999;104:466–72. doi: 10.1016/s0091-6749(99)70395-7. [DOI] [PubMed] [Google Scholar]
- 28.Bohle B, Zwölfer B, Heratizadeh A, Jahn-Schmid B, Antonia YD, Alter M, et al. Cooking birch pollen-related food: divergent consequences for IgE- and T cell-mediated reactivity in vitro and in vivo. J Allergy Clin Immunol. 2006;118:242–9. doi: 10.1016/j.jaci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Focke-Tejkl M, Weber M, Niespodziana K, Neubauer A, Huber H, Henning R, et al. Development and characterization of a recombinant, hypoallergenic, peptide-based vaccine for grass pollen allergy. J Allergy Clin Immunol. 2015;135:1207, 1207.e1–11. doi: 10.1016/j.jaci.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen allergic patients. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.03.034. [Epub ahead of print] doi: 10.1016/j.jaci.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. IgE reactivity to rBet v 1 and rBet v 1 fragments. Dot-blotted purified recombinant antigens (rBet v 1, rBet v 1 fragments F1 and F2, an rBet v 1 fragment mix [F1+F2], and BSA) were incubated with sera from the study subjects from groups 1 to 4 (1-30) or with buffer alone as a negative control (NC). Bound IgE antibodies were detected with iodine 125–labeled anti-human IgE antibodies and visualized by means of autoradiography.
FIG E2. Immediate-type skin reactions to rBet v 1 and rBet v 1 fragments in subjects with residual IgE reactivity to the F1+F2 mix in RAST-based dot blotting assay. SPTs were performed with birch pollen extract (1), rBet v 1 (2 and 3), and the mix of rBet v 1 fragments F1+F2 (4 and 5). SPTs were performed with antigen concentrations of 20 μg/mL (2 and 4) or 40 μg/mL (3 and 5).
FIG E3. Delayed-type skin reactions to rBet v 1 and rBet v 1 fragments in selected subjects. Shown are APT reactions to rBet v 1 and the rBet v 1 fragment mix (F1+F2) in patients from study groups 1 to 4 (group 1, subjects 26 and 19; group 2, subjects 13 and 11; group 3, subject 4; and group 4, subject 16). APTs were performed with 160 μg of rBet v 1 and a mix containing 80 μg of each rBet v 1 fragment (F1+F2).
FIG E4. Cytokine levels measured in PBMC cultures on stimulation with rBet v 1 shortly before SPTs and APTs. Shown are cytokine levels (in picograms per milliliter) determined for triplicate cultures as box-and-whisker plots showing minimum, quartiles, median, and maximum values (y-axes) for APT-positive patients with birch pollen allergy with positive APT reactions (A), patients with birch pollen allergy with negative APT reactions (B), and subjects without birch pollen allergy with negative APT reactions (C; x-axes). Statistically significant differences (P < .05) are indicated. G-CSF, Granulocyte colony-stimulating factor; MCP-1, monocyte chemoattractant protein 1; MIP-1b, macrophage inflammatory protein 1.
FIG E5. Cytokine levels measured in PBMC cultures on stimulation with the F1+F2 mix shortly before SPTs and APTs. Shown are cytokine levels (in picograms per milliliter) determined for triplicate cultures as box-and-whisker plots showing minimum, quartiles, median, and maximum values (y-axes) for APT-positive patients with birch pollen allergy with positive APT reactions (A), patients with birch pollen allergy with negative APT reactions (B), and subjects without birch pollen allergy with negative APT reactions (C; x-axes). Statistically significant differences (P < .05) are indicated. G-CSF, Granulocyte colony-stimulating factor; MCP-1, monocyte chemoattractant protein 1; MIP-1b, macrophage inflammatory protein 1.
TABLE E1. Clinical and serologic characterization of subjects