Abstract
Previous studies suggest dopamine (DA) D2-like receptor involvement in the reinforcing effects of food. To determine contributions of the three D2-like receptor subtypes, knockout (KO) mice completely lacking DA D2, D3, or D4 receptors (D2R, D3R, or D4R KO mice) and their wild-type (WT) littermates were exposed to a series of fixed-ratio (FR) food-reinforcement schedules in two contexts: an open economy with additional food provided outside the experimental setting and a closed economy with all food earned within the experimental setting. A behavioral economic model was used to quantify reinforcer effectiveness with food pellets obtained as a function of price (FR schedule value) plotted to assess elasticity of demand. Under both economies, as price increased, food pellets obtained decreased more rapidly (ie, food demand was more elastic) in DA D2R KO mice compared with WT littermates. Extinction of responding was studied in two contexts: by eliminating food deliveries and by delivering food independently of responding. A hyperbolic model quantified rates of extinction. Extinction in DA D2R KO mice occurred less rapidly compared with WT mice in both contexts. Elasticity of food demand was higher in DA D4R KO than WT mice in the open, but not closed, economy. Extinction of responding in DA D4R KO mice was not different from that in WT littermates in either context. No differences in elasticity of food demand or extinction rate were obtained in D3R KO mice and WT littermates. These results indicate that the D2R is the primary DA D2-like receptor subtype mediating the reinforcing effectiveness of food.
INTRODUCTION
Striatal dopamine (DA) D2-like (D2, D3, D4) receptor availability, assessed using positron emission or single photon emission computed tomography in humans, has been associated with body mass index (BMI) and several self-reported indices of overeating in obese participants (Caravaggio et al, 2015; de Weijer et al, 2011; Guo et al, 2014; Wang et al, 2001), suggesting a significant role of DA D2-like receptors in the reinforcing effects of food. However, positive (eg, Caravaggio et al, 2015) and negative (eg, Wang et al, 2001) associations have been reported, as well as an absence of an association (eg, Eisenstein et al, 2013). Several factors may influence whether an association is found between BMI and D2 receptor (D2R) binding including striatal region assessed, specificity of radiotracer, subject pools, competition with endogenous DA, and potentially definitions of regions of interest (Caravaggio et al, 2015; Guo et al, 2014). Possibly also important are the measures used to assess the reinforcing effects of food.
In mice, elimination of DA D2R expression by gene deletion (knockout; KO) produced lower food and water intake, lower body weights (Baik et al, 1995; Kim et al, 2010) and lower rates of food-reinforced responding (Caine et al, 2002; Chausmer et al, 2002) compared with wild-type (WT) controls suggesting decreases in reinforcing effects of food. In comparison, striatal DA D2R overexpression has produced both increases (Trifilieff et al, 2013) and decreases in food-reinforced responding (Drew et al, 2007; Simpson et al, 2011; Ward et al, 2012), with outcomes possibly depending on age of induction, degree of overexpression, and selectivity of the overexpression for subregions of the striatum (Trifilieff et al, 2013). Finally, diet-induced obesity in rats was associated with reduced striatal DA D2R expression and striatal DA D2R reduction was associated with the development of consumption of high-fat, high-sugar foods that was resistant to suppression by electric-shock-associated stimuli (Johnson and Kenny, 2010). Interpreting changes in absolute response rates as a function of changes in reinforcing effects is complicated, however, by the fact that rates of already acquired responses often are not influenced strongly by magnitude of reinforcement (eg, Catania, 1963).
The current study directly assessed the roles of the individual DA D2-like receptors in food reinforcement using a behavioral economic analysis of demand curves, where reinforcer consumption is calculated as a function of price (Hursh and Silberberg, 2008). In laboratory studies, price can be considered the number of responses required per unit of reinforcer. The exponential model of demand describes a monotonic decrease in consumption with increased price, and entails two distinct measures of reinforcement: Q0, estimated consumption at zero price, and α, elasticity of demand, which is inversely related to the effectiveness or ‘essential value' of the reinforcer (Hursh and Silberberg, 2008). The parameter α determines the rate at which demand decreases with increased price (ie, elasticity of demand; see Methods). Hursh and Silberberg (2008) have shown that the two measures, α and Q0, represent independent aspects of reinforcement with Q0 affected by variables such as magnitude of reinforcement and α affected by variables such as level of deprivation and economic conditions under which the reinforcer is obtained.
