Abstract
The striatum has an essential role in neural control of instrumental behaviors by reinforcement learning. Adenosine A2A receptors (A2ARs) are highly enriched in the striatopallidal neurons and are implicated in instrumental behavior control. However, the temporal importance of the A2AR signaling in relation to the reward and specific contributions of the striatopallidal A2ARs in the dorsolateral striatum (DLS) and the dorsomedial striatum (DMS) to the control of instrumental learning are not defined. Here, we addressed temporal relationship and sufficiency of transient activation of optoA2AR signaling precisely at the time of the reward to the control of instrumental learning, using our newly developed rhodopsin-A2AR chimeras (optoA2AR). We demonstrated that transient light activation of optoA2AR signaling in the striatopallidal neurons in ‘time-locked' manner with the reward delivery (but not random optoA2AR activation) was sufficient to change the animal's sensitivity to outcome devaluation without affecting the acquisition or extinction phases of instrumental learning. We further demonstrated that optogenetic activation of striatopallidal A2AR signaling in the DMS suppressed goal-directed behaviors, as focally genetic knockdown of striatopallidal A2ARs in the DMS enhanced goal-directed behavior by the devaluation test. By contrast, optogenetic activation or focal AAV-Cre-mediated knockdown of striatopallidal A2AR in the DLS had relatively limited effects on instrumental learning. Thus, the striatopallidal A2AR signaling in the DMS exerts inhibitory and predominant control of goal-directed behavior by acting precisely at the time of reward, and may represent a therapeutic target to reverse abnormal habit formation that is associated with compulsive obsessive disorder and drug addiction.
INTRODUCTION
The striatum has an essential role in neuronal control of the balance between flexible, goal-directed actions and repetitive, habitual behaviors to achieve optimal performance of task (Brown Gould and Graybiel, 2010; Yin and Knowlton, 2006). The striatum is distinguished into the dorsomedial striatum (DMS), which mediates the acquisition and expression of goal-directed behavior through action-outcome learning, and the dorsolateral striatum (DLS), which mediates habit formation through stimulus-response learning (Brown Gould and Graybiel, 2010; Yin and Knowlton, 2006). The shift between goal-directed and habitual actions is associated with changes in neural substrates from DMS to DLS (Yin and Knowlton, 2006) and critically involves the orbitofrontal and striatal circuits (Burguiere et al, 2013; Gremel and Costa, 2013). Dysfunction in normal shift between goal-directed and habit actions may contribute to obsessive compulsive disorder (Gillan et al, 2011), relapse of drug addiction (Ostlund and Balleine, 2008), habit learning deficit in Parkinson's patients (Knowlton et al, 1996), and preservative behaviors of Huntington's disease (Lawrence et al, 1998; Redgrave et al, 2010). Striatal control of instrumental learning involves critical functions of striatal dopamine and glutamate signaling (Lovinger, 2010; Yin et al, 2008): the nigrostriatal dopaminergic pathway provides a ‘prediction error' signal for instrumental learning through reinforcement (Rossi et al, 2013; Steinberg et al, 2013); the activation of glutamatergic corticostriatal pathway is critical to the ‘gain' control of cortical incoming information for action-outcome learning (Histed et al, 2009; Reynolds et al, 2001).
The adenosine A2A receptors (A2ARs) are highly enriched in the postsynaptic striatopallidal neurons (Svenningsson et al, 1999) where A2ARs interact with dopamine D2 receptors (D2Rs) (Canals et al, 2003) and NMDA receptors (Higley and Sabatini, 2010), as well as metabotropic glutamate 5 receptors (Ferre et al, 2002). Thus, striatopallidal A2ARs can integrate incoming information (glutamate) and neuronal sensitivity to this incoming information (dopamine) to control striatal synaptic plasticity and cognitions including goal-directed and habit behaviors (Chen, 2014). Indeed, genetic inactivation of striatal A2ARs impairs habit formation (Yu et al, 2009) and pharmacological reduction of A2AR-mediated cAMP-pCREB signaling in the DMS enhances goal-directed ethanol drinking (Nam et al, 2013). However, the contributions of the striatopallidal A2ARs in the DLS and DMS, two heterogeneous subregions underlying distinct DLS-related habitual or DMS-related goal-directed behavior, to the control of instrumental behavior are not defined.
Furthermore, the reward-based learning mechanism predicts that concurrent activation of the striatal neurons and reward-associated dopaminergic neuron activity is critical to reinforcement learning (Reynolds et al, 2001; Schultz et al, 1997). However, whether the transient activation of the striatopallidal A2AR signaling precisely at the time of reward is required or sufficient to modify instrumental learning is not known, largely because of the lack of methods to control A2AR signaling in intact animals with required spatiotemporal resolution. To overcome this limitation, we have developed chimeric rhodopsin-A2AR proteins (optoA2AR) by fusing the extracellular and transmembrane domains of rhodopsin with the intracellular loops of the A2AR (Li et al, 2015). We leveraged the spatiotemporal resolution of optoA2AR to activate striatopallidal A2AR signaling in a ‘time-locked' manner precisely at the time of the reward. Coupling the optoA2AR approach with a satiety-based instrumental learning procedure (Derusso et al, 2010), we defined the contribution of striatopallidal A2AR signaling in the DMS and DLS, precisely at or randomly in relation to the time of the reward, to the control of goal-directed and habitual behaviors. We further validated the striatopallidal A2AR control of instrumental learning by focal knockdown of striatopallidal A2ARs in the DMS and DLS using the AAV-Cre/flox strategy.
MATERIALS AND METHODS
Development of OptoA2AR Strategy
We have developed a optoA2AR, which retains the extracellular and transmembrane domains of rhodopsin (conferring light responsiveness), fused with the intracellular loops of A2AR (conferring specific A2AR signaling), as we described recently (Li et al, 2015). The specificity of the optoA2AR signaling was confirmed by light-induced selective enhancement of cAMP and phospho-MAPK levels, by the disappearance of light-induced optoA2AR signaling with a point mutation at the C-terminal region of A2AR, and by the demonstration that optoA2AR activation produced similar activation of signaling, synaptic plasticity, and behavioral responses in intact animals as the A2AR agonist CGS21680 (Li et al, 2015). We have constructed viral vectors for optoA2AR (AAV5-EF1α-DIO-mCherry-optoA2AR) and its control (AAV5-EF1α-DIO-mCherry) using a double-floxed inverted (DIO) strategy to target mCherry-optoA2AR fusions in Cre-expressing striatopallidal neurons. The AAV5-EF1α-DIO-mCherry-optoA2AR or AAV5-EF1α-DIO-mCherry was injected to adora2a-cre mice (MMRRC: 031168-UCD) in which the expression of Cre recombinase under the control of A2AR gene regulatory elements was restricted to the striatopallidal neurons (but not cholinergic interneurons or the cortical–striatal projection neurons) (Durieux et al, 2009).
