Abstract
The α3β4α5 nAChR has been recently shown to be a useful target for smoking cessation pharmacotherapies. Herein, we report on the development and characterization of the α3β4α5 nicotinic receptor column by frontal displacement chromatography. The binding affinity of the nicotine and minor alkaloids found in tobacco smoke condensates were determined for both the α3β4 and α3β4α5 nicotinic receptors. It was demonstrated that while no subtype selectivity was observed for nicotine and nornicotine, anabasine was selective for the α3β4α5 nicotinic receptor. The non-competitive inhibitor binding site was also studied and it was demonstrated while mecamylamine was not selective between subtypes, buproprion showed subtype selectivity for the α3β4 nicotinic receptor. The application of this methodology to complex mixtures was then carried out by screening aqueous-alcoholic solutions of targeted plant extracts, including Lycopodium clavatum L. (Lycopodiaceae) and Trigonella foenum graecum L. (Fabaceae) against both the α3β4 and α3β4α5 nAChRs.
Keywords: Frontal affinity chromatography, alkaloids, anabasine, subtype selectivity
1. Introduction
Neuronal nicotinic acetylcholine receptors (nAChRs) are ligand gated ion channels that are composed of five transmembrane subunits oriented around a central pore [1]. Two families of neuronal transmembrane subunits have been identified, the α subunit family composed of 9 members (α2–α10) and the β subunit family composed of 3 members (β2 – β4) [1,2]. Functional nAChRs are constructed from α subunits alone, homomeric nAChRs, or a mixture of α and β subunits, heteromeric nAChRs. The homomeric nAChRs are formed using the α7 through α10 subunits, with the α7 nAChR being the most well characterized and abundant subtype. The heteromeric nAChRs were initially believed to be composed of two α2, α3 or α4 subunits and three β subunits, with the α4β2 nAChR being the most abundant subtype in the central nervous system [1,2], and the α3β4 nAChR the most abundant subtype in the peripheral nervous system [2]. However, heteromeric nAChR subtypes containing two α subunits, the α2, α3 or α4 subunit plus the α5 or α6 subunit have also been described [3], with α5 subunits predominantly expressed in combination with α3 and β4 subunits [4,5].
It was initially believed that the α5 subunit played a purely structural role [3]. However, recent data suggest that the presence of the α5 subunit alters the activity of α3β4 nAChRs. Ciuraszkiewicz et al. have shown that the addition of α5 subunits to α3β4 nAChRs influences channel open time and burst duration [2]. Increased expression of α3β4α5 nAChR in the habenuolopeduncular pathway results in decreased nicotine aversion usually associated with decreased α3β4 nAChRs expression [4] suggesting that α3β4α5 nAChR inhibition may be a useful target for smoking cessation pharmacotherapies [1]. In addition, α3β4α5 nAChR subunits expressed in the respiratory system are believed to play a role for lung tumor initiation and growth [5] and may also be new targets for receptor-specific anti-tumor therapy.
Since ~70% of the drugs currently on the market are either nature-based or derived from a nature-based product [6], the screening of biological matrices for novel α3β4α5 nAChR inhibitors is a logical drug discovery program. However, the process is complicated by the complexity of the matrix and the variable concentrations of “active” components. Currently, dereplication is the most common approach for screening of complex matrixes (plant extracts) and has been approached using a variety of methods [7]. Another commonly used method to screen plant extracts is on-line screening with bioactive detection [8, 9]. These methods have been successfully used but predominantly to identify the most abundant compounds and not necessarily the most active. We have previously demonstrated that these issues can be addressed using bioaffinity chromatography and that this approach can be used for the isolation of active components from complex mixtures including tobacco smoke condensates and plant extracts [10–14]. The previous studies have utilized cellular membrane affinity chromatography (CMAC) including CMAC columns containing single or multiple nAChR subtypes [15, 16]. Thus, an objective of this study was the development and characterization of a CMAC column based upon immobilized cellular membranes obtained from cells lines expressing the α3β4α5 nAChR and the demonstration that this resulting column can be used to identify novel lead drugs for use in the treatment of smoking cessation.
Although the α3β4α5 nAChR is believed to play an important role in smoking cessation, the receptor has not been fully characterized. Previous studies have demonstrated that CMAC columns containing the α3β2 nAChR, α3β4 nAChR, α4β2 nAChR or α4β4 nAChR can be used to characterize the receptors at their orthosteric as well as their allosteric binding sites[15, 16]. Thus, a second objective of this study was to demonstrate that the CMAC(α3β4α5 nAChR) column can be used to probe competitive and non-competitive binding to the immobilized receptor.