Soto et al (2011) used demand curves to compare reinforcing effectiveness of food and found that the curves decreased more steeply in DA D2R KO compared with WT or heterozygote subjects indicating a lower reinforcing effectiveness of food with D2R deletion. The present study extends that assessment to the DA D3R and D4R subtypes. Soto et al (2011) used short experimental sessions in which the maximal number of reinforcers was held constant and mice were fed outside of the experimental session (open economy). However, as behavioral economic analyses were derived for situations in which maximal number of reinforcers is not restricted and all reinforcers are obtained within the experimental sessions (closed economy), the present study assessed the generality of effects with demand curves for food determined under open and closed economic conditions. Because the highest FR values (ie, prices) employed necessitated long periods of non-reinforcement in which extinction may have contributed to outcomes, the current study directly evaluated genotype-dependent differences in extinction of responding. Also, since food delivery can serve discriminative, in addition to reinforcing functions (Reid, 1958; Rescorla and Skucy, 1969; Skinner, 1938), two varieties of extinction were used: one without food deliveries and one with response-independent food deliveries. In the latter variety of extinction, only the response-reinforcer contingency was eliminated, whereas in the former variety of extinction, that contingency and the reinforcer delivery were eliminated.
MATERIALS AND METHODS
Subjects
Mice with genetic deletions of DA D2Rs (n=6), D3Rs (n=10), and D4Rs (n=6) and littermate controls (n=6, 10, and 6, respectively) were singly housed under a 12-h/12-h light/dark cycle (lights on: 0700 h). Subjects averaged 28.5±1.19 (SEM) and 81.3±3.39 weeks of age when experiments started and ended. The (incipient) congenic subjects used were the product of at least ten generations of mating heterozygous subjects with WT C57BL/6J mice. The homologous recombination techniques used and details of DA receptor targeting have been reported (Kelly et al, 1997, 1998; Rubinstein et al, 1997; Xu et al, 1997). Subjects were maintained at 85% of unrestricted-feeding weights for the entire study.
Apparatus
Experimental sessions were conducted in operant-conditioning chambers (Med-Associates, modified Model ENV307A, St Albans, VT) measuring 15.9 (length) × 14.0 (depth) × 12.7 (height) cm. On the front wall of each chamber, equidistant from midline, were two levers, 2.5 cm above the floor (each requiring a ~2 g force to depress). A food-pellet (20 mg Precision food pellets, BioServ, Frenchtown, NJ) delivery device and an electromechanical relay that provided audible clicks with lever presses were mounted behind the front wall. Food pellets were delivered to an accessible food-cup centered between the response levers. Centered above each lever was a row of three light-emitting diodes (LEDs), and centered at the top of the front wall was a single 28 V d.c. lamp (houselight) that illuminated the chamber.
Procedures
Initial training
Subjects were trained to lever press during daily (5 days/week) experimental sessions under conditions that changed gradually during training. Sessions initially began with the subject placed in the chamber, and with the houselight and LEDs on. Training was generally as described previously (Soto et al, 2011). Under final conditions, the FR value was five, the post-reinforcer timeout was 10 s and the timeout at the start of the session was five min. Each session ended after 20 pellets were delivered or 30 min, whichever occurred first.
Demand curves
For open-economy assessments, experimental sessions began between 0700–0900 h, and lasted 30 min or until 20 pellets had been delivered, whichever occurred first. Two sessions occurred at each of the following FR values: 1, 5, 15, 45, 90, 180, and 360. Sessions were conducted for each subject only when body weights were within the criterion range (85±5% of unrestricted weight) immediately before sessions. For the closed-economy assessments, sessions began at approximately 1900 h, lasted 11 h, and two sessions were conducted at each of the following FR values: 1, 5, 15, 45, 90, 180, 360, and 720. Subjects were weighed each day at 1200 h. If body weight was within the criterion range (85±5% of unrestricted weight) subjects were not fed and an experimental session was conducted. If body weight was outside 85±5% sessions were delayed for 24 h and subjects were fed an amount appropriate to bring weight within criterion by the next day.
Extinction
Before beginning the extinction with response-independent food deliveries, performances were stabilized on an FR 5 schedule for at least five sessions. Subsequently, subjects were exposed to 20 sessions of extinction with response-independent pellets followed by a 10 s timeout delivered at the same interpellet intervals as in the previous FR 5 session. All other aspects were identical to the previous FR 5 sessions.
Subsequently lever pressing was reconditioned and stabilized under the FR 5 schedule for at least five sessions. Subjects were then exposed to ten 30-min sessions of extinction, during which no pellets were delivered and responses had no scheduled consequences. All other aspects were identical to the previous FR 5 sessions.
Data analysis
Total responses excluding those during timeouts and total reinforcers delivered were calculated for each subject for each session. Averages and SEMs were then calculated for each genotype at each FR value. A repeated measures analysis of variance (ANOVA) was conducted on total responses separately for KO and WT pairs using FR value (within-subjects) and genotype (between-subjects) as factors. In all ANOVAs for total responses, there were significant effects of FR value (P-values <0.05) which are not detailed below for clarity. The logarithms of group averages of reinforcers obtained vs FR value were fitted (Prism version 5, Graphpad Software, Inc., San Diego, CA) with the equation 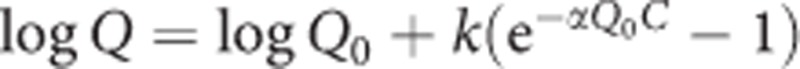 , where α represents decline rate of the curve and is inversely related to reinforcer effectiveness, Q represents the number of reinforcers consumed, Q0 represents maximum consumption at zero price, C represents the cost of each reinforcer (ie FR value), and k is the y axis range of the function (set to 4 for all data sets; Hursh and Silberberg, 2008). The parameters Q0 and α for each genotype were compared via an F-test to a global fit obtained utilizing common values of the two parameters (Motulsky and Christopoulos, 2004). A similar analysis was conducted on the three groups of WT subjects.