Stereotaxic AAV Injection, Optic Fiber Implantation, and Optogenetic Activation of OptoA2AR Signaling
For optoA2AR stimulation experiment, AAV5-EF1α-DIO-mCherry-optoA2AR or AAV5-EF1α-DIO-mCherry (200 nl per striatum) was injected to the DMS (AP, 0.98 mm; ML, 1.20 mm; DV, 2.50 mm) or DLS (AP, 0.98 mm; ML, 2.20 mm; DV, 2.60 mm) of adora2a-cre mice unilaterally. Optic fiber with 200 μm diameter was implanted into relevant brain tissue 0.5 mm above the virus injection site. The mice were maintained for 3 weeks to achieve sufficient virus expression before behavioral training.
Optogenetic stimulation of optoA2AR signaling was achieved by turning on light (473 nm, 10 mW power at the tip) for 2 s per reward (within average 30 or 60 s interval per reward session). To achieve ‘time-locked' activation of optoA2AR for 2 s precisely at the time of reward delivery, we programmed optical stimulation to be activated each time contingent on the mouse active lever pressing and delivery of sucrose reward (Figure 2b). ‘Random' light stimulation was programmed to randomly deliver light in relation to the reward (ie anytime within the interval periods between every two rewards) with same light stimulation parameters as ‘time-locked' stimulation (Figure 2b). Light stimulation manipulations were conducted only during random interval (RI) training sessions (Figures 2c, e and 3b).
The Cre-Flox-Mediated Conditional A2AR-Knockdown Strategy
Conditional knockdown of the A2AR gene was achieved by injecting Cre recombinase-expressing AAV into distinct striatal subregions of the A2AR-floxed (A2ARflox/flox) mice with the exon 2 of the A2AR gene being flanked by insertion of flox sequences, as we described recently (Lazarus et al, 2011). Specifically, AAV8-Cre-zsGreen (200 nl per striatum) was injected into the DMS and DLS of wild-type (WT, A2AR+/+) and the floxed (A2ARflox/flox) mice bilaterally.
Satiety-Based Instrumental Training
Training session (CRF→RI30→RI60)
Mice were subjected to satiety-based instrumental learning paradigm as we described previously (Yu et al, 2009). In brief, mice underwent 3 or 4 days of continuous reinforcement (CRF) training, followed by RI schedule, which promoted habitual behavior: mice were trained 2 days on RI 30 s schedule, followed by 4 days on the RI 60 s schedule (with a 0.1 probability of reward availability every 3 s (RI30) or 6 s (RI60) contingent upon lever pressing).
Devaluation test
Following the training sessions, a 2-day devaluation test was conducted. A specific satiety procedure was applied to alter the current value of a specific reward. On each day, the mice were allowed to have free access to home chows (at least 0.5 g per mouse) or sucrose solution (at least 1 ml per mouse) for at least an hour to achieve sensory-specific satiety. Immediately after the unlimited prefeeding session, mice were given a 5-min extinction test during which the lever was inserted and pressing times was recorded without reward delivery. For each mouse, lever press rate during the devaluation test was normalized to the lever press rate during the last day of RI60 training session before the devaluation test.
Immunofluorescence
Immunofluorescence was performed on free-floating sections (30 μm) using the procedure as we described recently (Augusto et al, 2013; Shen et al, 2013). Primary antibodies were incubated following the manufacturer's protocols: A2AR (Santa Cruz; 1 : 100), p-MAPK (Cell Signal; 1 : 200), mCherry (Clontech; 1 : 500), enkephalin (Abcam; 1 : 500), and substance-P (Abcam; 1 : 500). Sections were then rinsed and incubated with Alexa 488- or Alexa 594-conjugated secondary antibodies (Invitrogen; 1 : 1000). Slices were washed and mounted and images were acquired and quantified as mean integrated optical density using Image Pro Plus.
Statistical Analysis
Acquisition data were analyzed using two-way ANOVA for repeated measurements with training sessions as within-subjects effect and optoA2AR stimulation types or conditional knockdown genotypes as between-subjects effect. For the devaluation test, we performed two-way ANOVA for repeated-measures with optogenetic stimulation types or A2AR conditional knockdown genotypes as one factor and outcome devaluation as another factor. This was followed by simple main-effect analyses to determine the within-subject effect of devaluation test in each group. In addition, as per the experimental design, we also performed planned comparisons within each group between the devalued and valued conditions using a paired t-test.