We now report the development and initial characterization of the CMAC(α3β4α5 nAChR) column and its comparison to the CMAC(α3β4 nAChR) column. The results clearly demonstrate that there are differences in the binding affinity between the two subtypes. In addition, due to the interest in the identification of highly selective ligands for the α3β4α5 nAChR and to demonstrate the utility of this approach in screening of complex matrix, plant extracts were screened against the CMAC(α3β4α5 nAChR) and CMAC(α3β4 nAChR) columns. Several alkaloids have been previously identified as nicotinic receptor ligands. In fact, Huperzine A, an alkaloid commonly found in many club moss species, was found to slow the rate of recovery from desensitization of α7 nAChR [17]. Fayuk and Yakel postulated this alkaloid may play a role of cholinergic signaling modulator in hippocampus. As a result, Lycopodium clavatum L, a club moss species, was chosen as its known to contain a large amount of alkaloids, including but not limited to lycopodine, sieboldine, lycodine, fawcettimine.
2. Materials and Methods
2.1. Materials
D-MEM and Penicillin/streptomycin were purchased from Quality Biologicals (Gaithersburg, MD). Fetal Bovine serum was obtained from Atlanta Biologicals (Lawrenceville, Ga). [3H]-Epibatidine (>97 %) was purchased from Perkin Elmer (Waltham, MA). IAM (immobilized artificial membrane) particles were purchased from Regis Technologies Inc (Morton Grove, IL). Hygromycin (80%) was obtained from Amresco (Solon, OH). The HR 5/2 glass columns were purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). Tris-HCl and NaCl (>99 %) were obtained from Fischer Scientific (Jessup, MD). MgCl2 (≥ 98%),, CaCl2 (≥ 99%), KCl (>99 %), Sodium cholate hydrate (>99 %),, Leupeptin (> 90%), Ammonium acetate (>99 %), Benzamidine hydrochloride (>99 %), Phenylmethanesulfonyl fluoride (PMSF, ≥98.5%), G418, Nicotine (> 99%), Cytisine (≥ 99%), Nornicotine (≥ 98%), Anabasine (≥ 97%), Mecamylamine hydrochloride (> 96%), Bupropion hydrochloride (≥ 98%), and all other chemicals were purchased from Sigma-Aldrich unless otherwise stated (St. Louis, MO). Club Moss (Lycopodium clavatum L). aqueous-alcoholic extract was from Hawaii Pharm (Honolulu, Hawaii) and Trigonella foenum-graecum L. aqueous-alcoholic extracts wasfrom Herb Pharm (Williams, OR).
2.2. Methods
2.2. Preparation of α3β4 and α3β4βα5 stationary phases
2.2.1. Cell lines
The HEK293 cell line stably expressing the human α3β4 nAChR was developed as previously described [18]. The α3β4α5 membranes were obtained from HEK-293 cell lines were obtained from Jon Lindstrom from University of Pennsylvania generated by transfecting the stable α3β4 with constructs containing α5 subunits with hygromycin-selectable element [19]. D-MEM media with 1% Penicillin/streptomycin, 10% Fetal Bovine Serum and 0.7mg/mL of Geneticin (G418) were used to maintain the α3β4α5 cells. 400 μg per mL of hygromycin were used in the transfection steps.
2.2.2. Immobilization of α3β4α5 and α3β4 nicotinic receptors
The α3β4 membranes were prepared and immobilized following a previously described protocol [20]. The α3β4α5 membranes were prepared with slight modifications of this protocol [20]. Briefly, 1.8 × 107 Hek α3β4α5 cells were obtained and suspended in 20mL of Tris-HCl [50mM, pH 7.4] containing 5 mM EDTA, 3 mM benzamidine, 0.1 mM PMSF and 1/100 protease inhibitor cocktail. The suspension was then homogenized with a glass homogenizer 20 to 25 times. The mixture was then centrifuged for 30 minutes at 4° C at 100,000g. The resulting pellet was then suspended with 10mL of Tris-HCl [50 mM, pH 7.4] containing 100 mM NaCl, 2 mM MgCl2, 3 mM CaCl2, 5 mM KCl, 2% Cholate and 1/100 protease inhibitor cocktail P4589). This mixture was stirred for 18 hours at 4°C and subsequently centrifuged at 100,000 x g for 20 minutes and the supernatant which contains nAChR-Cholate solution was collected.
The supernatant was mixed with 200 mg of dried IAM-PC liquid chromatography stationary phase and stirred gently for 1 hour at 25°C. After an hour, the resulting mixture was transferred into dialysis tubing and dialyzed for 48 hours at 4°C against 1L of Tris-HCl [50mM, pH 7.4] containing 5mM EDTA, 100 mM NaCl, 0.1 mM CaCl2 and 0.1 mM PMSF.