, where α represents decline rate of the curve and is inversely related to reinforcer effectiveness, Q represents the number of reinforcers consumed, Q0 represents maximum consumption at zero price, C represents the cost of each reinforcer (ie FR value), and k is the y axis range of the function (set to 4 for all data sets; Hursh and Silberberg, 2008). The parameters Q0 and α for each genotype were compared via an F-test to a global fit obtained utilizing common values of the two parameters (Motulsky and Christopoulos, 2004). A similar analysis was conducted on the three groups of WT subjects.
For extinction, overall response rates (total responses divided by total time excluding timeouts) were calculated for the two FR 5 sessions (control response rates) that preceded the extinction sessions, and for each session of extinction expressed as a proportion of the average control response rate.
Because no broad consensus exists in the literature on a model for extinction, results were fitted to four models: (1) A hyperbolic decay equation of the form 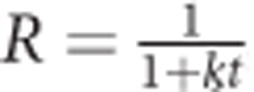 , with R representing the response rate as a proportion of control, t representing session number, and k representing the decay parameter, which reflects rate of change (ie, how quickly the behavior extinguishes); (2) an exponential decay model of the form R=e−kt in which R, t, and k are as in the hyperbolic decay equation; (3) the augmented extinction model of behavioral momentum (Nevin and Grace, 2000) of the form
, with R representing the response rate as a proportion of control, t representing session number, and k representing the decay parameter, which reflects rate of change (ie, how quickly the behavior extinguishes); (2) an exponential decay model of the form R=e−kt in which R, t, and k are as in the hyperbolic decay equation; (3) the augmented extinction model of behavioral momentum (Nevin and Grace, 2000) of the form 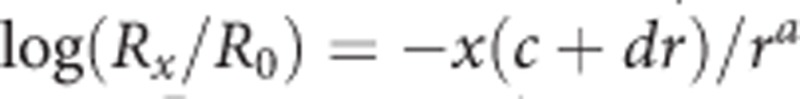 , with Rx representing response rate, R0 representing control response rate, x representing session number, r representing control reinforcement rate before extinction, and with a, c, and d as free parameters; (4) a single-parameter version of the Rescorla–Wagner equation of the form Rn=aRn−1, with R representing extinction response rates, n representing session number, and with a as a free parameter (Rescorla and Wagner, 1972). The hyperbolic model accounted for an average of 95.7 and 64.2% of the variance in response rates without and with response-independent food, respectively. The other three models accounted for an average of 40.7–94.0% and 35.4–63.6% of the variance in response rates for the two types of extinction, respectively. Thus, the hyperbolic model is the only model presented here, with fits assessed as described above for demand curves.
, with Rx representing response rate, R0 representing control response rate, x representing session number, r representing control reinforcement rate before extinction, and with a, c, and d as free parameters; (4) a single-parameter version of the Rescorla–Wagner equation of the form Rn=aRn−1, with R representing extinction response rates, n representing session number, and with a as a free parameter (Rescorla and Wagner, 1972). The hyperbolic model accounted for an average of 95.7 and 64.2% of the variance in response rates without and with response-independent food, respectively. The other three models accounted for an average of 40.7–94.0% and 35.4–63.6% of the variance in response rates for the two types of extinction, respectively. Thus, the hyperbolic model is the only model presented here, with fits assessed as described above for demand curves.