RESULTS
Targeted Expression of OptoA2AR and MAPK Signaling by OptoA2AR Activation in the Striatopallidal Neurons
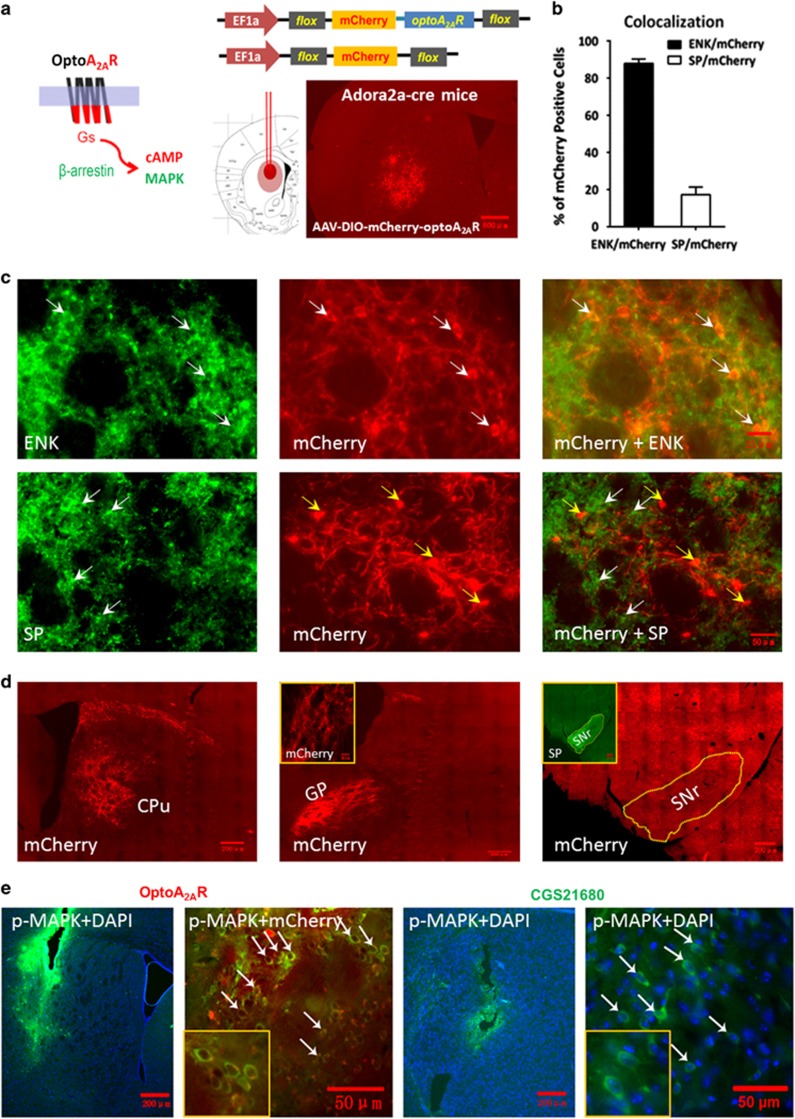
Two weeks after the injection of AAV5-EF1α-DIO-mCherry-optoA2AR and its control vector into the striatum of the adora2a-Cre mice (Figure 1a), we verified the selective expression of optoA2AR in the striatopallidal neurons. Quantitative analysis of double immunofluorescence staining result indicated that 88% of mCherry (optoA2AR-mCherry)-positive cells were colocalized with encephalin (a marker for the striatopallidal neurons), whereas only 17% mCherry-positive cells were colocalized with substance-P (a marker for the striatonigral neurons) in the striatum (Figure 1b). Representative double-immunofluorescence staining images illustrated the colocalization of optoA2AR-mCherry with enkephalin but not substance-P (Figure 1c). Furthermore, the red (mCherry) fluorescence was specifically expressed in the terminals of the striatopallidal neurons in the globus pallidus, but was absent in the terminals of striatonigral neurons in the substantia nigra pars reticularta where substance P are highly expressed (Figure 1d). These results confirmed the selective expression of optoA2AR in the striatopallidal neurons. Moreover, optoA2AR stimulation in the striatum for 5 min induced p-MAPK in the mCherry-positive cells underneath the optic fiber (Figure 1e) in a similar pattern as the A2AR agonist CGS21680. Quantified analysis showed that light-induced p-MAPK activation was detected in 57% mCherry-optoA2AR-positive cells (n=1218 from 4 mice). Thus, optoA2AR and CGS21680 produced indistinguishable p-MAPK signaling in the striatum.
Figure 1.
Targeted expression and phospho-MAPK (p-MAPK) signaling of optoA2AR in striatopallidal neurons. (a) Schematic illustration of the optoA2AR chimera construction by replacing the intracellular loops 1, 2, and 3 and C terminal of the bovine rhodopsin with that of the adenosine A2A receptor (A2AR) to achieve control of A2AR signaling by 473 nm light (left panel). Representative fluorescent image shows the expression of mCherry-optoA2AR in the striatum after injection of AAV5-DIO-mCherry-optoA2AR to adora2a-cre mice for 2 weeks (right panel). (b) The quantitative data shows that 88% mCherry-positive cells (n=114, from four mice) were colocalized with enkephalin (ENK), whereas only 17% mCherry-positive cells (n=106, from four mice) were colocalized with substance P (SP). (c) Double immunostaining with the mCherry and the specific antibodies (ENK or SP) showed that optoA2ARs were specifically expressed in ENK-positive striatopallidal neurons (white arrows, upper panels) but not SP-positive striatonigral neurons (yellow arrows, lower panels). (d) Following injection of AAV-DIO-mCherry-optoA2AR virus in the dorsomedial striatum (DMS) of adora2a-cre mice, the mCherry fluorescence of striatopallidal projection terminals was specifically expressed in the global pallidum (GP) but not in the substantia nigra pars reticulate (SNr). The green fluorescence of striatonigral projection terminals containing endogenous SP was specifically expressed in the SNr. (e) The expression of p-MAPK was induced by optoA2AR stimulation (white arrows, left panels) or CGS21680 injection (white arrows, right panels). Quantified analysis showed that light-induced p-MAPK activation was detected in 57% mCherry-optoA2AR-positive cells (n=1218 from four mice).
Optogenetic Activation of Striatopallidal A2AR Signaling in the DMS, Precisely at (but not Randomly in Relation to) the Time of the Reward, Suppressed Goal-Directed Behavior
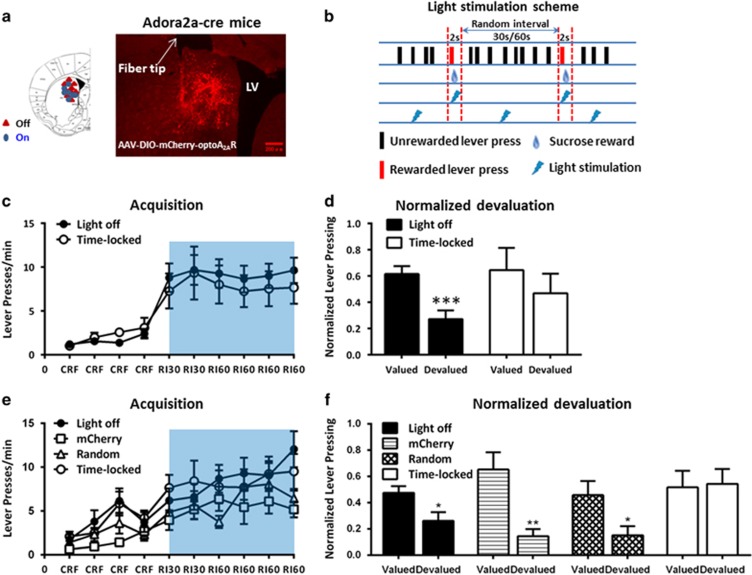
To determine the effect of optoA2AR signaling in the DMS and DLS on goal-directed and habitual actions using a satiety-based instrumental learning paradigm, we first performed an devaluation time-course study to select specific RI training schedule that were most likely sensitive to bidirectional manipulation of the A2AR activity in the DMS and DLS. Devaluation test revealed that after the CFR→RI30→RI60 training, mice showed a clear goal-directed behavior on the 3rd day, developed habitual behavior on the 4th day, and became a stable habitual behavior on the 5th day after RI60 training (Supplementary Figure 1). Since the mice on the 4th day of RI60 schedule were at the transition period from goal-directed to habitual behavior and were most sensitive to bidirectional manipulation of A2ARs in the DMS and DLS, we used the RI60 training for 4 days for the rest of the experiments.