The resulting mixture was centrifuged for 3 min at 4°C at 700 x g and the supernatant was discarded. The pellet (nAChR-IAM) was washed with 5mL of Tris-HCl [50mM, pH 7.4] and centrifuged. This process was repeated until the supernatant was clear. The nAChR-IAM (200 mg) was packed into a Tricorn 5/20 glass column GE Healthcare Life Sciences (Uppsala, Sweden) to yield a 150 mm × 5 mm (ID) chromatographic bed.
2.3. Frontal Chromatography on α3β4 and α3β4α5 nAChR IAM columns
The α3β4 and α3β4α5 nAChR-IAM packed columns were placed in a chromatographic system consisting of a Shimadzu LC-10 AD pump (Shimadzu, Columbia, MD) isocratic HPLC pump, a 50-mL sample superloop (Amersham Pharmacia Biotech) and an IN/US system β-ram model 3 on-line scintillation detector (IN/US, Tampa, FL, USA) with a dwell time of 2 s and running Laura Lite 3 software. 20 mL of ammonium acetate [10mM, pH 7.4] with varying concentrations of [3H]-epibatidine were injected into the column using a 50 mL sample superloop. Ammonium acetate buffer [10 mM, pH 7.4] was pumped through the system at 0.2 mL/min at room temperature and the elution profile was monitored by the on-line flow scintillation detector. The chromatographic data was summed up in 1 minute intervals and smoothed using the Microsoft excel program with a 10-point moving average. The mean and standard deviation could be obtained using the retention volume. The concentrations used with [3H]-epibatidine were 20 pM, 30 pM, 60 pM, 90 pM, 120 pM, 180 pM for the α3β4α5 and 30pM, 60 pM, 90 pM, 120 pM, 180pM, 300 pM for the α3β4 nicotinic receptor.
For the frontal displacement studies, 60 pM [3H]-epibatidine was used as the marker ligand for both the α3β4 and α3β4α5 nAChR, with varying concentrations of nicotine (25 nM, 50 nM, 75 nM, 100 nM, 125 nM, 150 nM, 200 nM); cytisine (5 nM, 7,5 nM, 10 nM, 25 nM, 50 nM, 75 nM, 100 nM); nornicotine (15 nM, 25 nM, 50 nM, 100 nM, 150 nM, 250 nM) and anabasine (α3β4: 250 nM, 500 nM, 600 nM, 750 nM, 1 μM, 2 μM concentrations; α3β4α5: 50 nM, 75 nM, 120 nM, 250 nM, 500 nM, 1 μM).
For the frontal displacement studies on the non-competitive inhibitor binding site for both the α3β4 and α3β4α5 nAChR, varying concentrations of ligands were tested. MCM (1 μM, 5 μM, 10 μM, 25 μM, 50 μM) was run on the α3β4 nAChR column and (0.5 μM, 1 μM, 5μM, 25μM) was run on the α3β4α5 nAChR column. Buproprion (1 μM, 5 μM, 10 μM, 25 μM, 50 μM, 100 μM) was run on the α3β4 nAChR column and (1 μM, 5 μM, 10 μM, 20 μM, 50 μM, 75 μM) was run on the α3β4α5 nAChR column.
MCM and buproprion were monitored in the positive ion mode using single ion monitoring at m/z = 168.1 [MW +H] ion and 240.10 [MW+H], respectively, with the capillary voltage at 4000 V, the nebulizer pressure at 45 psi, and the drying gas flow at 12 L/min at a temperature of 350°C.
2.4. Data analysis
The dissociation constants, Kd, for the marker and displacer ligands were calculated using a previously described approach [21]. The experimental approach is based upon the effect of escalating concentrations of a competitive binding ligand on the retention volume of a marker ligand that is specific for the target receptor. For example, for the nicotinic receptors, epibatidine is used as a marker ligand, then the binding affinity can be calculated for EB and a displacer using the following equation:
| (Eqn 1) |
where, V is retention volume of EB; Vmin, the retention volume of EB when the specific interaction is completely suppressed and P is the product of the Bmax and the (Kd/KdM). From the above plot and a plot of 1/(V − Vmin) vs. [EB], dissociation constant values, Kd, for [3H]-MPP+ and the drugs can be obtained. The data was analyzed by non-linear regression with a sigmoidal response curve using Prism 4 software (Graph Pad Software, Inc., San Diego, CA, USA) running on a personal computer.
2.5. Plant extracts
2.5.1. Trigonella foenum-graecum seed extract preparation
The extract was prepared by HerbPharm as previously described [12]. T. foenum-graecum seed was extracted by maceration for 3 weeks at 1:2.5 ratio at 72% ethanol, ratio expressed as mass raw plant material (T. foenum-graecum seed) in weight (g) per volume (mL) of extraction solvent.