RESULTS
Open Economy
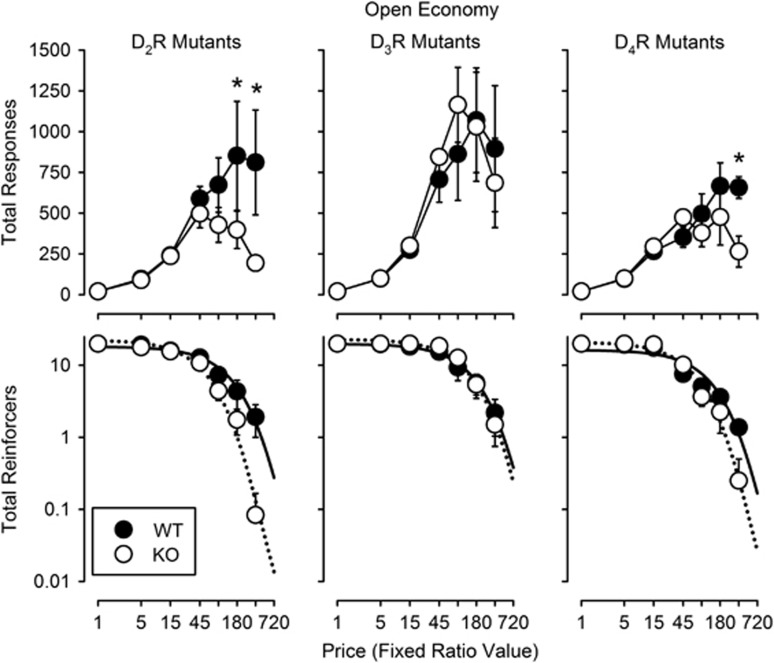
Increasing FR value produced equivalent increases in total response output up to FR 90 for WT and DA D2R KO subjects (Figure 1, top left panel). At FR 180 and 360, frequency of responding by WT subjects was greater than that for DA D2R KO subjects (Holm–Sidak post-hoc comparisons following a significant genotype by FR value interaction: F6,60=2.27, P=0.049). Concomitantly, as FR value increased, consumption decreased in WT and DA D2R KO subjects (Figure 1, bottom left panel). The rate of decline was greater in DA D2R KO compared with WT subjects (Figure 1, bottom left panel) with an α value in D2R KO subjects ~2.16-fold larger than that obtained with WT subjects (F1,10=27.6, P=0.004; Table 1). Values of Q0 (estimated consumption at zero price) for the WT and DA D2R KO subjects did not differ statistically (Table 1).
Figure 1.
Changes in total response output (top) and total reinforcers earned (bottom) as a function of fixed-ratio value in wild type and DA D2R (left), D3R (middle), and D4R (right) knockout subjects (n=6 per genotype except n=5 for D3R knockout subjects) during open economy sessions. Each data point represents the mean across subjects within a genotype. Error bars represent ±1.0 SEM. Solid and dotted lines in the bottom graphs represent best-fitting versions of the exponential model of demand for the wild-type subjects and knockout subjects, respectively. Asterisks indicate statistically significant differences (p<0.05) in total response output between wild-type and knockout subjects at each fixed-ratio value.
Table 1. Parameter Values with 95% Confidence Limits in Parentheses and %VAF by Best-Fits of the Exponential Demand Curve Under Open and Closed Economies.
| Genotype | α parameter (×10−5) | Q0 parameter | %VAF |
|---|---|---|---|
| Open economy | |||
| D2R WT | 4.64 (3.84–5.43) | 18.0 (14.4–21.7) | 97.4 |
| D2R KO | 10.0a (6.72–13.4) | 22.6 (12.1–33.2) | 97.6 |
| D3R WT | 3.92 (3.59–4.25) | 19.9 (18.0–21.8) | 99.3 |
| D3R KO | 4.11 (3.54–4.67) | 23.1 (19.1–27.0) | 98.5 |
| D4R WT | 5.86 (3.95–7.77) | 16.3 (9.65–23.0) | 92.1 |
| D4R KO | 8.48a (6.57–10.4) | 21.1 (14.4–27.8) | 98.3 |
| Closed economy | |||
| D2R WT | 0.269 (0.181–0.356) | 222 (131–312) | 91.9 |
| D2R KO | 0.505a (0.407–0.604) | 225 (165–285) | 98.7 |
| D3R WT | 0.250 (0.179–0.321) | 279 (175–382) | 94.8 |
| D3R KO | 0.226 (0.171–0.282) | 299 (204–394) | 95.9 |
| D4R WT | 0.314 (0.188–0.439) | 239 (113–365) | 90.7 |
| D4R KO | 0.362 (0.178–0.547) | 221 (70.9–371) | 86.7 |
Abbreviations: KO, knockout; %VAF, percentage of variance accounted for; WT, wild type.
Significant difference from parameter value in WT littermate subjects.
In WT and DA D3R KO subjects, increasing FR value produced similar increases in total response output up to intermediate values (FR 90 in DA D3R KO and FR 180 in WT subjects) with decreases at higher FR values in both groups of subjects (Figure 1, top middle panel). There were no significant differences in total responses between WT and DA D3R KO subjects. Increasing FR value decreased consumption similarly in WT and DA D3R KO subjects (Figure 1, bottom middle panel) and neither the difference between values of α nor values of Q0 was significant (Table 1).
Increases in FR value for WT and DA D4R KO subjects, produced similar increases in total response output up to FR 180 (Figure 1, top right panel). At FR 360, DA D4R KO subjects emitted fewer responses than WT subjects (Holm–Sidak post-hoc comparison following a significant genotype by FR value interaction: F6,52=3.15, P=0.010). Increases in FR value decreased consumption more rapidly in DA D4R KO compared with WT subjects (Figure 1, bottom right panel). The α value obtained with DA D4R KO subjects was ~1.45 times greater than the α value obtained with WT subjects (F1,10=5.95, P=0.035; Table 1). Values of Q0 did not differ statistically among these groups (Table 1).