We verified that the locations of the optical fiber implantation sites and expression of optoA2AR were restricted to the DMS by immunofluorescence (Figure 2a). At the RI sessions, we used the ‘time-locked' method to deliver optoA2AR stimulation (for 2 s per reward) precisely at the time of reward delivery (Figure 2b). Mice with ‘light off' serviced as controls. All mice gradually increased their lever pressing rates to obtain reward and reached the lever pressing plateau at the second day of RI training. There was no main effect of optoA2AR stimulation (F1,14=0.371, p>0.05) nor optoA2AR stimulation × RI training course interaction effect (F5,70=0.098, p>0.05) by repeated-measures ANOVA. Thus, optogenetic activation of the striatopallidal A2AR signaling in the DMS did neither impair lever pressing performance nor affect acquisition of instrumental learning (Figure 2c).
Figure 2.
‘Time-locked' but not random optogenetic activation of striatopallidal adenosine A2A receptor (A2AR) signaling in the dorsomedial striatum (DMS) suppresses goal-directed behavior. (a) Left panel: Schematic illustration of the locations of the fiber tips for each animal in the ‘light-off' group (the red triangles) and ‘time-locked' activation group (the blue circles). Right panel: Typical coronal section of mCherry-optoA2AR expression in the DMS of adora2a-cre(+) mice. The white arrow indicates the optical fiber tip. (b) Schematic illustration of timing of lever pressing, sucrose reward delivery, and optical stimulation. Light stimulation (the blue flash) was delivered to the DMS during a 2-s period in ‘time-locked' manner with (the flashes between the two red dotted vertical lines) or in ‘random' manner with (the flashes in the random interval periods) reward delivery (the liquid drops). (c) Two groups of mice expressing optoA2AR in the DMS were subjected to either ‘time-locked' light stimulation or ‘light off' (n=8 per group) during the random interval (RI) training session (as indicated by the blue bar). The two groups performed indistinguishably in the acquisition phase of instrumental learning by repeated-measures analysis of variance (ANOVA)—RI period × optoA2AR stimulation interaction effect: F5,70=0.098, p>0.05; optoA2AR stimulation main effect: F1,14=0.371, p>0.05. (d) Following the RI training sessions, a 2-day devaluation test without any experimental (optoA2AR activation) manipulation was conducted as described in the Materials and Methods section. Mice without optoA2AR activation during the RI training sessions significantly reduced their lever presses in devalued condition compared with valued condition (normalized devaluation: t1,7=6.861, ***p<0.001, preplanned t-test). By contrast, mice with optoA2AR ‘time-locked' stimulation showed no significant devaluation effect (normalized devaluation: t1,7=0.709, p>0.05, preplanned t-test). However, there was no normalized devaluation × optoA2AR interaction effect by repeated-measures ANOVA analysis (F1,14=0.429, P=0.523). (e) We further performed instrumental behavioral analyses of a separate set of four experimental groups: mice expressing mCherry with ‘time-locked' light stimulation (n=7), mice expressing optoA2AR with ‘light off' (n=9), mice expressing optoA2AR with ‘time-locked' light stimulation (n=8), and mice expressing optoA2AR with random light stimulation (n=8). Consistent with the result in (c) repeated-measures ANOVA analysis indicated that there was neither between-subject effect (F3,28=1.481, p=0.241) nor RI training sessions × manipulation groups interaction effect (F15,140=1.284, p=0.220) in the acquisition phase. (f) Repeated-measures ANOVA analyses of the devaluation test revealed that there was significant effect of optogenetic manipulation × (normalized) devaluation interaction effect: F3,28=3.258, p=0.036. Similarly, the simple main-effect analyses of the devaluation test in four groups indicated that only mice with optoA2AR expression in the DMS and time-locked light stimulation performed habitually, whereas other groups displayed goal-directed behavior (simple effect analyses: F1,8=7.141, *p<0.05 for ‘light off' and F1,7=6.074, *p<0.05 for ‘random' stimulation groups, and F1,6=16.050, **p<0.01 for mCherry group). Data are presented as the mean±SEM. The color reproduction of this figure is available on the Neuropsychopharmacology journal online.
The devaluation test (Figure 2d) revealed that there was no normalized devaluation × optoA2AR interaction effect (F1,14=0.429, p=0.523) by repeated-measures ANOVA. However, preplanned t-test showed that the optoA2AR mice with ‘light off' displayed a goal-directed behavior with sensitivity to devalued reward (t1,7=6.861, ***p<0.001, n=8). The goal-directed behavior in the ‘light-off' group probably reflects unstable (transient) nature of instrumental behavior for the 4-day RI60 training schedule and might be partially attributed to the relatively low level of lever pressing in this group (and the total rewards received) when the optical fiber implanted in the DMS compared with other experimental groups. Importantly the optoA2AR with ‘time-locked' stimulation during the RI sessions failed to show sensitivity to outcome devaluation (preplanned t-test, t1,7=0.709, p>0.05, n=8), indicating that their responding was habitual.