2.5.2. Lycopodium clavatum L extract preparation
The extract was prepared at Hawaii Pharm (Honolulu, HI). Briefly, Lycopodium clavalatum L. was extracted by maceration for 3–6 months at a 1:3 ratio at 60% ethanol, ratio expressed as mass raw plant material (Lycopodium clavatum L.) in weight (g) per volume (mL) of extraction solvent.
2.5.3. Displacement Studies
Lycopodium clavatum L. and Trigonella foenum-graecum L. aqueous-alcoholic extracts were tested for the presence of potential nAChR ligands. Both extracts were injected at two different concentration levels: 0.1% (v/v) and 0.5% (v/v) with 60 pM [3H]-epibatidine for the agonist-binding sites and 1% (v/v) and 5% (v/v) with 1 μM MCM for the non-competitive inhibitor binding site.
3. Results and Discussion
3.1. Characterization of the α3β4α5 nAChR
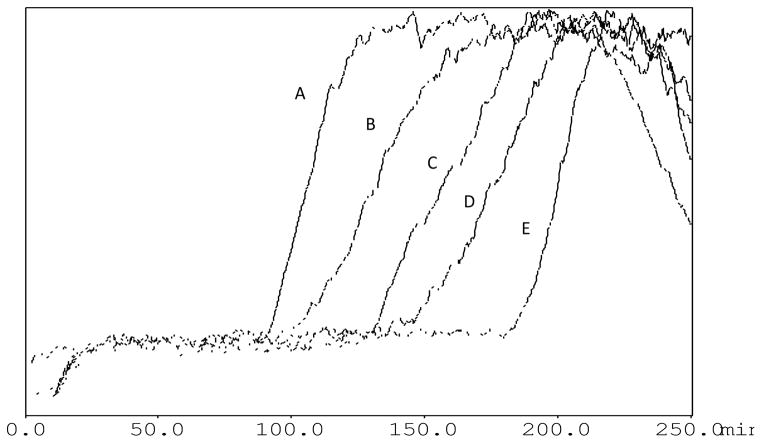
The CMAC (α3β4α5 nAChR) column was prepared through the direct immobilization of crude membrane preparations from HEK-293 cell lines transfected with the stable constructs containing α3, β4 and α5 subunits. No attempt was made to isolate or purify the individual receptors, as we have previously shown that sufficient quantities of the nAChR were solubilized using 2% cholate. In order to characterize the immobilized α3β4α5 nAChR, frontal affinity chromatography was carried out. This technique is used to characterize the binding of small molecules to the immobilized membrane-bound target (nAChR) and allows the determination of the binding affinities (Kd) and the number of active binding sites on the column (Bmax). A typical chromatogram of increasing concentrations of [3H]-epibatidine ([3H]-EB) obtained using frontal chromatographic techniques on the CMAC (α3β4α5 nAChR) stationary is presented in Figure 1. The concentration-dependent decreases in the retention time of the chromatographic traces demonstrate that specific binding interaction between epibatidine and the α3β4α5 nAChR was observed (Figure 1), with a Kd of (20.7 ± 12.6) pM for α3β4α5 nAChR and (15.43 ± 10.4) pM for α3β4 nAChR, similar to previously reported values [18].
Figure 1.
Frontal chromatogram of increasing concentrations of [3H]-epibatidine on the α3β4α5 nicotinic receptor column, where: A =240pM, B = 150 pM, C = 100 pM, D=80 pM and E=60pM. Ammonium acetate buffer [10 mM, pH 7.4] was used as eluent at a flow rate of 0.2 mL/min.
As α3β4α5 nAChR was shown to be immobilized in a functional state, the binding affinity of nicotine and two minor alkaloids found in tobacco smoke condensates (anabasine and nornicotine), as well as cytisine, an alkaloid found in Cytisus laburnum L. (Fabaceae) with known nAChR activity, was tested. In the displacement studies, increasing concentrations of the test ligands were added to the mobile phase with a constant concentration of [3H]-EB and the change in its retention volume, measured at the midpoint of the breakthrough curves, was used to determine the binding affinity of the ligand (Figure 1). The Kd values for tested alkaloids for both the α3β4 and α3β4α5 nAChR subtypes are reported in Table 1. While nicotine, cytisine and nornicotine were similar between both subtypes (28 vs 15 nM; 8.5 vs 5.4 nM; 36 vs 21 nM) respectively, the difference observed with anabasine was significant. Anabasine had a 10 fold higher affinity for the α3β4α5 nAChR vs the α3β4 nAChR (68 vs 530 nM, respectively). This would be consistent with previous findings that the levels of anabasine typically found in cigarette smoke, resulted in a significant increase in nicotine self-administration in rats [22], suggesting that anabasine could contribute to the reinforcing effects of tobacco. Although, the α5 subtype is believed to play a predominantly structural role, the results of this study indicate, that the α5 can significantly alter the binding affinity of nicotinic ligands and as a result can be selectively targeted for the identification of novel nicotinic ligands that may play a role in smoking cessation.