Closed Economy
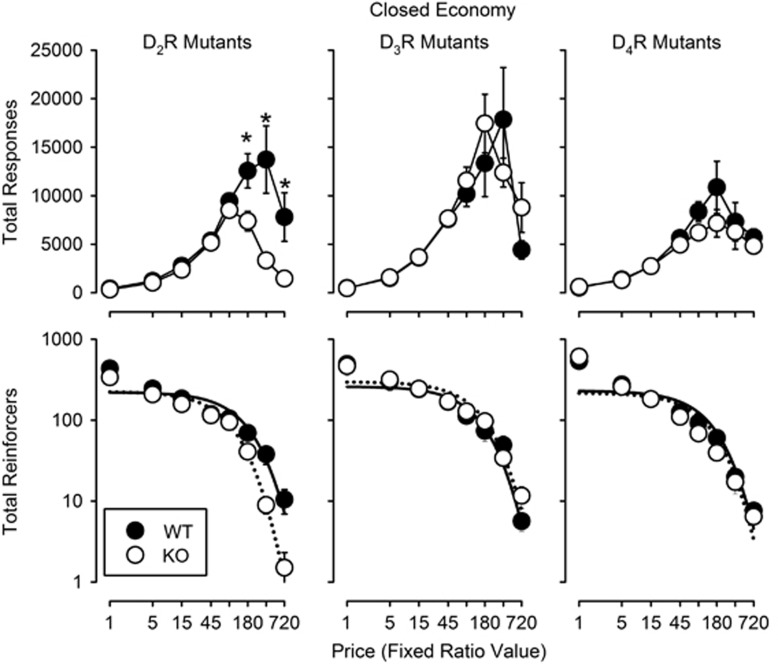
In WT and DA D2R KO subjects, increasing FR value produced similar increases in total response output up to FR 90 (Figure 2, top left panel). At higher FR values the D2R KO subjects emitted significantly fewer total responses than WT littermates (Holm–Sidak post-hoc comparisons following a significant genotype by FR value interaction: F7,70=7.06, P<0.001). Increasing FR value produced decreases in consumption in WT and DA D2R KO subjects, with a higher rate of decrease in KO compared with WT subjects (Figure 2, bottom left panel). The α value in DA D2R KO subjects was ~1.88-fold greater than the α value in WT subjects (F1,12=17.0, P=0.001; Table 1). Values of Q0 for the WT and DA D2R KO subjects did not differ statistically (Table 1).
Figure 2.
Changes in total response output (top) and total reinforcers earned (bottom) as a function of fixed-ratio value in wild type and DA D2R (left), D3R (middle), and D4R (right) knockout subjects (n=6 per genotype) during closed economy sessions. All other details are as in Figure 1.
Increasing FR value in WT and DA D3R KO subjects produced similar increases in total response output up to intermediate values (FR 180 in DA D3R KO and FR 360 in WT subjects) with decreases at higher FR values in both groups of subjects (Figure 2, top middle panel). There were no significant differences in total responses between WT and DA D3R KO subjects. Increasing FR value produced similar decreases in consumption for WT and DA D3R genotypes (Figure 2, bottom middle panel). Correspondingly, there were neither significant differences in α values nor Q0 values among the genotypes (Table 1).
In WT and DA D4R KO subjects, increasing FR value produced similar increases in total response output up to FR 180, with decreases at higher values (Figure 2, top right panel). There were no significant differences in total responses between WT and DA D4R KO subjects. Increasing FR value produced equivalent decreases in total consumption in both WT and DA D4R KO subjects (Figure 2, bottom right) with no significant differences between genotypes in the values of α or Q0 (Table 1).