To better define the temporal importance of optoA2AR signaling precisely at the time of reward and to exclude the nonspecific effect caused by light, we have performed behavioral analyses with separate set of four experimental groups: mice expressing mCherry with ‘time-locked' light stimulation (n=7), mice expressing optoA2AR with ‘light off' (n=9), mice expressing optoA2AR with ‘time-locked' light stimulation (n=8), and mice expressing optoA2AR with ‘random' (n=8) light stimulation. The light stimulation scheme was illustrated in Figure 2b. Consistent with the result in Figure 2c, there was neither between-subject effect (F3,28=1.481, p=0.241) nor RI training sessions × manipulation groups interaction effect (F15,140=1.284, p=0.220) in the acquisition phase by repeated-measures ANOVA (Figure 2e). However, analyses of the devaluation test (Figure 2f) revealed that there was a significant effect of optogenetic manipulation × (normalized) devaluation interaction effect (repeated-measures ANOVA, F3,28=3.258, p=0.036). The simple main-effect analyses of the devaluation test, respectively, in each group confirmed that only mice with optoA2AR expression in the DMS and time-locked light stimulation performed habitually (F1,8=7.141, *p<0.05 for light off and F1,7=6.074, *p<0.05 for random stimulation groups, F1,6=16.050, **p<0.01 for mCherry group). Taken together, statistical analyses of both sets of the experiments (Figure 2d by the preplanned t-test and Figure 2f by the repeated-measures ANOVA) support that optogenetic activation of striatopallidal A2AR signaling in the DMS modulated the mode of instrumental behaviors by acting precisely at the time of the reward.
Optogenetic Activation of Striatopallidal A2AR Signaling in the DLS had Relatively Limited Effects on Habitual Formation
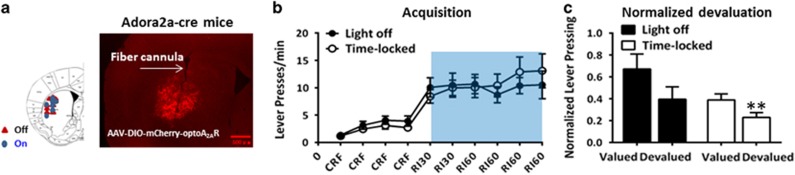
Next, we examined the effect of optoA2AR signaling in the DLS on instrumental behaviors. Similarly, we confirmed the optical fiber implantation sites and expression of optoA2AR to be restricted to DLS by immunofluorescence (Figure 3a). Following the RI training sessions, optoA2AR mice with ‘light off' (n=10) or with ‘time-locked' stimulation (n=13) gradually increased lever presses. There was no main effect of optoA2AR stimulation (F1,21=0.156, p>0.05) and no interaction effect of training session × optoA2AR stimulation in the RI sessions (F5,105=0.916, p>0.05) by repeated-measures ANOVA (Figure 3b). After the 4th day of RI60 training, repeated-measures ANOVA analyses of the devaluation test revealed that there was no optogenetic manipulations × normalized devaluation interaction effect (F1,21=0.022, p=0.884). However, the preplanned t-test showed that optoA2AR mice with ‘time-locked' stimulation tended to perform goal-directed behavior (normalized devaluation test, t1,12=3.725, **p<0.01 (Figure 3c); devaluation test, t1,12=2.030, p>0.05 (Supplementary Figure 2c)). Conversely, optoA2AR mice with ‘light off' displayed habitual behavior (normalized devaluation test, t1,9=1.270, p>0.05 (Figure 3c); devaluation test, t1,9=1.868, p>0.05 (Supplementary Figure 2c)). Thus, optogenetic activation of striatopallidal A2AR signaling in the DLS tended to promote goal-directed behavior, but its effect was relatively limited.
Figure 3.
Optogenetic activation of striatopallidal adenosine A2A receptor (A2AR) signaling in the dorsolateral striatum (DLS) exerts relatively limited and possibly opposite control over habitual action compared with the optoA2AR in the dorsomedial striatum (DMS). (a) Left: Schematic illustration of the sites of optical fibers implantation. Right: A representative image of mCherry-optoA2AR expression and fiber implantation. (b) Mice were under continuous reinforcement (CRF) training followed by RI30 and then RI60 training with or without optoA2AR stimulation as described in the Materials and Methods section. The performances of optoA2AR mice with ‘time-locked' stimulation (n=13) or with ‘light off' (n=10) during the acquisition phase were indistinguishable (repeated-measures analysis of variance (ANOVA), random interval (RI) training course × optogenetic stimulation interaction: F5,105=0.916, p>0.05; optoA2AR stimulation main effect: F1,21=0.156, p>0.05). (c) OptoA2AR mice with ‘time-locked' stimulation or ‘light off' during the RI training sessions were subjected to devaluation test as described in the Materials and Methods section. Repeated-measures ANOVA analyses revealed that there was no normalized devaluation × optogenetic stimulation interaction effect (F1,21=0.022, p=0.884). However, preplanned t-test analysis revealed that optoA2AR mice receiving ‘time-locked' stimulation tended to perform goal-directed behavior (only for the normalized devaluation test: t1,12=3.725, **p<0.01; but not for devaluation test: t1,12=2.030, p>0.05; Supplementary Figure 2c). Whereas optoA2AR mice with ‘light off' displayed habitual behavior (normalized devaluation test: t1,9=1.270, p>0.05; devaluation test: t1,9=1.868, p>0.05; Supplementary Figure 2c). Data are presented as the mean±SEM. The color reproduction of this figure is available on the Neuropsychopharmacology journal online.