Table 1.
Binding affinities (Ki) determined using frontal displacement chromatography on both the α3β4 and α3β4α5 nAChRs using [3H]-epibatidine as the marker ligand.
| Compound | α3β4α5 | α3β4 |
|---|---|---|
| Epibatidine | 20.7 ± 12.6 pM (r2=0.96) | 15.43 ± 10.4 pM (r2=0.89) |
| Nicotine | 15.4 ± 9.6 nM (r2=0.98) | 28.15 ± 14.6 nM (r2=0.97) |
| Cytisine | 5.4 ± 3.5 nM (r2=0.89) | 8.52 ± 5.7 nM (r2=0.95) |
| Nornicotine | 20.8 ± 11.7nM (r2=0.88) | 35.6± 18.9 nM (r2=0.98) |
| Anabasine | 67.5± 25.8 nM (r2=0.92) | 528.1 ± 135.6 nM (r2=0.86) |
In addition to probing the interactions at the competitive agonist binding site, we examined the non-competitive inhibitor site, as several drugs that show promise for smoking cessation are non-competitive inhibitors and in some cases are in the clinic. For example, buproprion (Zyban) has been clinically approved for smoking cessation [23], while MCM shows effectiveness in smoking cessation [24, 25].
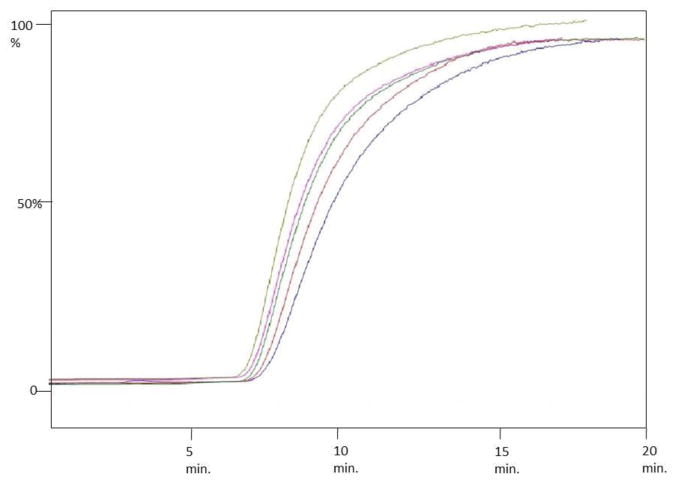
To this end, we developed a frontal displacement chromatographic method for the screening the NCI binding site for the first time. The binding affinity of MCM was determined on the CMAC (α3β4 nAChR) to be 8.5 μM (Fig. 2), which is consistent with previously reported values [15]. A similar binding affinity was obtained for the α3β4α5 nAChR with a Kd of 3.4 μM obtained (Figure 2). Buproprion binding affinity for both subtypes was also determined with a Kd of 9.2 μM and 40.4 μM obtained for the α3β4 and α3β4α5 nAChRs, respectively, indicating a slightly higher affinity for the α3β4 nicotinic receptor. The data indicates that subtype selectivity between the α3β4 and α3β4α5 nAChRs can be easily differentiated using frontal displacement chromatographic techniques.
Figure 2.
Frontal chromatogram of increasing concentrations of mecamylamine on the CMAC (α3β4 nAChR) column (from right to left: 1 μM, 5 μM, 10 μM, 25 μM, 50 μM). Ammonium acetate buffer [10 mM, pH 7.4] was used as eluent at a flow rate of 0.2 mL/min.