Extinction without Food
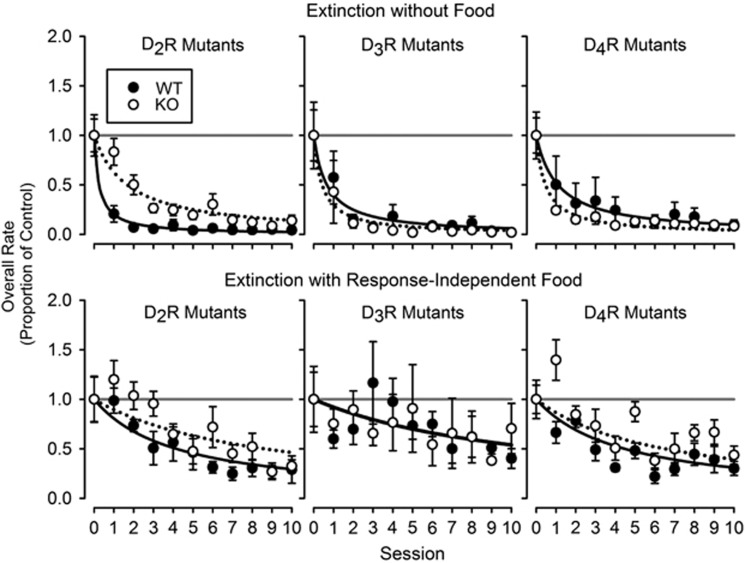
Response rates decreased more slowly in DA D2R KO compared with WT subjects across sessions (Figure 3, top left panel). Consequently, fits of the hyperbolic model produced a ~6.68-fold lower value of k, the decay parameter, for the DA D2R KO compared to WT subjects (Table 2; F1,20=80.7, P<0.0001). For WT and DA D3R KO subjects, there were no apparent differences in rates of extinction (Figure 3, top middle panel) and no significant difference in values of k (Table 2). Finally, for WT and DA D4R KO subjects, there was an apparent difference in rates of extinction, but that difference resulted from one of six WT subjects that exhibited response rates that were higher than control values (all other WT subjects exhibited rates below control values) during the first several sessions of extinction (Figure 3, top right panel). Excluding that subject there was no significant difference in the values of k between the WT and DA D4R KO subjects (Table 2).
Figure 3.
Overall response rate as a proportion of control response rate during sessions of extinction without food (top row) and extinction with response-independent food (bottom row) for wild type and DA D2R (left), D3R (middle), and D4R (right) knockout subjects (n=6 per genotype except n=7 for D3R genotypes in extinction without food). Each data point represents the mean across subjects within a genotype. Error bars represent ±1.0 SEM. Solid and dotted lines in the bottom graphs represent best-fitting versions of the hyperbolic model of demand for the wild-type subjects and knockout subjects, respectively. Horizontal gray lines are for reference and indicate control response rates.
Table 2. Parameter Values with 95% Confidence Limits in Parentheses and %VAF From Best Fits of the Hyperbolic Decay Equation to Response Rates as Proportion of Control Response Rate Across Sessions of Extinction Without Food Presentations and Extinction with Response-Independent Food Presentations.
| Genotype | Extinction without food presentations | Extinction with response-independent food presentations | ||
|---|---|---|---|---|
| k parameter | %VAF | k parameter | %VAF | |
| D2R WT | 3.91 (3.01–4.82) | 99.3 | 0.255 (0.188–0.323) | 90.9 |
| D2R KO | 0.585a (0.399–0.771) | 92.1 | 0.121a (0.053–0.137) | 68.9 |
| D3R WT | 1.49 (0.836–2.14) | 92.9 | 0.082 (0.027–0.137) | 35.0 |
| D3R KO | 2.36 (1.41–3.31) | 96.3 | 0.086 (0.047–0.124) | 45.6 |
| D4R WT | 0.895 (0.721–1.07) | 97.1 | 0.242 (0.154–0.331) | 77.1 |
| 2.80b (1.92–3.68) | 97.8 | |||
| D4R KO | 2.16 (1.44–2.89) | 96.4 | 0.162 (0.091–0.233) | 68.0 |
Abbreviations: KO, knockout; %VAF, percentage of variance accounted for; WT, wild type.
Significant difference from parameter value in WT littermate subjects.
Fit obtained after excluding one D4R WT subject that exhibited increases in responding above control rates for the first three sessions of extinction (all other D4R WT subjects exhibited decreases in responding relative to control rates).
Extinction with Response-Independent Food
Response rates decreased more slowly in DA D2R KO compared with WT subjects (Figure 3 bottom left panel), resulting in a ~2.11-fold lower value of k in D2R KO vs WT subjects (Table 2; F1,20=8.15, P=0.010). Decreases in rates of responding were similar (Figure 3, bottom middle and right panels) and there were no significant differences in values of k (Table 2) within the DA D3R or DA D4R KO subjects and their WT littermates.
Extinction with response-independent food exhibited more variability in response rates than extinction without food; in all cases, the variance accounted for by the hyperbolic decay model was less in the former condition (see Table 2). In addition, the rate of decline in responding (k) with response-independent food was ~4.83 to ~27.4-fold lower than that obtained with food completely eliminated (Table 2).