Knockdown of A2ARs in the DMS Enhanced Goal-Directed Behavior, Whereas Knockdown of the A2ARs in the DLS had a Limited Effect on Habitual Behavior
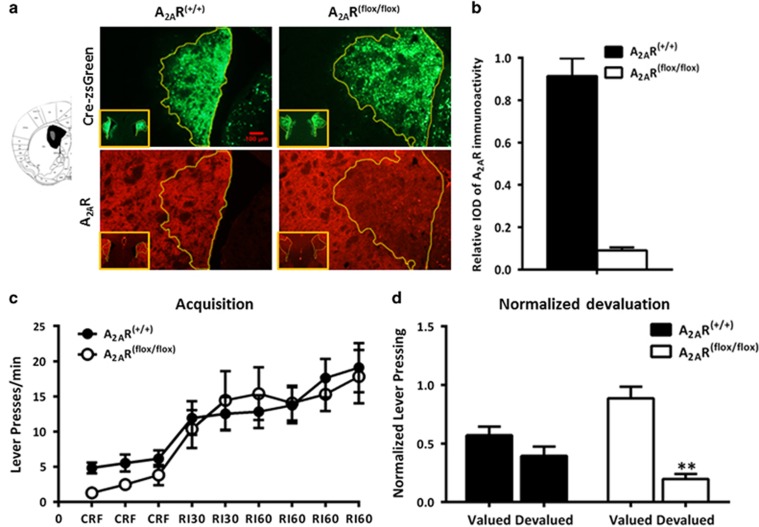
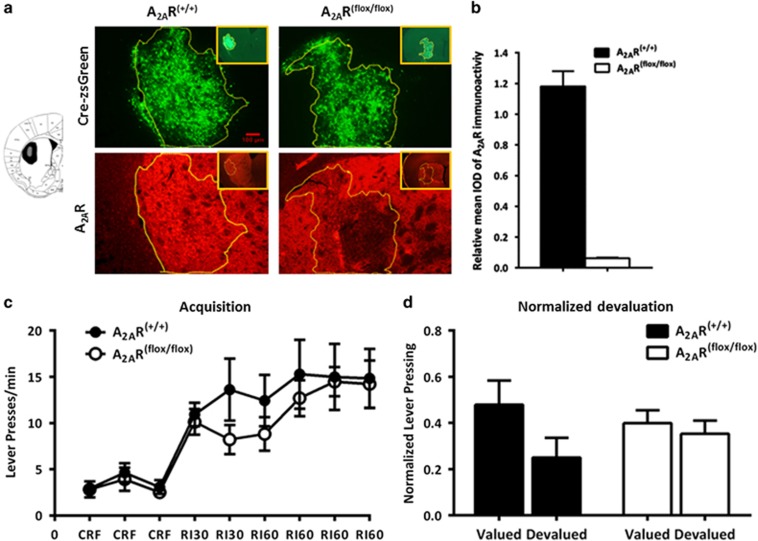
We further evaluated the effects of focal knockdown of the A2ARs in the DMS and DLS on instrumental learning. Figures 4a and 5a provided representative outline of the AAV transfection and A2AR focal knockdown areas of the DMS and DLS. Fluorescent images showed that A2ARs expression (the red fluorescence) was reduced selectively in the Cre-expressing regions (indicated by green fluorescence). Quantitative analysis of the A2AR immunoreactivity (Figures 4b and 5b) confirmed selective knockdown of A2ARs in the DMS (by 91%) and DLS (by 94%) after transfection with AAV-Cre-zsGreen only in A2ARflox/flox mice but not in WT mice (A2AR+/+).
Figure 4.
Focal knockdown of adenosine A2A receptors (A2ARs) in the dorsomedial striatum (DMS) enhances goal-directed behavior. (a) Left: Schematic illustration of the maximal (black) and minimal (gray) A2AR knockdown areas in the DMS. Right: Representative immunofluorescent photomicrographs show focal knockdown expression of A2ARs in the DMS after injection of AAV-Cre-zsGreen into the A2AR(flox/flox) (right panels) and A2AR(+/+) mice (left panels). Intensity of A2ARs (red) were significantly deceased in the overlapping area with zsGreen expression (the yellow circle) in A2AR(flox/flox) mice but not in A2AR(+/+) mice. (b) Quantitative analysis showed that A2AR expression were markedly reduced in the virus-transfected regions of A2AR(flox/flox) mice compared with A2AR(+/+) mice. (c) Two–three weeks after bilateral injection of AAV-Cre-zsGreen into the DMS, A2AR(flox/flox) mice and A2AR(+/+) mice (n=8 per group) were under CRF-RI30-RI60 training paradigm as described in the Materials and Methods section. Both groups similarly increased their lever pressing rate during the acquisition phases (repeated-measures analysis of variance (ANOVA) revealed no random interval (RI) period × genotype interaction effect: F5,65=0.859, p>0.05; and no genotype main effect: F1,13<0.001, p>0.05). (d) Mice with DMS A2AR knockdown significantly reduced their lever pressing in the devalued condition compared with that of the valued condition, but the A2AR(+/+) mice responded insensitively to the selective satiety devaluation treatment (normalized devaluation × genotype interaction effect: F1,13=9.161, p=0.01; simple effect analysis: F1,6=35.683, **p<0.01 for A2AR focal knockdown mice by repeated-measures ANOVA). Data are presented as the mean±SEM. CRF, continuous reinforcement. The color reproduction of this figure is available on the Neuropsychopharmacology journal online.
Figure 5.
Focal knockdown of the adenosine A2A receptors (A2ARs) in the dorsolateral striatum (DLS) exerts relatively limited effects on habitual behaviors. (a) Representative image shows that A2ARs were knocked down selectively in the area with the AAV-Cre-zsGreen expression in the DLS of A2AR(flox/flox) mice but not in A2AR(+/+) mice. The yellow circle depicted the boundary of the AAV-Cre-zsGreen expression and A2AR knockdown area. (b) Quantitative analysis shows that A2AR expression was markedly reduced in the AAV-Cre-zsGreen transfected regions of A2AR(flox/flox) mice compared with A2AR(+/+) mice. (c) Focal A2AR knockdown in the DLS (n=7) did not affect lever pressing during the acquisition phase compared with their A2AR(+/+) controls (n=6) (repeated-measures analysis of variance (ANOVA) revealed no random interval (RI) period × genotype interaction effect: F5,55=1.234, p>0.05; and no genotype main effect: F1,11=0.534, p>0.05). (d) There was no genotype × devaluation interaction effect (F1,11=1.993, p=0.186, repeated-measures ANOVA) for the normalized devaluation test. Both groups similarly showed insensitivity to outcome devaluation (DLS A2AR knockdown mice: normalized devaluation; t1,6=0.646, p>0.05; wild-type (WT) mice: normalized devaluation; t1,5=2.017, p>0.05). Data are presented as the mean±SEM. CRF, continuous reinforcement.