3.2. Plant Screening
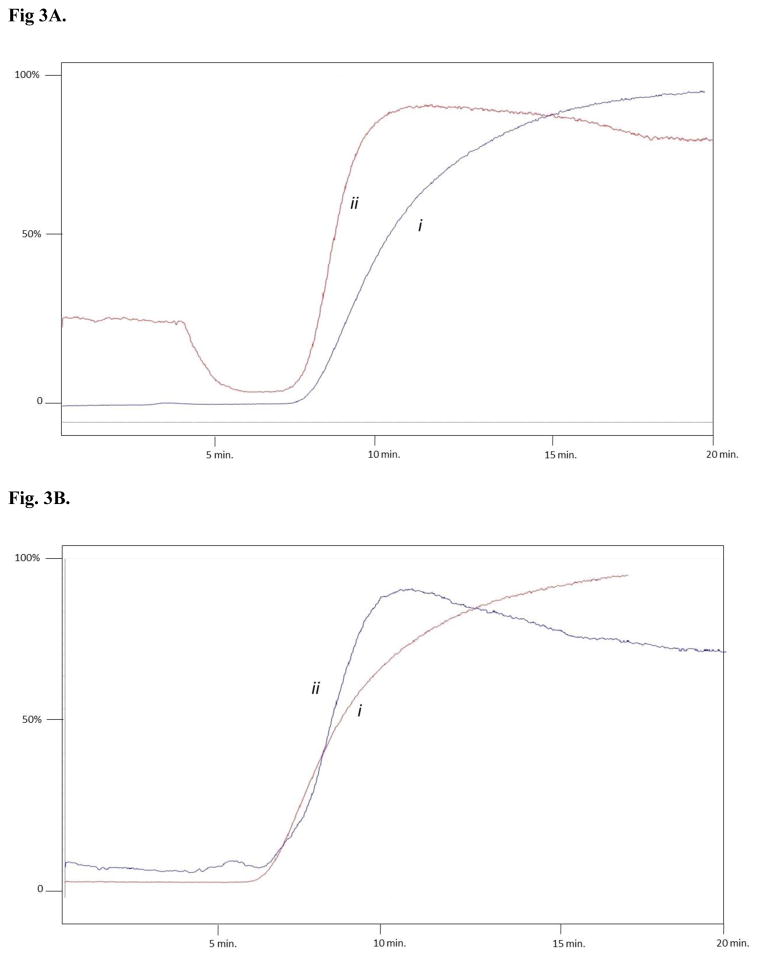
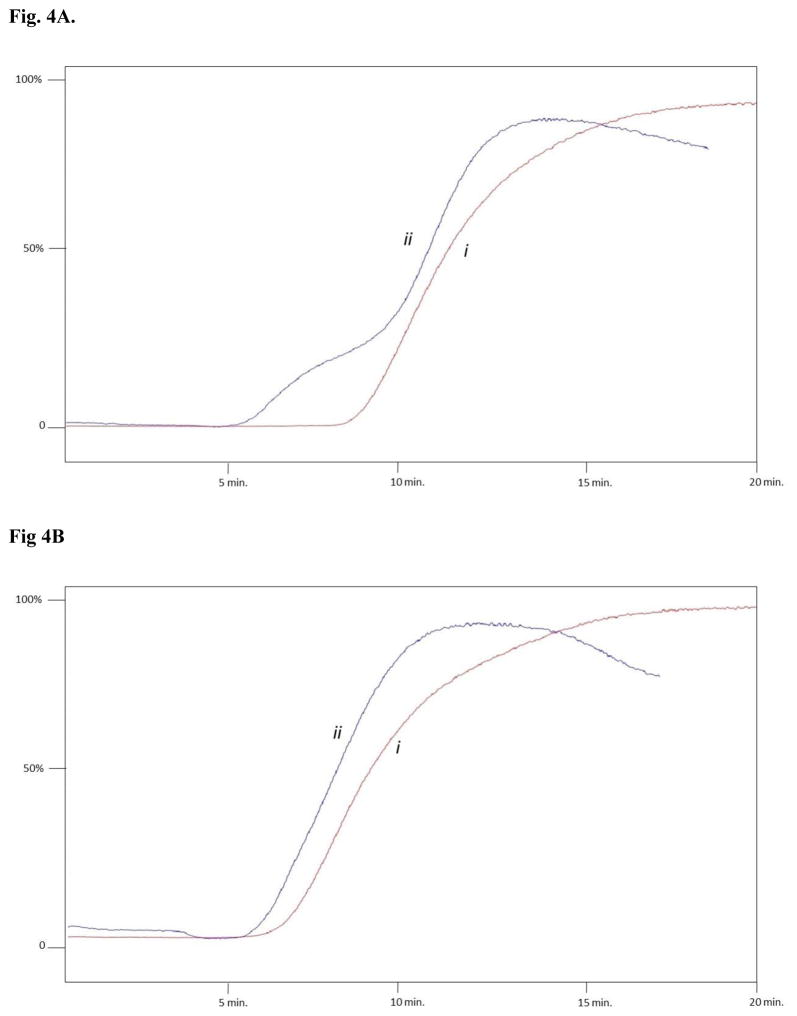
The role of the α3β4α5 nAChR in smoking addiction has been clearly demonstrated [4], and has resulted in the α3β4α5 nAChR being targeted for smoking cessation. To date, there are very few ligands that have been demonstrated to have high selectivity for the α3β4α5, as a result, the identification of novel α3β4α5 nAChR inhibitors is desired. A potential source for such a compound could be botanical matrices, for example, to date several alkaloids, have been identified as nAChRs ligands, e.g.: (S)-nicotine, (R,S)- anatabine, anabasine, cytisine, nornicotine. As a result, we have screened two different concentrations (0.1% and 0.5%) of aqueous-alcoholic solutions of targeted plant extracts, including Lycopodium clavatum L., and Trigonella foenum graecum L. against both the α3β4 and α3β4α5 nAChRs. Prior to analysis of the plant extracts, an equivalent amount of ethanol present in the extract was added to the mobile phase for both the agonist binding site (60 pM [3H]-epibatidine) and the non-competitive inhibitor binding site (1 μM MCM), and no displacement of the marker ligand was observed in either case (data not shown). Lycopodium clavatum L. was chosen as an extract, as it is known to contain a large amount alkaloids, while Trigonella foenum graecum L. was chosen as it is known to contain no significant amounts of alkaloids, with the exception of Trigonelline in Trigonella foenum graecum L. In both cases, only slight displacements of [3H]-EB was observed with 0.1% of the extract, as a result all extracts were run at 0.5%. As expected, a 0.5% solution of Lycopodium clavatum L. resulted in a significant decrease of the retention of epibatidine to the α3β4α5 (data not shown), indicating the presence of competing ligands for the nicotinic agonist binding site from Lycopodium L.. In the case of the non-competitive inhibitor binding site, no displacement was observed with a 0.5 or 1 % solution of Lycopodium clavatum L. on either the α3β4 or α3β4α5 nAChR (data not shown), as a result, the concentration of the extract was increased to 5%. This is consistent with the fact that non-competitive inhibitors binding