DISCUSSION
A behavioral economic analysis indicated that food demand was more elastic in DA D2R KO mice than in their WT littermates under both open and closed economic conditions. In contrast, elasticity of food demand was equivalent in mice lacking DA D3Rs and their WT littermates under both economic conditions. Food demand was more elastic in DA D4R KO mice compared to WT littermates under the open, but not closed economic condition. A primary role of DA D2Rs compared with D3Rs and D4Rs in mediating the reinforcing effectiveness of food is consistent with outcomes from previous studies that did not directly assess reinforcer effectiveness. For example lower rates of food-maintained responding and impaired discrimination reversal performance using food reinforcement have been reported in DA D2R KO compared with WT mice (Caine et al, 2002; Kruzich and Grandy, 2004; Kruzich et al, 2006). Further, differences in discrimination reversal performance may have been secondary to a difference in reinforcing effectiveness of food. Similarly, DA D2R KO responded less than WT mice on a progressive-ratio schedule of food reinforcement, although the effect lacked statistical significance (Kruzich et al, 2006). Consistent with the present results, no differences were reported in preference for a sucrose solution over tap water or rates of food-maintained responding in DA D3R KO compared with WT mice (Caine et al, 2012; Chourbaji et al, 2008), or in rates of food-maintained responding or the percentage of trials, in which a pellet was obtained in an operant two-choice food reinforcement task in DA D4R KO compared with WT mice (Katz et al, 2003; Nemirovsky et al, 2009; Thanos et al, 2010). Interestingly, a recent study reported that mice lacking only DA D2 autoreceptors responded more than controls under progressive ratio and FR 100 schedules of food reinforcement, though there were no differences in responding under FR schedules at lower values (Bello et al, 2011). Because autoreceptor deletion would enhance postsynaptic DA D2R signaling, those results also are consistent with the present findings.
The conclusion that elimination of DA D2Rs reduces the reinforcing effectiveness of food is in apparent contrast to previous imaging studies suggesting increased reinforcing effects of food in humans with lower DA D2R density (de Weijer et al, 2011; Guo et al, 2014; Wang et al, 2001). As those studies did not directly measure the reinforcing effectiveness of food per se, a behavioral economic analysis could possibly resolve apparent inconsistencies. In addition, using foods high in fat and sugar, one study reported higher rates of food-maintained response in human subjects with genetic markers associated with lower DA D2R number (Epstein et al, 2007). Possibly the role of DA D2Rs in the reinforcing effects of food varies with the type of food.
An interaction between effects of DA D2R change and type of food is not unprecedented. Johnson and Kenny (2010) found that virally mediated reduction of striatal DA D2R expression increased consumption of a high-fat, high-sugar diet and that consumption was resistant to suppression by presentation of a shock-paired stimulus. In contrast, consumption of standard rodent chow was equally suppressed by presentation of the shock-paired stimulus in both virally treated rats and controls. Of course, inherent differences between humans and mice, differences in the procedures used, and differences in outcome measures also may contribute to apparent discrepancies between the current study and human studies reporting increased food consumption and food-maintained responding in individuals with lower DA D2R density.
Life-long KO of a receptor can produce biological changes (eg, Dickinson et al, 1999; Zahniser et al, 2000), which may compensate for its absence. As compensation is thought to, at least, partially rectify a deficiency, such changes if present would result in an underestimate of the differences in the reinforcing effectiveness of food between DA D2R KO and WT mice. Such compensations might potentially eliminate differences between KOs and WTs in the other lines of subjects. It seems doubtful however that such compensations would change the present conclusion that D2Rs are more involved than D3 or D4 Rs. Further, previous studies have reported motor impairment and less activity in DA D2R KO compared with WT subjects (Baik et al, 1995; Jung et al, 1999; Kelly et al, 1998), which might be suggested to contribute to the differences between DA D2R KO and WT mice. However, the bitonic function obtained for responding with change in FR value, exhibiting both increases and decreases in responding with increases in price, indicates that differences in elasticity of food demand between these lines were not due to a simple difference in motor capacity. The slower rate of extinction in DA D2R KO subjects suggests that D2Rs has a role in response persistence per se, but most importantly demonstrates that the role of D2Rs in the reinforcing effectiveness of food is not secondary to differences in extinction. Combined, these findings demonstrate a primary role of DA D2Rs compared to D3Rs and D4Rs in mediating the reinforcing effectiveness of food.
According to the theoretical interpretation of α, lower values of α are predicted under closed vs open economy conditions, because exclusive food availability within sessions increases food's reinforcing effectiveness (Hursh and Silberberg, 2008). Consistent with this theoretical interpretation, obtained values of α were ~16–23 times lower under closed vs open economy conditions for all genotypes. Further, values of Q0 were ~10–14 times larger within the closed economy compared with the open economy, consistent with the greater consumption levels. Interestingly, there were no differences in Q0 values between WT and KO subjects under any conditions, indicating that none of the DA D2-like receptor subtypes contribute to maximal consumption and that the two reinforcement-related parameters of the model (Q0 and α) entail independent aspects of reinforcement, consistent with theoretical interpretations of the model (Hursh and Silberberg, 2008).