Consistent with the optoA2AR results, focal knockdown of A2ARs in the DMS (Figure 4c) and DLS (Figure 5c) did not affect the acquisition of instrumental learning as the A2ARflox/flox and WT mice transfected with AAV-Cre-zsGreen showed identical instrumental learning course at RI training session (DMS: genotype main effect, F1,13<0.001, p>0.05, RI period × genotype interaction effect: F5,65=0.859, p>0.05; DLS: genotype main effect, F1,11=0.534, p>0.05, RI period × genotype interaction effect: F5,55=1.234, p>0.05; by repeated-measures ANOVA). For the devaluation test, repeated-measures ANOVA analyses revealed that there was genotypes × devaluation interaction effect in the DMS experiment (Figure 4d, normalized devaluation, F1,13=9.161, p=0.01, simple main-effect analyses, F1,6=35.683, **p<0.01 for A2AR focal knockdown mice; Supplementary Figure 2d, devaluation, F1,13=10.231, p=0.007, simple main-effect analyses, F1,6=40.197, **p<0.01 for A2AR focal knockdown mice). This indicated that the control mice displayed a clear habitual action without sensitivity to devaluation condition, whereas focal A2AR knockdown in the DMS altered sensitivity to devaluation by markedly reducing lever presses in the devalued condition. In contrast to the DMS A2AR-knockdown effect, focal knockdown of A2AR in the DLS did not affect instrumental behavior and showed no sensitivity to devaluation condition (Figure 5d: genotypes × normalized devaluation interaction effect, F1,11=1.993, p=0.186 by repeated-measures ANOVA, and t1,6=0.646, p>0.05 for DLS A2AR-knockdown mice, t1,5=2.017, p>0.05 for WT mice by preplanned t-test; the devaluation test showed a similar result; Supplementary Figure 2e). Thus, consistent with the results of the optoA2AR, these findings validate that focal knockdown of striatopallidal A2ARs in the DMS selectively enhanced goal-directed behavior, whereas focal knockdown of striatopallidal A2ARs in the DLS had little effect on habitual behavior.
DISCUSSION
Transient and ‘Time-Locked' Activation of optoA2AR Signaling Precisely at the Time of Reward is Required and Sufficient to Modulate Goal-Directed Behavior
The contemporary theory of striatum-dependent learning postulates that the concurrent activation of presynaptic nigral–striatal dopamine (reinforcement) signaling and corticostriatal glutamate (sensorimotor) signaling and postsynaptic striatopallidal neuronal activity (modulated by neuromodulator such as adenosine) is critical to striatal synaptic plasticity and instrumental learning (Yagishita et al, 2014; Reynolds et al, 2001; Schultz et al, 1997). Indeed, modification of instrumental learning by optogenetic manipulation of striatal neurons was only effective in a narrow temporal window (ie before or concurrent with the onset of cue (Tai et al, 2012), or in the time segment (1.5 s) between action selection and outcome (Aquili et al, 2014)), supporting the temporal importance of dopamine, glutamate, and neuromodulator signaling in striatum-dependent instrumental learning. Different from rapid neurotransmitter release such as dopamine and glutamate, extracellular adenosine is generated by conversion of ATP to adenosine through a set of ectonucleotidases and by bidirectional nucleotide transporters (Chen et al, 2013). Striatopallidal A2AR activity may modulate instrumental learning by acting precisely at the time of the reward to integrate dopamine or glutamate signaling for coding the action-outcome contingency. Alternatively, striatopallidal A2ARs control instrumental learning by modulating the vigor of actions (Desmurget and Turner, 2010), by providing permissive role in learning association (Brainard and Doupe, 2000), or by modulating the ‘off-line' processing of incoming signaling (glutamate) (Pomata et al, 2008). In these alternative schemes, the temporal relationship between striatopallidal activity (ie A2AR activity) and the reward is not essential. Thus, a critical question is whether the transient activation of A2AR precisely at the time of reward delivery was required and sufficient to modulate instrumental learning. This question has not been addressed owing to the lack of methods to control A2AR signaling in behaving animals with required temporal resolution. Our development of the optoA2AR (Li et al, 2015) offers the opportunity to optogenetically control the A2AR signaling with sufficient temporal resolution. We showed that transient (2 s per reward) and ‘time-locked' light activation of the optoA2AR signaling in the striatopallidal neurons precisely at the time of the reward (but not random light stimulation) was required and sufficient to modify the sensitivity to outcome devaluation without affecting the acquisition. The requirement and sufficiency of ‘time-locked' and transient activation of optoA2AR signaling at the time of the reward to modify instrumental learning demonstrated a temporally specific relationship between adenosine A2AR signaling and nigrostriatal dopamine signaling in association with the reward delivery and possibly corticostriatal glutamate signaling that converged on the striatopallidal neurons. Considering the extensive interaction between A2ARs, D2Rs, and NMDA receptors in the striatopallidal neurons (Lovinger, 2010), we speculate that concurrent activation of A2ARs, D2Rs, and NMDA receptors in the striatopallidal neurons allows the integration of adenosine, dopamine, and glutamate signaling, and coding of the mode of instrumental learning behavior (Abeliovich et al, 1992; Tai et al, 2012).
The Striatopallidal A2AR Signaling in the DMS Provides a ‘Break' Mechanism to Constrain Instrumental Learning
As the DMS and DLS are distinctly involved in goal-directed and habitual behaviors, respectively (Balleine et al, 2009; Brown Gould and Graybiel, 2010; Yin and Knowlton, 2006), another important question is whether striatopallidal A2ARs exert DMS- and DLS-specific control over instrumental learning. Our bidirectional manipulation of the striatopallidal A2ARs by optogenetic activation of A2AR signaling and Cre-mediated knockdown of A2ARs in the DMS and DLS demonstrated that A2ARs in the DMS exerted an inhibitory and predominant control of goal-directed, whereas striatopallidal A2ARs in the DLS had relatively limited but possibly opposite effects on habit formation. This is consistent with the associative corticostriatal–DMS loop being ‘default' model of striatal function (Thorn et al, 2010) and with previous finding that deletion of the indirect pathway in the DMS (but not DLS) produces pronounced psychomotor and cognitive effects (Durieux et al, 2012). This view is also supported by recent pharmacological study that reduction of A2AR-mediated PKA-pCREB signaling in the DMS enhanced acquisition of goal-directed ethanol drinking behaviors in mice (Nam et al, 2013). Given the prominent role of the DMS in control of goal-directed behavior, our finding that focal knockdown of striatopallidal A2ARs in the DMS captures the goal-directed characteristics of striatum-specific A2AR knockout (KO) mice argue that striatum-A2AR KO mice displayed enhanced goal-directed behavior, but manifested as impaired habit formation (Yu et al, 2009). Although our analysis is designed to isolate the striatopallidal A2AR action from other action sites, this does not preclude the contribution of the A2ARs in extrastriatal or cholinergic neurons to the control of instrumental learning, which needed to be further defined.