affinity are typically in the μM range, while competitive agonist/antagonist are typically in the nM range. Of interest, a 5% solution of Lycopodium clavatum L. caused a significant decrease in the retention time of 1 μM MCM for the α3β4 nAChR, while having no effect on the α3β4α5 nAChR (Figure 3), indicating that there a selective non-competitive inhibitors for the α3β4 nAChR present in the extract of Lycopodium clavatum L.. While Trigonella foenum graecum L. showed no displacement of [3H]-EB for the agonist binding site, a small displacement (<8%) of 1 μM MCM was observed for both α3β4 and α3β4α5 nAChRs (Fig. 4B and 4B, respectively), the displacement was significantly less than what was observed for Lycopodium clavatum L. (~25%), indicating that this method can be used to screen for active complex mixtures, prior to isolation of the active components.
Figure 3.
Representative frontal elution profile of 1 μM MCM (i) and 1 μM MCM + 5% Lycopodium clavatum L. (ii) on the CMAC (α3β4 nAChR) column (A) and on the CMAC (α3β4α5 nAChR) column (B). Ammonium acetate buffer [10 mM, pH 7.4] was used as eluent at a flow rate of 0.2 mL/min.
Figure 4.
A. Representative frontal elution profile of 1 μM MCM (i) and 1 μM MCM + 5% Trigonella foenum graecum L. (ii) on the CMAC (α3β4 nAChR) column (A) and the CMAC (α3β4α5 nAChR) column (B). Ammonium acetate buffer [10 mM, pH 7.4] was used as eluent at a flow rate of 0.2 mL/min.
Highlights.
The α3β4α5 nicotinic receptor was fully characterized
Anabasine is selective for the α3β4α5 versus the α3β4 nicotinic receptor
Characterization of non-competitive inhibitor binding site by frontal chromatography
Application to the screening of plant extracts was demonstrated
Acknowledgments
This work was supported in part by funds from the NIA Intramural Research Program and from the IACTRS FDA/NIH Collaborative Grant. Research reported in this publication was supported by the NIH intramural research program and the FDA through funds obtained under the Family Smoking Prevention and Tobacco Control Act. The content was not reviewed by the Food and Drug Administration, but underwent the standard manuscript clearance process for scientific papers published from the NIH intramural research program.