In summary, the current results are consistent with the interpretation that DA D2R-mediated signaling contributes significantly to the reinforcing effectiveness of food, whereas DA D3Rs and D4Rs contribute not at all or to a more limited extent. As noted above, differences between interpretations of human imaging results and the present mouse study might be resolved using behavioral economic measures of reinforcing effects, but other differences may also contribute. Extrapolating, the present findings suggest the development of behavioral interventions that may alter α values for food or novel DA D2R-selective pharmacological agents for the treatment of overeating, which would in turn limit medical consequences such as heart disease, obesity and diabetes.
FUNDING AND DISCLOSURE
The current studies were supported by funding from the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) to JLK and by R01-DA025088 and R21-DA036921 to MX. Neither the NIDA IRP nor the extramural program had a role in the design of the study, collection and analysis of data, or the decision to publish. SRH has received funding from the Federal Aviation Administration, Federal Rail Road Administration, Washington Metropolitan Area Transit Authority, NASA, UPS, Airline Pilots Association, and FedEx. PLS has received funding from Shands Hospital at the University of Florida. The authors declare no conflict of interest.
Acknowledgments
These studies were conducted with support of the National Institute on Drug Abuse Intramural Research Program. We also gratefully acknowledge the contributions of Dawn French-Evans in conducting these studies.
References
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A et al (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424–428. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ et al (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 14: 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J et al (2002). Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22: 2977–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Thomsen M, Barrett AC, Collins GT, Grundt P, Newman AH et al (2012). Cocaine self-administration in dopamine D(3) receptor knockout mice. Exp Clin Psychopharmacol 20: 352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A (2015). Ventral striatum binding of a dopamine d2/3 receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry 77: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania AC (1963). Concurrent performances: a baseline for the study of reinforcement magnitude. J Exp Anal Behav 6: 299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL (2002). Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 163: 54–61. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Mueller R, Drescher KU et al (2008). Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacol Res 58: 302–307. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM et al (2011). Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 1: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA et al (1999). Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem 72: 148–156. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S et al (2007). Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci 27: 7731–7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA et al (2013). A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse 67: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ (2007). Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci 121: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD (2014). Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior. Mol Psychiatry 19: 1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008). Economic demand and essential value. Psychol Rev 115: 186–198. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J et al (1999). Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience 91: 911–924. [DOI] [PubMed] [Google Scholar]
- Katz JL, Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK (2003). Cocaine-induced locomotor activity and cocaine discrimination in dopamine D4 receptor mutant mice. Psychopharmacology (Berl) 170: 108–114. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR et al (1997). Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19: 103–113. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G et al (1998). Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci 18: 3470–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW et al (2010). Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J Biol Chem 285: 8905–8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Grandy DK (2004). Dopamine D2 receptors mediate two-odor discrimination and reversal learning in C57BL/6 mice. BMC Neurosci 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzich PJ, Mitchell SH, Younkin A, Grandy DK (2006). Dopamine D2 receptors mediate reversal learning in male C57BL/6J mice. Cogn Affect Behav Neurosci 6: 86–90. [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A (2004) Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press: New York. [Google Scholar]
- Nemirovsky SI, Avale ME, Brunner D, Rubinstein M (2009). Reward-seeking and discrimination deficits displayed by hypodopaminergic mice are prevented in mice lacking dopamine D4 receptors. Synapse 63: 991–997. [DOI] [PubMed] [Google Scholar]
- Nevin JA, Grace RC (2000). Behavioral momentum and the law of effect. Behav Brain Sci 23: 73–90 discussion 90-130. [DOI] [PubMed] [Google Scholar]
- Reid RL (1958). The role of the reinforcer as a stimulus. Br J Psychol 49: 202–209. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Skucy JC (1969). Effect of response-independent reinforcers during extinction. J Comp Physiol Psych 67: 381–389. [Google Scholar]
- Rescorla RA, Wagner AR (1972) A theory of Pavolvian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black HA, Prokasy WF (eds), Classical Conditioning II: Current Research and Theory. Appleton-Century Crofts: New York, pp 64–99. [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G et al (1997). Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90: 991–1001. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S et al (2011). Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry 69: 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner BF (1938) The Behavior of Organisms: An Experimental Analysis. Appleton-Century-Crofts: New York. [Google Scholar]
- Soto PL, Grandy DK, Hursh SR, Katz JL (2011). Behavioral economics of food reinforcement and the effects of prefeeding, extinction, and eticlopride in dopamine D(2) receptor mutant mice. Psychopharmacology (Berl) 215: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Habibi R, Michaelides M, Patel UB, Suchland K, Anderson BJ et al (2010). Dopamine D4 receptor (D4R) deletion in mice does not affect operant responding for food or cocaine. Behav Brain Res 207: 508–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM et al (2013). Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry 18: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357. [DOI] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI et al (2012). Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology 37: 1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT et al (1997). Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19: 837–848. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Simosky JK, Mayfield RD, Negri CA, Hanania T, Larson GA et al (2000). Functional uncoupling of adenosine A(2A) receptors and reduced responseto caffeine in mice lacking dopamine D2 receptors. J Neurosci 20: 5949–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]