It is worth noting that similar to striatal A2AR KO (Yu et al, 2009), either optoA2AR activation or focal A2AR knockdown of striatopallidal A2AR activity did not affect the acquisition (Figures 2c, 3b, 4c and 5c) or omission/extinction (Supplementary Figure 3) phase of instrumental learning, but specifically affect sensitivity to outcome devaluation. The lack of the optoA2AR effect during the acquisition and extinction/omission phases indicates that striatopallidal A2ARs unlikely affect general arousal status or attention to influence instrumental learning, but instead it may modify the motivation control of action selection. This notion is consistent with the critical role of striatopallidal A2ARs in the modulation of effort expenditure and motivation (Mingote et al, 2008; Nunes et al, 2013).
Lastly, bidirectional manipulation of the striatopallidal A2ARs by optoA2AR and Cre-mediated A2AR knockdown demonstrates a critical role of the postsynaptic striatopallidal A2ARs and the striatopallidal pathway in the DMS in control of instrumental learning. This collaborates with the recent finding that transient optogenetic stimulation of striatopallidal neurons introduces opposing biases during decision making in mice (Tai et al, 2012), and that loss of striatal long-term depression largely restricted to striatopallidal neurons is associated with a shift in behavioral control from goal-directed action to habitual responding (Nazzaro et al, 2012). Taken together with increasing evidences from diverse learning paradigms that striatopallidal A2ARs assume an inhibitory control over working memory (Wei et al, 2011; Zhou et al, 2009), fear condition (Singer et al, 2013; Wei et al, 2014), reversal learning (Wei et al, 2011), and instrumental learning (Yu et al, 2009), we postulate that postsynaptic striatopallidal A2AR function may provide a ‘break' mechanism to constrain some cognitions including instrumental learning (Chen, 2014). If the postulated ‘break' mechanism of the striatopallidal A2AR is validated by future experiments, this provides a framework for a pharmacological strategy by blocking striatopallidal A2AR activity to reverse abnormal habit formation that is associated with compulsive obsessive disorder and relapse of drug addiction.
FUNDING AND DISCLOSURE
This study was sponsored by the Start-up Fund from Wenzhou Medical University (No. 89211010 JFC; No. 89212012, JFC and KYQD121004, ZL), the Zhejiang Provincial Special Funds (No. 604161241), the Special Fund for Building National Clinical Key Resource (Key Laboratory of Vision Science, Ministry of Health, No. 601041241), the Central Government Special Fund for Local Universities' Development (No. 474091314), the Zhejiang Provincial Natural Science Foundation of China (No. LQ15H090007), and by NIH Grants (NS041083-11 and NS073947) and special BUSM research fund DTD 4-30-14. The authors have no proprietary interest in any materials or methods described within this article.
Acknowledgments
We thank Liu Ya for her assistance in image analysis of optical fluorescent density.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abeliovich A, Gerber D, Tanaka O, Katsuki M, Graybiel AM, Tonegawa S (1992). On somatic recombination in the central nervous system of transgenic mice. Science 257: 404–410. [DOI] [PubMed] [Google Scholar]
- Aquili L, Liu AW, Shindou M, Shindou T, Wickens JR (2014). Behavioral flexibility is increased by optogenetic inhibition of neurons in the nucleus accumbens shell during specific time segments. Learn Mem 21: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusto E, Matos M, Sevigny J, El-Tayeb A, Bynoe MS, Muller CE et al (2013). Ecto-5'-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci 33: 11390–11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Liljeholm M, Ostlund SB (2009). The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 199: 43–52. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ (2000). Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766. [DOI] [PubMed] [Google Scholar]
- Brown Gould B, Graybiel AM (2010). Afferents to the cerebellar cortex in the cat: evidence for an intrinsic pathway leading from the deep nuclei to the cortex. 1976. Cerebellum 9: 1–13. [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Feng G, Graybiel AM (2013). Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 340: 1243–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR et al (2003). Adenosine A2A-dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749. [DOI] [PubMed] [Google Scholar]
- Chen JF (2014). Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol 119: 257–307. [DOI] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB (2013). Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12: 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derusso AL, Fan D, Gupta J, Shelest O, Costa RM, Yin HH (2010). Instrumental uncertainty as a determinant of behavior under interval schedules of reinforcement. Front Integr Neurosci 4: 17 (doi: 10.3389/fnint.2010.00017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Turner RS (2010). Motor sequences and the basal ganglia: kinematics, not habits. J Neuroscience 30: 7685–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M et al (2009). D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci 12: 393–395. [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A (2012). Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J 31: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA et al (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA 99: 11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW et al (2011). Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 168: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Costa RM (2013). Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL (2010). Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Pasupathy A, Miller EK (2009). Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron 63: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR (1996). A neostriatal habit learning system in humans. Science 273: 1399–1402. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW (1998). Cognitive functions and corticostriatal circuits: insights from Huntington's disease. Trends Cogn Sci 2: 379–388. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE et al (2011). Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067–10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X et al (2015). Optogenetic activation of intracellular adenosine A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 1–11 (doi:10.1038/mp.2014.182). [DOI] [PubMed]
- Lovinger DM (2010). Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM et al (2008). Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci 28: 9037–9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A et al (2013). Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci 33: 4329–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M et al (2012). SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15: 284–293. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD (2013). Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev 37(Part A): 2015–2025. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW (2008). On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models 5: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomata PE, Belluscio MA, Riquelme LA, Murer MG (2008). NMDA receptor gating of information flow through the striatum in vivo. J Neurosci 28: 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H et al (2010). Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 11: 760–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR (2001). A cellular mechanism of reward-related learning. Nature 413: 67–70. [DOI] [PubMed] [Google Scholar]
- Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH (2013). Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One 8: e65799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Shen HY, Canas PM, Garcia-Sanz P, Lan JQ, Boison D, Moratalla R et al (2013). Adenosine A(2)A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One 8: e80902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer P, Wei CJ, Chen JF, Boison D, Yee BK (2013). Deletion of striatal adenosine A(2A) receptor spares latent inhibition and prepulse inhibition but impairs active avoidance learning. Behav Brain Res 242: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH (2013). A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 16: 966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999). Distribution, biochemistry and function of striatal adenosine A2A receptors. Progr Neurobiol 59: 355–396. [DOI] [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L (2012). Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci 15: 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM (2010). Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Augusto E, Gomes CA, Singer P, Wang Y, Boison D et al (2014). Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol Psychiatry 75: 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK et al (2011). Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem 18: 459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H (2014). A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345: 1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nat Rev Neurosci 7: 464–476. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW (2008). Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci 28: 1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH (2009). Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci 29: 15100–15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT et al (2009). Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res 1303: 74–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.