Footnotes
The content was not reviewed by the Food and Drug Administration, but underwent the standard manuscript clearance process for scientific papers published from the NIH intramural research program
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.George AA, Lucero LM, Damaj MI, Lukas RJ, Chen X, Whiteaker P. Function of human α3β4α5 nicotinic acetylcholine receptors is reduced by the α5(D398N) variant. J Biol Chem. 2012;287:25151–25162. doi: 10.1074/jbc.M112.379339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciuraszkiewicz A, Schreibmayer W, Platzer D, Orr-Urtreger A, Scholze P, Huck S. Single-channel properties of α3β4, α3β4α5 and α3β4α2 nicotinic acetylcholine receptors in mice lacking specific nicotinic acetylcholine receptor subunits. J Physiol. 2013;591:3271–3288. doi: 10.1113/jphysiol.2012.246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;15:8785–8790. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, Filkin S, Pons SS, Fontaine JF, Tsetlin V, Maskos U, Ibańez-Tallon I. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer. Multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudencio SP, Pereira F. Dereplication: racing to speed up the natural products discovery process. Nat Prod Rep. 2015;32:779–810. doi: 10.1039/c4np00134f. [DOI] [PubMed] [Google Scholar]
- 8.Rhee IK, Appels N, Luijendijk T, Irth H, Verpoorte R. Determining acetylcholinesterase inhibitory activity in plant extracts using a fluorimetric flow assay. Phytochem Anal. 2003;14:145–149. doi: 10.1002/pca.695. [DOI] [PubMed] [Google Scholar]
- 9.Jong CF, Derks RJE, Bruyneel B, Niessen W, Irth H. High-performance liquid chromatography–mass spectrometry-based acetylcholinesterase assay for the screening of inhibitors in natural extracts. J Chromatogr A. 2006;1112:303–310. doi: 10.1016/j.chroma.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda M, Wilson DR, Fugmann SD, Moaddel R. Synthesis and characterization of SIRT6 protein coated magnetic beads: identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts. Anal Chem. 2011;83:7400–7407. doi: 10.1021/ac201403y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanzolini KL, Jiang Z, Zhang X, Curcino Vieira LC, Gonçalvez Corrêa A, Lucia Cardoso C, Bezerra Cass Q, Moaddel R. Acetylcholinesterase immobilized capillary reactors coupled to protein coated magnetic beads: A new tool for plant extract ligand screening. Talanta. 2013;116:647–652. doi: 10.1016/j.talanta.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh N, Ravichandran S, Norton DD, Fugmann SD, Moaddel R. Synthesis and characterization of a SIRT6 open tubular column: Predicting deacetylation activity using frontal chromatography. Anal Biochem. 2013;436:78–83. doi: 10.1016/j.ab.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciuk A, Moaddel R, Haginaka J, Wainer IW. Screening of tobacco smoke condensate for nicotinic acetylcholine receptor ligands using cellular membrane affinity chromatography columns and missing peak chromatography. J Pharm Biomed Anal. 2008;48:238–246. doi: 10.1016/j.jpba.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moaddel R, Rosenberg A, Spelman K, Frazier J, Frazier C, Nocerino S, Brizzi A, Mugnaini C, Wainer IW. Development and characterization of immobilized cannabinoid receptor (CB1/CB2) open tubular column for on-line screening. Anal Biochem. 2011;412:85–91. doi: 10.1016/j.ab.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moaddel R, Jozwiak K, Whittington K, Wainer IW. Conformational mobility of immobilized α3β2, α3β4, α4β2, and α4β4 nicotinic acetylcholine receptors. Anal Chem. 2005;77:895–901. doi: 10.1021/ac048826x. [DOI] [PubMed] [Google Scholar]
- 16.Singh NS, Habicht KL, Dossou KSS, Shimmo R, Wainer IW, Moaddel R. Multiple protein stationary phases: A review. J Chromatogr B. 2014;968:64–68. doi: 10.1016/j.jchromb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharm. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Xiao Y, Kellar KJ, Wainer IW. Immobilized nicotinic receptor stationary phase for on-line liquid chromatographic determination of drug–receptor affinities. Anal Biochem. 1998;264:22–25. doi: 10.1006/abio.1998.2828. [DOI] [PubMed] [Google Scholar]
- 19.Tammimäki A, Herder P, Li P, Esch C, Laughlin JR, Akk G, Stitzel Jerry A. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharm. 2012;63:1002–1011. doi: 10.1016/j.neuropharm.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baynham MT, Patel S, Moaddel R, Wainer IW. Multidimensional on-line screening for ligands to the α3β4 neuronal nicotinic acetylcholine receptor using an immobilized nicotinic receptor liquid chromatographic stationary phase. J Chromatogr B. 2002;772:155–161. doi: 10.1016/s1570-0232(02)00070-3. [DOI] [PubMed] [Google Scholar]
- 21.Moaddel R, Wainer IW. Immobilized nicotinic receptor stationary phases: Going with the flow in high-throughput screening and pharmacological studies. J Pharm Biomed Anal. 2003;30:1715–1724. doi: 10.1016/s0731-7085(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 22.Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED. Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats. Pharmacology Biochemistry and Behavior. 2014;120:103–108. doi: 10.1016/j.pbb.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boshier A, Wilton LV, Shakir SA. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. Eur J Clin Pharmacol. 2003;59:767–773. doi: 10.1007/s00228-003-0693-0. [DOI] [PubMed] [Google Scholar]
- 24.Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR. Mecamylamine (Inversine): an old antihypertensive with new research directions. 2002;16:453–457. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- 25.Jiloha RC. Pharmacotherapy of smoking cessation. Indian J Psychiatry. 2014;56:87–95. doi: 10.4103/0019-5545.124726. [DOI] [PMC free article] [PubMed] [Google Scholar]